Abstract
Toxoplasma gondii, the causative agent of toxoplasmosis, is an obligate intracellular protozoan that can infect a wide range of vertebrate cells. Here, we describe the cytotoxic effects of the dinuclear iron compound [Fe(HPCINOL)(SO4)]2-μ-oxo, in which HPCINOL is the ligand 1-(bis-pyridin-2-ylmethyl-amino)-3-chloropropan-2-ol, on T. gondii infecting LLC-MK2 host cells. This compound was not toxic to LLC-MK2 cells at concentrations of up to 200 μM but was very active against the parasite, with a 50% inhibitory concentration (IC50) of 3.6 μM after 48 h of treatment. Cyst formation was observed after treatment, as indicated by the appearance of a cyst wall, Dolichos biflorus lectin staining, and scanning and transmission electron microscopy characteristics. Ultrastructural changes were also seen in T. gondii, including membrane blebs and clefts in the cytoplasm, with inclusions similar to amylopectin granules, which are typically found in bradyzoites. An analysis of the cell death pathways in the parasite revealed that the compound caused a combination of apoptosis and autophagy. Fluorescence assays demonstrated that the redox environment in the LLC-MK2 cells becomes oxidant in the presence of the iron compound. Furthermore, a reduction in superoxide dismutase and catalase activities in the treated parasites and the presence of reactive oxygen species within the parasitophorous vacuoles were observed, indicating an impaired protozoan response against these radicals. These findings suggest that this compound disturbs the redox equilibrium of T. gondii, inducing cystogenesis and parasite death.
INTRODUCTION
Toxoplasma gondii is an intracellular parasitic protozoan and the causative agent of toxoplasmosis, with a worldwide distribution in warm-blooded animals, including humans (1). The following forms of T. gondii can infect hosts: tachyzoites, which are present during the acute phase of toxoplasmosis; bradyzoites, which are typically found inside tissue cysts in the brain and skeletal muscles during the chronic phase of the infection; and sporozoites, which are present inside oocysts that are produced during the sexual cycle that occurs in the intestines of felines, which are the definitive hosts (2). As the host adaptive immune response weakens, parasite tissue cysts rupture and release bradyzoites through an unknown mechanism. These recrudescent infections permit parasite conversion to the rapidly dividing tachyzoite stage and cause significant morbidity, including Toxoplasma encephalitis (3, 4).
Transmission in humans occurs via the ingestion of food or water contaminated with oocysts shed by cats, via the ingestion of undercooked or raw meat containing tissue cysts, or congenitally, particularly when the mother acquires the infection for the first time during pregnancy (5). In immunocompetent organisms, T. gondii infection is rarely severe and is often asymptomatic. In contrast, in immunocompromised individuals, the most common condition associated with this infection is encephalitis, which causes headache, disorientation, lethargy, hemiparesis, altered reflexes, and convulsions (6). Pneumonia and myocarditis may also occur in these individuals. In children infected in utero, the parasite invades the brain and retina, resulting in potentially severe consequences, including reduced visual acuity, mental retardation, intracranial calcifications, and hydrocephalus (6). Associations have recently been made between parasite infection and neurological disorders, such as schizophrenia (7).
Currently, the most effective therapy for toxoplasmosis is the administration of antifolate compounds, such as the combination of pyrimethamine and sulfadiazine. Despite the efficacy of this therapy, these drugs are often associated with many side effects, which are primarily observed in AIDS patients and include bone marrow suppression and hematological toxicity, which occur in association with pyrimethamine, and/or hypersensitivity and allergic skin reactions, which are associated with sulfadiazine (8–10).
In light of the medical relevance of toxoplasmosis, the development of new therapies for this parasitic disease is essential. However, the main challenge in this field is the development of compounds that are capable of reaching the protozoan inside the host cell at concentrations that are toxic to the parasite but safe for the host.
Some reports in the literature demonstrate that coordination compounds may be an interesting alternative for antiparasite therapy. For example, compounds containing copper or cobalt ions bound to the HmtpO ligand {HmtpO, [5-methyl-1,2,4-triazol[1,5-a]pyrimidin-7(4H)-one]} strongly affect the energy metabolism of Leishmania infantum and Leishmania braziliensis cells, disrupting the membrane structure of organelles and inducing cell death (11). These compounds were also active in vitro against the trypomastigote and amastigote forms of Trypanosoma cruzi at concentrations similar to those of drugs that are commonly used in clinical therapy, such as benznidazole; however, these compounds were associated with reduced toxicity to host cells and an improved selectivity index. Furthermore, in vivo tests demonstrated that these compounds promoted a significantly lower parasite burden than that with benznidazole treatment (12).
Horn, Jr., et al. (13) reported that HPCINOL [1-(bis-pyridin-2-ylmethyl-amino)-3-chloropropan-2-ol] is a promising ligand for the development of metallopharmaceuticals, because the associated copper and iron complexes exhibit interesting biological activities. The associated copper complex [Cu(HPCINOL)Cl]+ exhibited nuclease activity and was cytotoxic to leukemia cancer cells (14). Iron complexes with the same ligand were also biologically tested, and the mononuclear compound [Fe(HPCINOL)(Cl)2] protected Saccharomyces cerevisiae cells against oxidative stress, mimicking superoxide dismutase and catalase (15). This same compound and its dinuclear counterparts [Fe(HPCINOL)(SO4)]2-μ-oxo and [Fe(HPCINOL)Cl]2-μ-oxo accelerated DNA hydrolysis approximately 108-fold compared to the spontaneous DNA cleavage rate, revealing impressive nuclease activity. However, the activities of these compounds against cancer cells were modest and associated with very low toxicity for normal human peripheral blood mononuclear cells (16). This lack of toxicity for normal cells prompted us to evaluate the activity of these compounds in antiparasitic therapies, because the main challenge of these therapies is the preservation of host cell viability.
Thus, we report here the evaluation of the anti-Toxoplasma activity of the compound [Fe(HPCINOL)(SO4)]2-μ-oxo (Fig. 1), which significantly reduced the level of parasite infection in the host cell. Furthermore, the associated iron complex induces the production of reactive oxygen species in the cell and promotes a dramatic reduction in the activity of the parasite antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT), indicating that the mode of action of this compound involves the impairment of this protective system.
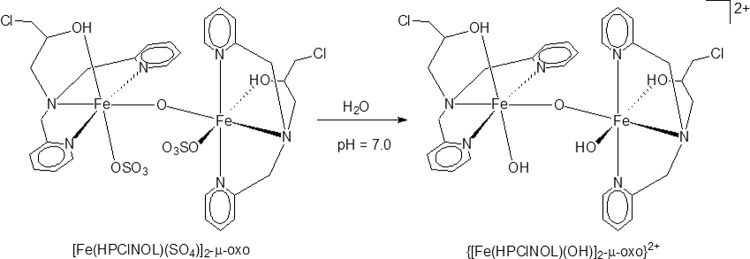
FIG 1.
