Abstract
An increase in fluoroquinolone resistance and transrectal ultrasound-guided prostate (TRUS) biopsy infections has prompted the need for alternative effective antibiotic prophylaxis. We aimed to compare ciprofloxacin and other single-agent therapies to combination therapy for efficacy and adverse effects. Men who underwent a TRUS biopsy within the VA Boston health care system with documented receipt of prophylactic antibiotics periprocedure were eligible for inclusion. Postprocedure infections within 30 days were ascertained by chart review from electronic records, including any inpatient, outpatient, or urgent-care visits. Among 455 evaluable men over a 3-year period, there were 25 infections (5.49%), with sepsis occurring in 2.4%, urinary tract infections (UTI) in 1.54%, and bacteremia in 0.44% of patients. Escherichia coli was the most common urine (89%) and blood (92%) pathogen, with fluoroquinolone resistance rates of 88% and 91%, respectively. Ciprofloxacin alone was associated with significantly more infections than ciprofloxacin plus an additional agent (P = 0.014). Intramuscular gentamicin alone was also significantly associated with a higher infection rate obtained with all other regimens (P = 0.004). Any single-agent regimen, including ciprofloxacin, ceftriaxone, or gentamicin, was associated with significantly higher infection rates than any combination regimen (odds ratio [OR], 4; 95% confidence interval [CI], 1.47, 10.85; P = 0.004). Diabetes, immunosuppressive condition or medication, hospitalization within the previous year, and UTI within the previous 6 months were not associated with infection risk. Clostridium difficile infections were similar. These findings suggest that ciprofloxacin, ceftriaxone, and gentamicin alone are inferior to a combination regimen. Institutions with high failure rates of prophylaxis for TRUS biopsies should consider combination regimens derived from their local data.
INTRODUCTION
A transrectal ultrasound-guided prostate biopsy (TRUS-guided prostate biopsy) is the most commonly used technique to diagnose prostate cancer (1). The most common complication of TRUS biopsies is infection, specifically urinary tract infection (UTI), bacteremia, and septicemia. Therefore, antibiotic prophylaxis is standard practice prior to the procedure to prevent postbiopsy infections. The American Urological Association (AUA) recommends single-agent prophylaxis with fluoroquinolones (FQs) or cephalosporins (drugs of choice) or trimethoprim-sulfamethoxazole or aminoglycosides (alternatives) for periprocedure prophylaxis. Fluoroquinolones, specifically ciprofloxacin, are most commonly used due to their broad spectrum of activity, oral administration, good penetration to the prostate gland, and long-lasting activity (2).
The most common organism responsible for post-TRUS biopsy infection complications is Escherichia coli (3). Unfortunately, fluoroquinolone resistance among E. coli organisms is increasing, with reported rates as high as 22% in patients undergoing a urological procedure (4). A recent study showed the rate of postprocedure infection after TRUS biopsy to be 2.65% and on an upward trend from 2006 to 2010 (5). Based on these data and other studies of alternative prophylactic regimens, the preferred prophylactic regimen at our institution was changed from ciprofloxacin to ciprofloxacin plus cefpodoxime in late 2012.
Previous studies have compared different prophylaxis regimens for TRUS biopsy, mostly comparing ciprofloxacin to another single agent. One study evaluated the addition of gentamicin as a second agent to standard ciprofloxacin and found a reduced postprocedure hospitalization rate (6). However, no study has evaluated a general strategy of single-agent- versus combination-based therapy. The risks of using a two-drug regimen includes increased rates of adverse effects and an increase in cost. Therefore, we sought to evaluate the comparative effectiveness of single-agent therapy and combination therapy for reduction in postbiopsy infection rates.
MATERIALS AND METHODS
We conducted a retrospective cohort study of patients who underwent a TRUS biopsy within the VA Boston Healthcare System between January 2011 and October 2013. All patients receive periprocedure prophylactic antibiotics as the standard of care. Patients who had documentation of taking the prescribed regimen were eligible for inclusion. Patients were excluded if they underwent a simultaneous procedure at the time of prostate biopsy, such as bladder endoscopy, if they did not have clear documentation of antibiotics received, or if the procedure was not completed. The Veterans Administration (VA) institutional review board approved the study protocol.