Molecular structure of the iron(III) compound [Fe(HPCINOL)(SO4)]2-μ-oxo, as solved using X-ray diffraction (left) and in aqueous solution at pH 7.0 (right).
MATERIALS AND METHODS
Parasites.
The tachyzoites used in this study were from the virulent RH strain of T. gondii and were maintained via intraperitoneal infections in Swiss mice. After 48 h of infection, the parasites were collected via a peritoneal wash with phosphate-buffered saline (PBS) (pH 7.2) and then centrifuged at 1,000 × g for 10 min. The pellet was washed twice with PBS and RPMI 1640 medium. The parasites were used within 30 to 40 min of their removal from the peritoneal cavity. All animal studies were reviewed and approved by the ethics committee of animal use of the Biophysics Institute Carlos Chagas Filho (code IBCCF99).
LLC-MK2 cells.
LLC-MK2 kidney epithelial cells (rhesus monkey [Macaca mulatta]) were grown in 25-cm2 culture flasks (SPL Life Sciences) containing RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) at 37°C in a 5% CO2 atmosphere. Infection by the parasite was conducted in subconfluent cultures in flasks or over coverslips in 24-well tissue culture plates (SPL Life Sciences).
Iron(III) compound.
The iron(III) compound [Fe(HPCINOL)(SO4)]2-μ-oxo was obtained as previously described (17). For in vitro studies, this compound was dissolved in RPMI 1640 medium and stored at −20°C.
Cell viability assays.
The possible toxic effects of the compound on the host cell were evaluated based on the reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] and the release of lactate dehydrogenase (LDH). For these assays, 1 × 105 cells per well were seeded in 96-well plates and cultured with RPMI 1640 medium supplemented with 5% FBS. After 24 h, the cells were washed and directly subjected to compound treatment at concentrations derived from serial dilutions in RPMI 1640 medium supplemented with 5% FBS; the dilutions included concentrations from 200 to 1.69 μmol liter−1. As a negative control, the cells were cultured in RPMI 1640 medium supplemented with 5% FBS without the addition of the compound. As a positive control, the cells were cultured with 10% Triton X-100, as previously described.
After 24 h of treatment, the culture supernatant was removed and used for LDH measurement (see below), and 15 μl of MTT (5 mg/ml) in RPMI 1640 medium solution was added to each well for 4 h. The formazan crystals were subsequently solubilized by the addition of 100 μl of pure dimethyl sulfoxide (DMSO). The plate was centrifuged at 400 × g for 7 min, and 100 μl of the supernatant was collected, transferred to a new 96-well plate, and read at 570 nm in a VersaMax microplate reader (Molecular Devices) using the 6.0 SoftMax Pro software. LDH concentrations (18, 19) were measured in the culture supernatants of the LLC-MK2 cells using the Doles kit with 50 μl of the cultured supernatant, according to the manufacturer's protocol. The data were plotted using the GraphPad Prism 6.0 software. The presented data are representative of the results from three independent experiments.
Antiproliferative assays.
Approximately 2 × 105 LLC-MK2 cells per well were seeded over coverslips in a 24-well plate 1 day before the assay. The cells were infected with parasites in RPMI 1640 medium using a 5:1 parasite-to-host cell ratio based on the host cell count on the day of the infection. Tachyzoites were allowed to interact with the host cells for 1 h, the cell monolayer was washed twice with PBS to remove nonadhered parasites, and the iron(III) compound was added at different concentrations (2.5 to 25 μM) in RPMI 1640 medium supplemented with FBS. After 48 h or 6 days of treatment, the cells were fixed with fresh 4% formaldehyde in PBS, stained with Giemsa, and observed by light microscopy. The samples subjected to the 6-day treatment received new culture medium containing the compound every 2 days. This procedure was repeated for all of the 6-day assays described in this paper. The proliferation index was calculated by multiplying the mean number of internalized T. gondii organisms per cell by the percentage of infected cells on two different coverslips per experiment (20). The data were plotted using the GraphPad Prism 6.0 software. The presented results represent the means ± standard deviations of the results from at least three independent experiments, and differences were considered statistically significant at a P value of <0.05. For the calculations of the 50% inhibitory concentration (IC50), the percentage of growth inhibition was plotted as a function of the drug concentration by fitting the values for nonlinear curve analysis. The regression analyses were performed using the SigmaPlot 8.0 software (Systat Software, Inc., Chicago, IL, USA).
Electron microscopy analysis.
To observe the ultrastructure of intracellular parasites using transmission electron microscopy, cells in culture flasks were fixed for 1 h in a solution containing 2.5% glutaraldehyde and 4% recently prepared formaldehyde in 0.1 mol liter−1 sodium cacodylate buffer (pH 7.4) after 48 h or 6 days of treatment with 10 μmol liter−1 iron(III) compound. The cells were scraped from the flasks with a rubber policeman, washed with sodium cacodylate buffer, and postfixed for 1 h in the dark with a solution containing 1% osmium tetroxide in 0.1 M sodium cacodylate buffer. The cells were subsequently washed in the same buffer, dehydrated in acetone, and embedded in Epon. Ultrathin sections were stained with uranyl acetate and lead citrate and observed under a Zeiss 900 transmission electron microscope.
For scanning electron microscopy, cells over coverslips were fixed and postfixed after 48 h or 6 days of treatment with 10 μmol liter−1 or 25 μmol liter−1 compound, as described above, and dehydrated in a graded acetone series. The cells were critical point dried and mounted on stubs, and the upper portion of the cells was scraped off with an adhesive tape, revealing the internal organization of the parasitophorous vacuole (21). The samples were then coated with gold (20 to 30 nm) and observed using a Jeol JSM 6490LV scanning electron microscope.
Immunofluorescence and cell death assays.
LLC-MK2 cells over coverslips were infected with tachyzoites, treated with 10 μmol liter−1 compound or left untreated for 48 h or 6 days, washed with PBS, and fixed with 4% freshly prepared formaldehyde in PHEM buffer {60 mmol liter−1 PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid)], 20 mmol liter−1 HEPES, 10 mmol liter−1 EGTA, 5 mmol liter−1 MgCl2, and 70 mmol liter−1 KCl [pH 7.2]}. After fixation, the cells were washed, permeabilized with 2% Triton X-100 in PHEM buffer for 10 min, incubated with 100 mmol liter−1 NH4Cl for 30 min, and then incubated with PHEM buffer containing 3% bovine serum albumin (PHEM-BSA) for 30 min at room temperature. The cells were incubated for 1 h in the presence of Dolichos biflorus lectin conjugated with fluorescein isothiocyanate (DBA-FITC) (10 μg ml−1) (Sigma Aldrich Co., St. Louis, MO, USA) or with the LC3B rabbit polyclonal antibody (1:1,000 dilution). After labeling with LC3B, the cells were incubated with goat anti-rabbit Alexa Fluor 546 antibody (1:100 dilution) (Molecular Probes). After labeling, the cells were washed with PHEM, and the coverslips with cells were mounted in ProLong Gold with or without 4′,6-diamidino-2-phenylindole (DAPI). The percentage of cells with vacuoles positive for LC3B was quantified by a direct count of the total number of positive cells among 100 infected cells on two different coverslips per experiment. DNA fragmentation was also assayed after infection and treatment. Infected cells were treated with 10 μmol liter−1 compound or left untreated for 6 days, washed, and fixed, as described above. After fixation, the cells were washed, permeabilized with cold 70% ethanol for 10 min, and processed, as recommended by the manufacturer of the Click-iT terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) Alexa Fluor 488 imaging assay kit (Molecular Probes). After labeling, the coverslips were prepared as described above and observed using a Zeiss LSM-710 confocal laser scanning microscope.
DCFH-DA assay.
In order to evaluate the possible generation of reactive oxygen species in the host cells after treatment, the LLC-MK2 cells over coverslips were infected or noninfected with tachyzoites and treated with the compound or left untreated for 6 days. The cells were washed in PBS and incubated for 30 min with 0.5 μmol liter−1 2′,7′-dichlorofluorescein diacetate (DCFH-DA) at 37°C in a 5% CO2 atmosphere. The cells were subsequently fixed using 4% recently prepared formaldehyde in PBS. After 1 h, the cells were washed in PBS and mounted with ProLong Gold containing DAPI (adapted from Jakubowski and Bartosz [22]). The cells were analyzed using a Zeiss LSM-710 confocal laser scanning microscope.
Determination of T. gondii catalase and superoxide dismutase activities.
To measure parasite enzyme activities, LLC-MK2 cells were seeded in a 25-cm2 culture flask and infected with tachyzoites or left uninfected. After 1 h of infection, the samples were treated with 10 μmol liter−1 iron(III) compound or left untreated for 6 days. Uninfected host cells that were treated with the iron(III) compound or left untreated were used as controls. T. gondii cells were mechanically isolated by scraping the infected cells with a rubber policeman and passing the cells through syringes (1-in. BD precision glide needle, 22 gauge); the host cells were also scraped, as described above. The T. gondii tachyzoites and host cells were then recovered by centrifugation at 1,000 × g for 10 min and washed in 50 mmol liter−1 sodium phosphate buffer (pH 6.0). The cells were lysed via two freeze-thaw cycles in lysing solution (20% PBS [pH 7.0] and 80% distilled water), as described by Murray and Cohn (23). Protein concentrations were evaluated, as described previously (24). Catalase activity was determined spectrophotometrically based on H2O2 consumption (25). The activity was expressed as units per milligram of protein, where 1 U corresponds to the consumption of 1 μmol H2O2 per minute. SOD activity was indirectly evaluated using native polyacrylamide gel electrophoresis by measuring the enzyme's capacity to inhibit the reduction of nitroblue tetrazolium (NBT) by O2− produced photochemically by riboflavin. Reduced NBT appears as a uniformly blue color in the gel. If SOD is present, this reaction is inhibited, and achromatic bands are formed. After electrophoresis, the gel was soaked in 2.5 mmol liter−1 NBT for 20 min, transferred to a development solution (36 mmol liter−1 K2HPO4 and 36 mmol liter−1 KH2PO4 [pH 7.8] as a buffer solution, 28 mmol liter−1 N,N,N′,N′-tetramethylethylenediamine [TEMED], and 86 μmol liter−1 riboflavin) for another 15 min and exposed to light (60 W) until the bands appeared. The activity was obtained via densitometry of the bands scanned from the gel and expressed as the fold increase in SOD activity for comparisons of LLC-MK2 cells that were uninfected and treated with the iron compound, infected and left untreated, infected and treated with the compound, and uninfected and left untreated (i.e., control host cells).
RESULTS
The tested metallocomplex is a neutral coordination compound that contains an inorganic diiron(III) core (Fig. 1) (18). The toxicity of the Fe(III) compound for LLC-MK2 host cells was initially evaluated after 24 h of treatment. The data presented in Fig. 2 demonstrate that this compound does not affect the viability of the host cells after 24 h of treatment, even at a concentration of 200 μmol liter−1, as indicated by the two assays employed in this study (i.e., MTT and LDH) (Fig. 2A and B, respectively).
FIG 2.
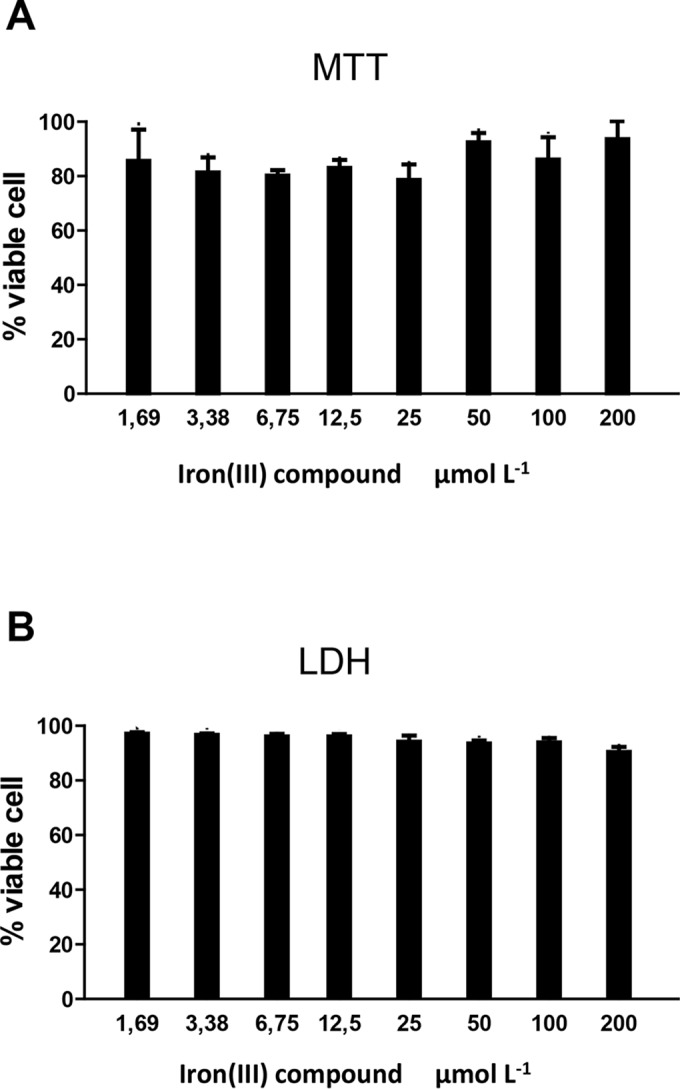
Viability of LLC-MK2 host cells after treatment with the iron(III) compound. Host cells were treated with increasing concentrations of the iron(III) compound up to 200 μmol liter−1 for 24 h. The toxicity of the complex for the host cells was evaluated based on the reduction of MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (A) and on the release of lactate dehydrogenase (LDH) (B). The presented results represent the means ± standard deviations of the results from at least three independent experiments.