The primary objective was to compare the rates of infection-related complications in patients receiving ciprofloxacin and in those receiving alternative regimens, such as ciprofloxacin plus a cephalosporin or a non-FQ regimen. Assessment for an infection-related complication was limited to 30 days postprocedure to accurately include only those patients with a procedure-related event. Infection-related complications included symptomatic UTI with fever, bacteremia, or sepsis. UTI with fever was defined as bacteriuria postprocedure associated with clinical signs of UTI (dysuria, frequency, and urgency) and a temperature of ≥38°C. Bacteremia was defined as the presence of bacteria in the blood culture, irrespective of clinical signs. Sepsis was defined as systemic inflammatory response syndrome (SIRS) caused by infection (SIRS is defined as two or more of the following: temperature of ≥38°C [centigrade] or less than 36°C, heart rate more than 90 beats/minute, respiratory rate more than 20 breaths/minute or respiratory alkalosis, white blood cell count more than 12,000, or immature forms more than 4,000 or more than 10%) (2).
Baseline demographic data (age and race), clinical data (diabetes, immunosuppressive condition, or medications), and information regarding hospitalization within the previous year, antibiotic use within the previous 6 months, UTI within the previous year (symptoms plus a positive culture), antibiotics received prior to the procedure and infection postprocedure, adverse drug effects, and episodes of Clostridium difficile infection within 30 days of the procedure were collected by manual review of the electronic medical record. If a patient was admitted to the hospital for an infection, blood and urine culture data with pathogen and sensitivities, antibiotics received, and discharge diagnosis were collected utilizing manual chart review. Data for patients admitted to outside hospitals were available through a non-VA care progress note that is routinely entered by a nurse.
The statistical analyses were conducted in JMP (version 12; JMP, Cary, NC). Multivariate analyses were performed using logistic regression with infection as the primary outcome. Covariates were determined by their univariate significance. We hypothesized that the incidence of infection with alternative combination regimens would be 1%, compared to 5 to 10% with ciprofloxacin alone; thus, for 80% power with an α of 0.05, a range of 200 to 570 patients were needed.
RESULTS
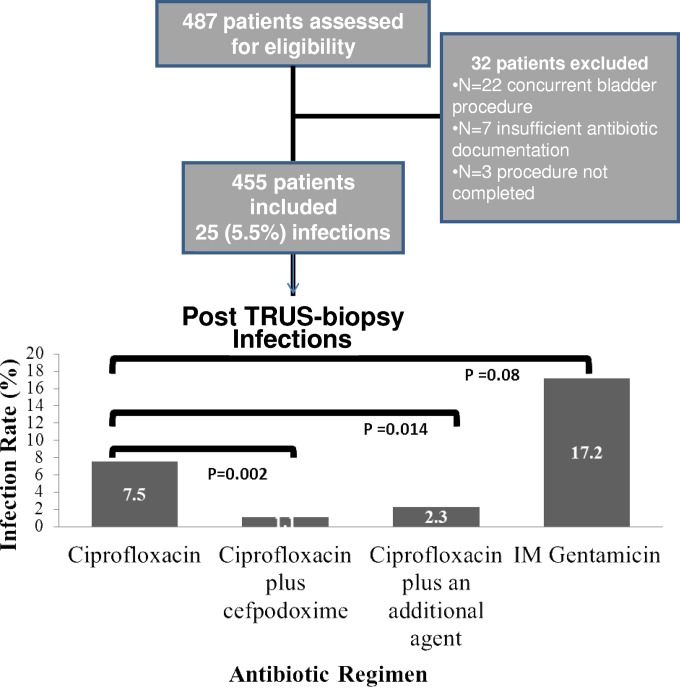
A total of 487 subjects were evaluated during the study period (Fig. 1). Of these men, 22 were excluded because of undergoing concurrent bladder procedures, 7 had insufficient antibiotic documentation, and 3 did not complete the procedure. The characteristics of the 455 included subjects are shown in Table 1. The mean age was 65 years, the majority were Caucasian, and almost half had undergone prostate biopsy previously. Among the 455 men included in the study cohort, almost half (200, 43.9%) received ciprofloxacin alone, 182 received ciprofloxacin plus cefpodoxime, and the remaining 73 received another regimen. The other regimens included ceftriaxone alone (6), intramuscular (i.m.) gentamicin alone (29), or combination regimens. As expected based on the institutional policy change, the use of a combination regimen was more common in years 2 and 3 than in year 1 of the study.
FIG 1.