The anti-Toxoplasma activity of the iron(III) compound against the growth of intracellular T. gondii tachyzoites within LLC-MK2 cells was evaluated after 48 h and 6 days of treatment. Figure 3 shows that the iron(III) compound induces a dose-dependent inhibition of parasite growth at concentrations from 2.5 to 25 μmol liter−1 (Fig. 3A). An IC50 of 3.6 μmol liter−1 was obtained after 48 h of treatment (Fig. 3A). After 6 days of treatment at a concentration of 10 μmol liter−1, the infection index was three times lower than that of the untreated cells (Fig. 3B).
FIG 3.
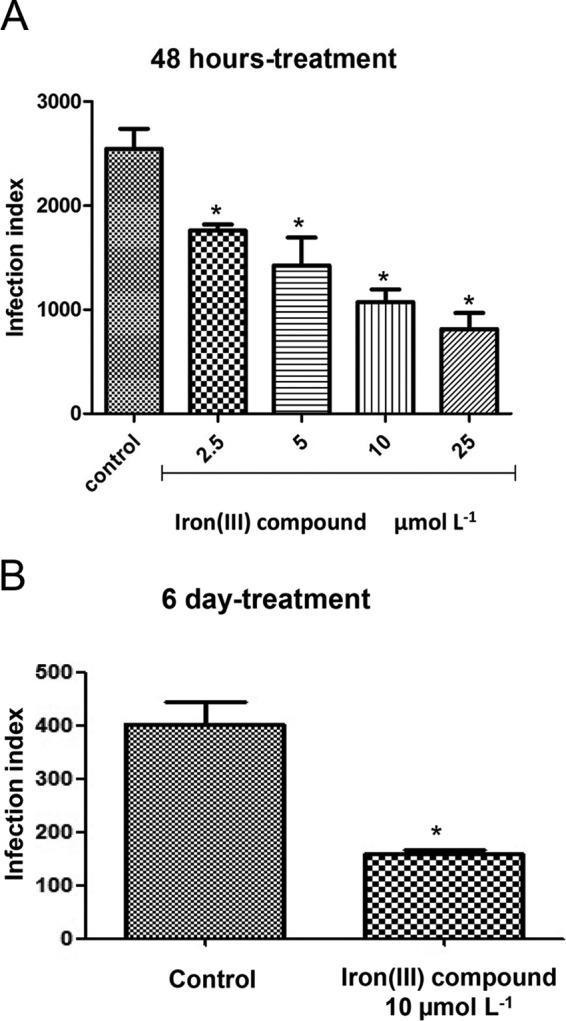
Treatment with the iron(III) compound lowers the infection index (II) of T. gondii in LLC-MK2 cells. Shown are the II after treatment with different concentrations of the iron(III) compound for 48 h (A) or after 6 days (B) of treatment with 10 μmol liter−1 iron(III) complex. *, significantly different from the control (P ≤ 0.05) using analysis of variance (ANOVA). The presented results represent the means ± standard deviations of the results from at least three independent experiments.
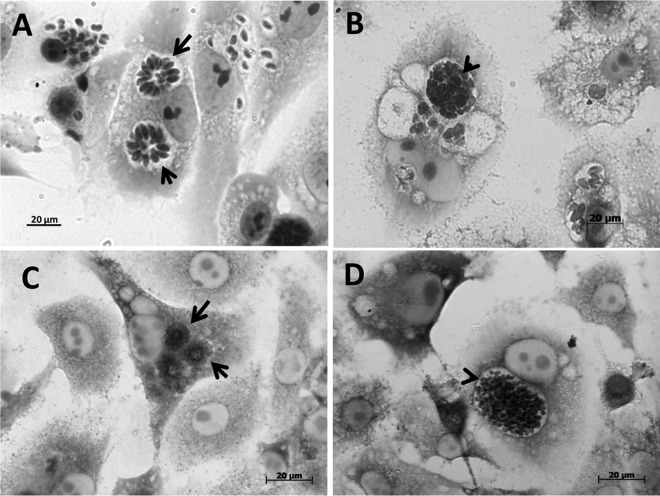
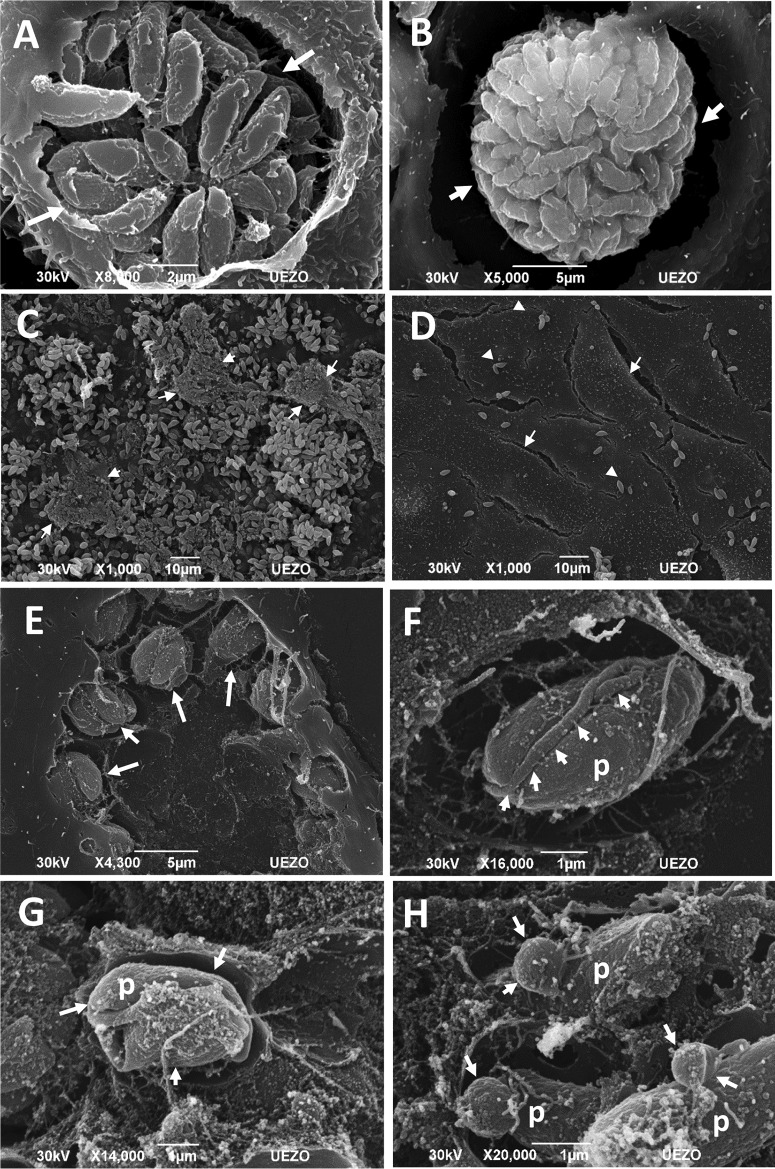
Light microscopy images revealed that treatment with the iron(III) compound for 48 h and 6 days affected the characteristic organization of tachyzoites inside the parasitophorous vacuoles. As expected, in untreated cells, the parasites were organized in rosettes (Fig. 4A and C); however, after treatment with the compound, the parasites were grouped in a structure with a thick vacuolar membrane that was similar to that of bradyzoite cysts (Fig. 4B and D). Scanning electron microscopy confirmed the organization of the parasites into cysts after 6 days of treatment. Untreated cells contained tachyzoites in rosettes (Fig. 5A), and after treatment, nearly all of the parasites were clustered in structures similar to those of bradyzoite cysts (Fig. 5B). A large number of parasites were also observed in infected untreated cells (Fig. 5C) due to the nature of the lytic cycle of tachyzoites of T. gondii. After treatment with 10 μmol liter−1 iron(III) compound, the cell monolayers were preserved, with a clear reduction in the number of attached extracellular parasites (Fig. 5D), showing that the iron(III) compound controlled the growth of T. gondii. In addition, this treatment appeared to generate abnormalities related to the T. gondii division process (Fig. 5E). Interestingly, treatment with 25 μmol liter−1 compound also induced changes in parasite shape (Fig. 5F and G), with the appearance of unusual structures attached to the parasite body, such as membrane blebs, which are usually seen in apoptotic cells (Fig. 5H).
FIG 4.
Optical microscope images of LLC-MK2 cells infected with T. gondii and treated with the iron(III) compound at a concentration of 10 μmol liter−1 or left untreated. (A) Rosettes (arrow) inside untreated cells after 48 h. (B) Parasites grouped in a structure similar to that of a cyst (arrowhead) after 48 h of treatment. (C) Rosettes (arrows) inside untreated cells after 6 days. (D) Cyst structure after 6 days of treatment (arrowhead). Scale bars = 20 μm.
FIG 5.
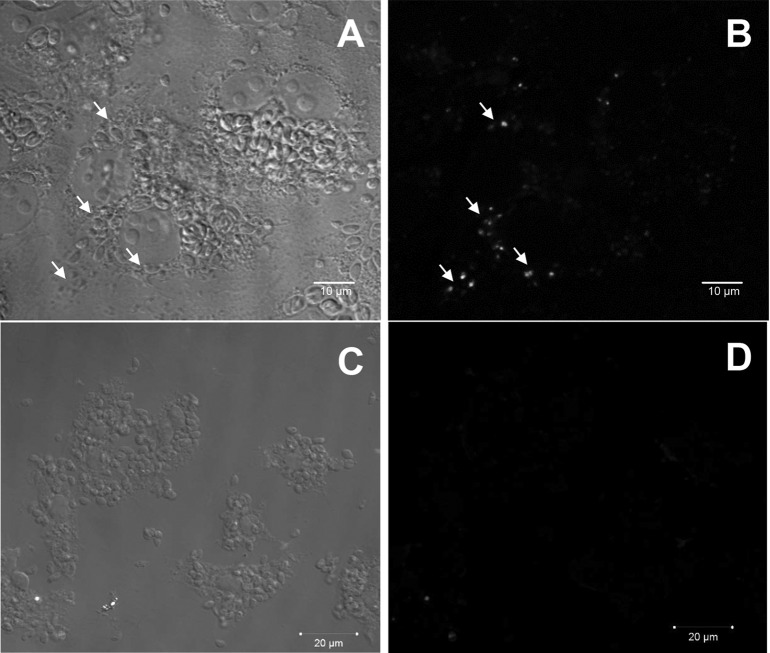
Scanning electron microscopy images of LLC-MK2 cells infected by T. gondii after 6 days of treatment with 10 μmol liter−1 or 25 μmol liter−1 iron(III) compound. The tachyzoites in the parasitophorous vacuoles of untreated cells were organized in rosettes (arrows) (A); however, after treatment, the parasites were grouped in structures similar to those of the cysts of bradyzoites (arrows) (B). (C) Infected and untreated cells contained many parasites (arrows), and the cells were impaired. (D) In contrast, after treatment, the monolayers were conserved (arrows), and fewer extracellular parasites (arrowheads) were observed as the result of the control of the natural lytic cycle of T. gondii. (E) Treatment generated abnormalities that may be related to the T. gondii division process (arrows). Treatment with 25 μmol liter−1 iron(III) compound induced the appearance of unusual structures attached to the parasite body (arrows) (F), changes in the parasite shape (arrows) (G), and blebs in the parasite membrane (arrows) (H). Scale bars: A and B = 10 μm; C = 2 μm; D and E = 5 μm; F to H = 1 μm.