Flow chart of study participants and risk of infections post-TRUS biopsy based on antibiotic regimen.
TABLE 1.
Characteristics of the study cohort
| Patient characteristic | Total no. of patients (n = 455) |
|---|---|
| Age (mean; yrs) | 65 |
| Race | |
| Caucasian (%) | 86.9 |
| African American (%) | 9.4 |
| Diabetes mellitus (%) | 20.7 |
| Immunosuppressive medication or disease (%) | 8.1 |
| Hospitalization within the previous 1 yr (%) | 21.9 |
| Antibiotic use within the previous 6 mo (%) | 21.2 |
| UTI within the previous 1 yr (%) | 4.7 |
| History of prior biopsy (%) | 41.8 |
| Periprocedure prophylaxis regimen | |
| Ciprofloxacin | 200 |
| Ciprofloxacin plus cefpodoxime | 182 |
| Other regimen | 73 |
There were a total of 25 infections (5.49%) in the study cohort, with sepsis occurring in 2.4%, UTI in 1.54%, and bacteremia in 0.44% of patients. E. coli was the most common blood (92%) and urine (89%) pathogen, with fluoroquinolone resistance rates of 91% and 88%, respectively. Fluoroquinolone resistance among E. coli isolates in general at our institution during the study period was approximately 25%. Klebsiella was the only other pathogen isolated. All patients who developed an infection-related complication presented to the hospital within 3 days of their procedure.
There were significantly more infections in the ciprofloxacin group (15 [7.5%]) than in the group receiving dual therapy with ciprofloxacin plus cefpodoxime (2 [1.1%]) (OR, 7.29; 95% CI, 1.65 to 32.37; P = 0.002) (Fig. 1). There were also significantly more infections with ciprofloxacin alone than there were with ciprofloxacin plus any additional agent (5 [2.3%]) (P = 0.014). Patients given intramuscular gentamicin alone had significantly more infections (5/29 [17.2%]) than did those receiving any alternative regimen (20/426 [4.69%]) (odds ratio [OR], 4.23; 95% confidence interval [CI], 1.5 to 12.2; P = 0.004). Ciprofloxacin alone was associated with a statistical trend for fewer infections than observed with gentamicin alone (OR, 0.39; 95% CI, 0.13 to 1.17; P = 0.08).
In univariate analyses, the rates of infection were not significantly different among patients who were hospitalized or had UTI in the past year and those who did not. In addition, diabetes, immunocompromising medications or disease, and previous biopsy were not found to be significantly associated with postbiopsy infection risk. The rate of infection was 3-fold higher in 2011-2012 than in 2013, 6.8% versus 2.3% (P = 0.06).
In multivariate analyses, year of infection was no longer significantly associated with infection risk after accounting for a single-agent versus combination antibiotic regimen. Use of any single-agent regimen, including ciprofloxacin, ceftriaxone, or i.m. gentamicin, was associated with significantly higher infection rates than obtained with any combination regimen (OR, 4.0; 95% CI, 1.47 to 10.85; P = 0.004), independent of UTI history and year of study.
There were no drug-related adverse events in any of the treatment groups. There was one episode of C. difficile infection in a patient who received combination therapy (1/220 [0.5%]) with ciprofloxacin and cefpodoxime and none in the single-agent treatment group (0/235 [0%]), for a nonsignificant difference of 0.5% between groups.
DISCUSSION
Prostate biopsy-related infections are largely preventable events if appropriate and active antimicrobial prophylaxis is utilized. This has become more challenging with increasing rates of multidrug-resistant Gram-negative organisms. Designing a regimen that is safe, effective, and easily deliverable to an outpatient population and that minimizes unnecessary antimicrobial exposure requires consideration of two-drug regimens rather than the traditional single-agent prophylaxis that has been the standard of care. The data presented here support the use of a combination regimen in terms of both efficacy and safety. In fact, the two single-agent regimens recommended by the AUA were both significantly associated with inferior outcomes. The use of any combination antibiotic regimen was highly protective and demonstrated a 75% reduction in infection rate compared to those obtained with ciprofloxacin or i.m. gentamicin or ceftriaxone alone. Importantly, there were no drug-related adverse events in either group, and no significant difference in C. difficile rates between the groups.