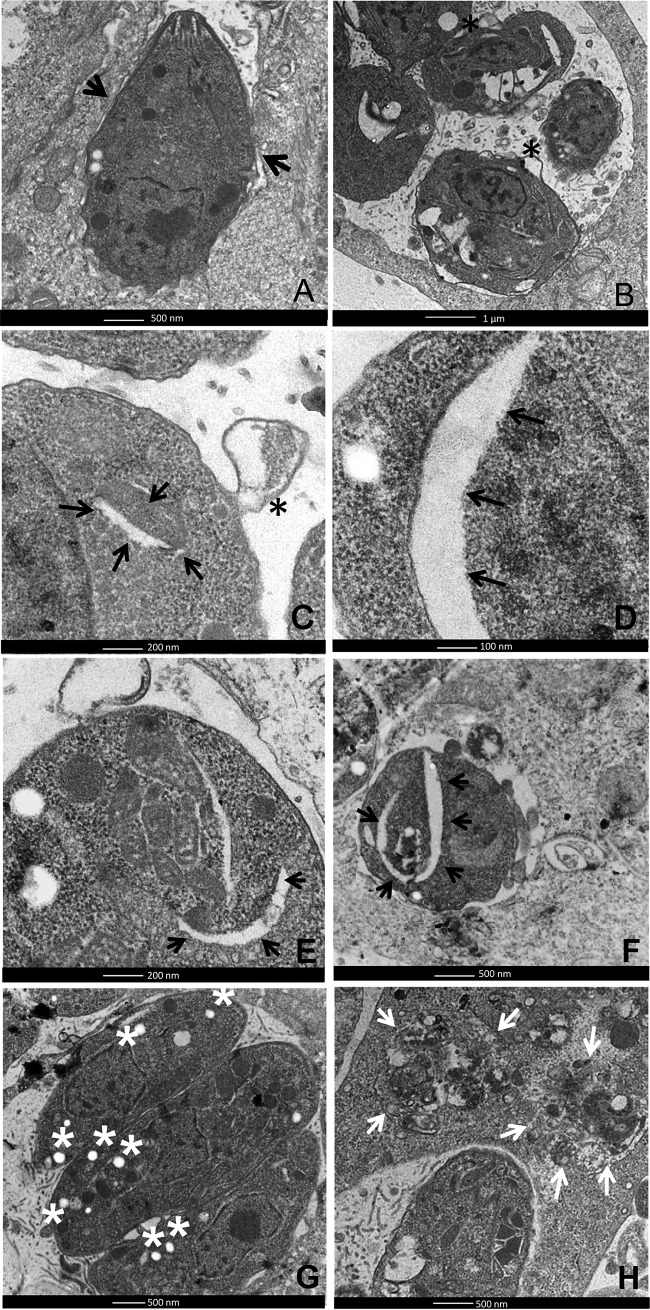
Transmission electron microscopy of the control cells revealed preserved tachyzoite structures (Fig. 6A). However, after 6 days of treatment with 10 μmol liter−1 iron(III) compound, the parasites exhibited alterations in the cytoplasm, such as extensive clefts (Fig. 6B to F) and membrane blebs (Fig. 6B and C), which are hallmarks of apoptosis. In addition, DNA strand breaks were also detected in intravacuolar parasites after 6 days of treatment with the compound (Fig. 7A and B). This effect was not observed in the untreated cells (Fig. 7C and D).
FIG 6.
Transmission electron microscopy images of LLC-MK2 cells infected with T. gondii and treated with 10 μmol liter−1 iron(III) compound or left untreated. (A) T. gondii within untreated LLC-MK2 cells after 6 days of infection. Note that the typical structure of the tachyzoites was preserved (arrows). After 6 days of treatment with the iron(III) compound, the parasites presented alterations in the cytoplasm, such as extensive clefts (arrows in C to F) and membrane blebs (asterisks in B and C). (G) After 6 days of treatment, the appearance of an abnormal quantity of amylopectin-like granules was observed in the parasite cytoplasm (asterisks). (H) After 48 h of treatment, degraded parasites were observed inside some vacuoles (arrows). Scale bars: A and F to H = 500 nm; B and D = 100 nm; C and E = 200 nm.
FIG 7.
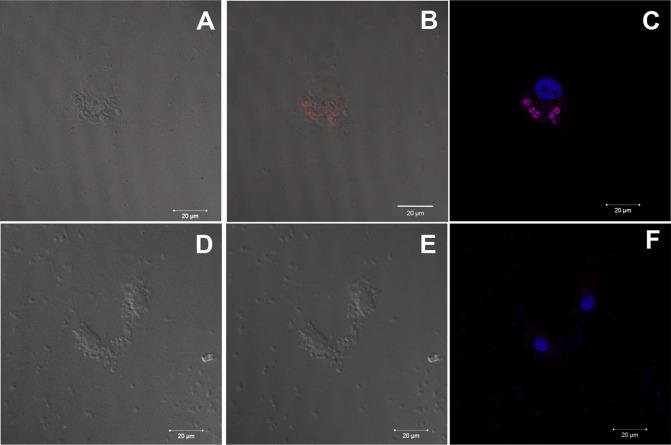
T. gondii cell death by apoptosis after treatment with the iron(III) compound. Confocal laser scanning microscopy images show that after 6 days of treatment with 10 μmol liter−1 iron(III) compound, some parasites exhibited positive TUNEL staining (arrows in A and B), while untreated cells were not stained (C and D). (A and C) Differential interference contrast (DIC) microscopy images. (B and D) TUNEL labeling by fluorescence microscopy. Scale bars = 20 μm.
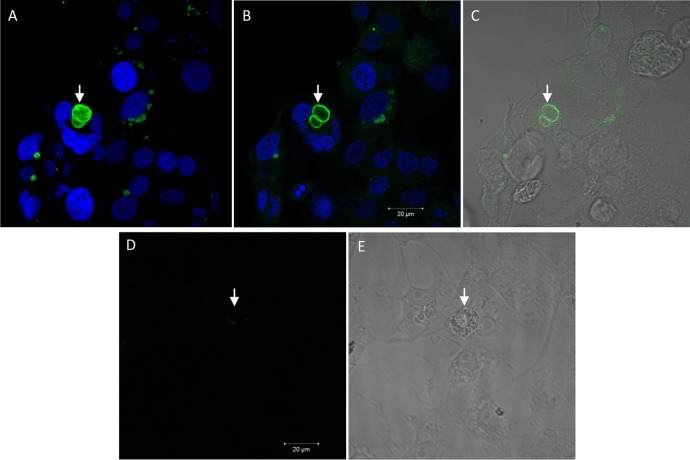
After 6 days of treatment, the parasites exhibited an abnormal quantity of cytoplasmic structures similar to amylopectin inclusions, which are typically found in bradyzoites (Fig. 6G). The presence of bradyzoites was confirmed after labeling the cells with DBA lectin, which specifically binds to the cyst wall of bradyzoite cysts (26). A large number of parasitophorous vacuoles labeled with DBA were observed in cells incubated in the presence of the iron(III) compound for 6 days (Fig. 8A to C), while almost no DBA labeling was detected in the infected and untreated cells (Fig. 8D and E). Quantification revealed that approximately 12% of the infected treated cells had structures that were DBA positive; in contrast, only 2% of the infected untreated cells were labeled.
FIG 8.
Confocal laser scanning microscopy images showing the conversion of T. gondii tachyzoites to bradyzoites after 6 days of treatment with 10 μmol liter−1 iron(III) compound. (A, B, and D) D. biflorus lectin (DBA) and DAPI labeling by fluorescence microscopy. (C and E) Differential interference contrast microscopy images. Shown are the cystic wall (A to C, arrow) in treated cells and unstained rosettes (D and E, arrow) in untreated cells. Scale bars = 20 μm.
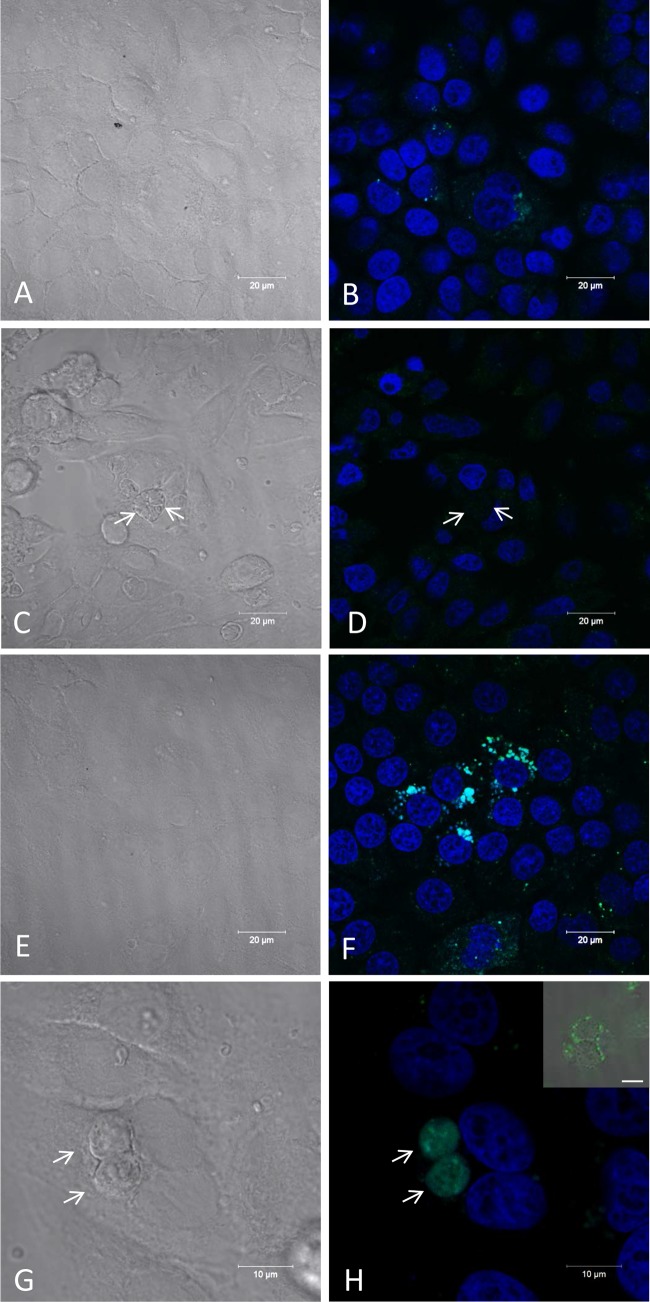
The appearance of degraded parasites inside the parasitophorous vacuoles (Fig. 6H) led us to investigate whether autophagy was involved in the death of the parasites. After 6 days of treatment with the iron(III) compound, some parasites were positive for LC3B, which labels autophagic vesicles (Fig. 9A to C), while infected untreated cells showed very little staining by LC3B (Fig. 9E and F). The labeled tachyzoites were counted, and 5% of the tachyzoites were LC3B positive after treatment. In contrast, only 0.7% of the parasites were positive in untreated infected cells.
FIG 9.
T. gondii cell death by autophagy after treatment with the iron(III) compound. Confocal laser scanning microscopy images of LLC-MK2 cells infected by T. gondii showing labeled autophagic vesicles (LC3B positive) after 6 days of treatment with 10 μmol liter−1 iron(III) compound. Parasites in treated cells were positive (B and C), and untreated cells were not stained for the LC3B marker (E and F). (A and D) Differential interference contrast (DIC) microscopy images. (B and E) LC3B labeling and DIC microscopy images. (C and F) LC3B and DAPI labeling. Scale bars = 20 μm.
To determine whether the oxidative status of the cellular environment after treatment with the compound was related to the death of the parasites, we incubated infected or uninfected cells with the compound for 6 days. Intracellular oxidation was monitored using H2DCFDA, which becomes internalized by the cells and subsequently reacts with esterases and reactive oxygen species (ROS); these reactions convert the molecule into the highly fluorescent molecule dichlorofluorescein (DCF) (22). Untreated cells showed no DCF signal, regardless of whether they were uninfected (Fig. 10A and B) or infected (Fig. 10C and D) with T. gondii. However, treatment with the compound induced ROS production in the host cells, as indicated by a fluorescent signal in the cytoplasm of uninfected (Fig. 10E and F) and infected (Fig. 10G and H) host cells; labeling was observed primarily around the parasitophorous vacuoles (Fig. 10H, inset).
FIG 10.
Assessment of intracellular oxidation in infected host cells treated with 10 μmol liter−1 iron(III) compound for 6 days. Confocal laser scanning microscopy images of DCF staining were performed to assess the intracellular oxidation status of the host cells in different conditions: DCF staining of uninfected (A, B, E, and F) and infected host cells (C, D, G, and H) that were untreated (A to D) or treated (E to H) with the iron(III) compound. (A, C, E, and G) Differential interference contrast microscopy images. (B, D, F, and H) DCF and DAPI labeling by fluorescence microscopy. (H, inset) DIC and fluorescence microscopy. Note the positive staining in the parasites within the vacuole (arrows in H) and in some vesicles around the vacuole (other section of the same sample [inset in H]), while infected and untreated cells were not stained around the parasites (arrows in C and D). Scale bars: A to F = 20 μm; G and H = 10 μm; inset of H = 10 μm.
To investigate whether these changes in ROS production might affect an important antioxidant defense strategy of the parasite, we analyzed the SOD and CAT activities of the host cells and T. gondii. Both of these enzymes are antioxidant metalloenzymes that are strongly related to the primary defense of the parasite against the harmful generation of ROS (27). The SOD activity did not differ between treated and untreated host cells (Fig. 11A). However, parasites obtained after 6 days of treatment with the iron(III) compound exhibited a 50% reduction in SOD activity in comparison to parasites obtained from untreated infected host cells (Fig. 11A). Moreover, CAT activity was nearly completely abolished in parasites obtained from compound-treated host cells compared to that in parasites from untreated host cells and untreated host cells alone (Fig. 11B). These results indicate that oxidative stress is a principal mechanism by which this iron(III) compound exerts its cytotoxicity.