The optimal combination regimen likely varies by institutional rates and patterns of Gram-negative resistance and is not something we were able to further tease out from our data. The majority of patients in our study received a fluoroquinolone with a beta-lactam because that was the regimen we recommended after review of microbiology and breakthrough infections that occurred when ciprofloxacin alone was the primary regimen. Use of perirectal swabs to determine optimal prophylaxis is another approach that experts have proposed, but the logistics of obtaining swabs and results prior to the procedure were not deemed feasible at our institution, particularly with a large number of outside referrals that present on the day of the procedure. Thus, combination therapy may be an approach other facilities could utilize if faced with similar logistical problems determining preprocedural rectal flora. Assessing individual risk factors, such as recent hospitalization or previous biopsy, may also be useful in identifying patients at higher risk of infection (7, 8, 9).
Our study is limited by being performed in a single institution, although the patient population represents the entire New England region since our VA facility is a referral center for this procedure. The retrospective design can be biased by unmeasured confounders. In addition, although the VA electronic medical record is very robust, we may have missed events occurring at non-VA facilities. Finally, although our study was well-powered for our primary outcome, the power for secondary analyses on risk factors for infection and adverse events may be limited and warrant further evaluation.
In conclusion, this 3-year study of more than 450 patients demonstrates that combination therapy is associated with reduced post-TRUS biopsy infections compared to those obtained with single-drug therapy, with no difference in adverse effects. With increasing reports of failures of standard prophylaxis, this general strategy may be helpful in reducing a costly and potentially life-threatening adverse outcome (7).
REFERENCES
- 1.Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS, Feng Z, Parnes HL, Coltman CA. 2006. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 98:529. doi: 10.1093/jnci/djj131. [DOI] [PubMed] [Google Scholar]
- 2.Zani EL, Clark OA, Rodrigues Netto N Jr. 2011. Antibiotic prophylaxis for transrectal prostate biopsy. Cochrane Database Syst Rev 2011:CD006576. [DOI] [PubMed] [Google Scholar]
- 3.Zaytoun OM, Vargo EH, Rajan R, Berglund R, Gordon S, Jones JS. 2011. Emergence of fluoroquinolone-resistant Escherichia coli as cause of postprostate biopsy infection: implications for prophylaxis and treatment. Urology 77:1035–1041. doi: 10.1016/j.urology.2010.12.067. [DOI] [PubMed] [Google Scholar]
- 4.Taylor AK, Zembower TR, Nadler RB, Scheetz MH, Cashy JP, Bowen D, Murphy AB, Dielubanza E, Schaeffer AJ. 2012. Targeted antimicrobial prophylaxis using rectal swab cultures in men undergoing transrectal ultrasound guided prostate biopsy is associated with reduced incidence of postoperative infectious complications and cost of care. J Urol 187:1275–1279. doi: 10.1016/j.juro.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 5.Lay A, Kaplan J, Williams S, Chang S, Siroky Orlando R. 2012. Risk factors for complications after prostate biopsy at the VA. J Urol 187:e438. [Google Scholar]
- 6.Adibi M, Hornberger B, Bhat D, Raj G, Roehrborn CG, Lotan Y. 2013. Reduction in hospital admission rates due to post-prostate biopsy infections after augmenting standard antibiotic prophylaxis. J Urol 189:535–540. doi: 10.1016/j.juro.2012.08.194. [DOI] [PubMed] [Google Scholar]
- 7.Carignan A, Roussy JF, Lapointe V, Valiquette L, Sabbagh R, Pépin J. 2012. Increasing risk of infectious complications after transrectal ultrasound guided prostate biopsies: time to reassess antimicrobial prophylaxis? Eur Urol 62:453–459. doi: 10.1016/j.eururo.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 8.Ehdaie B, Vertosick E, Spaliviero M, Giallo-Uvino A, Taur Y, O'Sullivan M, Livingston J, Sogani P, Eastham J, Scardino P, Touijer K. 2014. The impact of repeat biopsies on infectious complications in men with prostate cancer on active surveillance. J Urol 191:660–664. doi: 10.1016/j.juro.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 9.Patel U, Dasgupta P, Amoroso P, Challacombe B, Pilcher J, Kirby R. 2012. Infection after transrectal ultrasonography-guided prostate biopsy: increased relative risks after recent international travel or antibiotic use. BJU Int 109:1781–1785. doi: 10.1111/j.1464-410X.2011.10561.x. [DOI] [PubMed] [Google Scholar]