FIG 11.
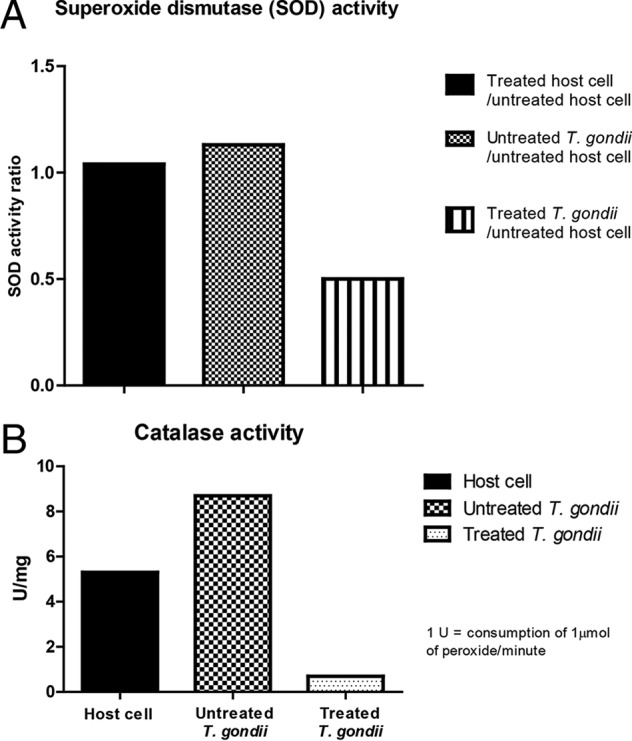
Reduction of superoxide dismutase (SOD) and catalase (CAT) activities. Uninfected or infected host cells were subjected to different conditions to evaluate the effect of treatment with 10 μmol liter−1 iron(III) compound for 6 days on the SOD (A) and CAT (B) antioxidant activities.
DISCUSSION
The worldwide occurrence of toxoplasmosis, the lack of a definitive cure for this disease, and the current use of a treatment with several side effects (9, 10) highlight the urgent need to design and investigate new compounds with increased specificity and effectiveness against T. gondii. In this context, we investigated whether a compound containing iron(III) as a coordination center exerts toxic effects on this parasite.
Our results demonstrate that the iron(III) compound had low toxicity to LLC-MK2 host cells, which is consistent with previous results obtained using normal human peripheral blood mononuclear cells (16). However, the compound had an IC50 of 3.6 μmol liter−1, which is notably lower than the values described in the literature for currently used clinical drugs. For example, sulfadiazine, which is a reference drug for the clinical treatment of T. gondii, has a variable IC50 that is dependent on the host cell used. The IC50s against T. gondii (RH strain, tachyzoite form) in fibroblastic cell lines, such as HFF (28) and MRC-5 (29), are 0.2 mmol liter−1 and 26 μmol liter−1, respectively; in epithelial cell lines, such as HeLa (30), Vero (31), and HEp2 cells (32), the IC50s are 7.1 mmol liter−1, 0.3 mmol liter−1, and 26 μmol liter−1, respectively. Thus, in addition to being safe, the iron(III) compound under investigation was efficient. Because the cell line used in this work (i.e., LLC-MK2) is an epithelial cell line with characteristics similar to those described above, we believe that the iron(III) compound tested in this study is a promising candidate as a new alternative drug for toxoplasmosis therapy.
In the population of T. gondii that was resistant to the treatment, the iron(III) compound also induced the transformation of T. gondii from the tachyzoite (i.e., active) form to the bradyzoite (i.e., latent) form. This conversion was indicated by the presence of cyst-like structures in conventional optical and electron microscopy samples. Furthermore, the appearance of bradyzoite ultrastructural markers, such as amylopectin granules and the tissue cyst wall, which are labeled by DBA lectin, was also observed. The formation of tissue cysts of T. gondii in vitro after the conversion of tachyzoites to bradyzoites has also been described for other molecules (e.g., pterocarpanquinone LQB-118 [33] and azasterols [34], for which the IC50s after 48 h of treatment were 2.5 μmol liter−1 and between 0.8 and 4.7 μmol liter−1, respectively). It is important to note that the T. gondii tachyzoites used in this set of assays belong to the virulent strain RH, which does not usually generate tissue cysts in mice (35–37). However, some studies have shown that tachyzoites that belong to the RH strain, when infecting primary cultures of embryonic muscle cells, are able to spontaneously convert into the bradyzoite stage in vitro, although at a 3% rate (38, 39). In addition, in an infection model with mice, treatment with atovaquone and pyrrolidine dithiocarbamate (40) or sulfadiazine (41) induces the conversion of tachyzoites of the RH strain to bradyzoites. Other compounds with in vitro and in vivo anti-T. gondii activities were able to convert tachyzoites into bradyzoites, such as an inhibitor of cyclic GMP-dependent protein kinase (42, 43). This result suggests that the iron(III) compound employed in this study induced a stressful condition for the parasite, resulting in this type of conversion.
The scanning electron microscopy images clearly show that after 6 days of infection of the host cells, the lytic cycle of the parasite was established with the release of large amounts of parasites, resulting in disruption of the host cells (44). This is characteristic of the cell cycle of tachyzoites of T. gondii RH. Treatment with the iron(III) compound was able to preserve the host cell monolayer, as seen by the much smaller number of extracellular parasites (Fig. 5D), probably due to the induction of T. gondii cystogenesis and the control of parasite proliferation caused by the metallocomplex.
In addition to cystogenesis, treatment with the compound also induced ultrastructural damage, resulting in death of part of the parasite population. This damage included the appearance of structures with a myelin-like aspect and membrane structures in the parasite cytoplasm that are typical of autophagic processes. Autophagy was confirmed in some parasites by labeling with the LC3B marker. Approximately 5% of the infected and treated cells contained autophagic vesicles, and some vacuoles contained parasites with autophagic ultrastructural changes. These features have been observed in T. cruzi cells treated with geranylgeraniol (45) and in T. gondii cells incubated with monensin (46). In addition, our scanning and transmission electron microscopy analyses revealed the appearance of blebs in the parasite membrane, which is a hallmark of cells undergoing apoptotic cell death. This type of cell death was confirmed using the TUNEL test, indicating that the compound used in this study activated multiple cell death mechanisms. The simultaneous occurrence of different types of cell death, such as autophagy, apoptosis, and necrosis, has been described in other parasites, such as T. cruzi (47, 48). Interestingly, this phenomenon appears to occur in T. gondii after treatment with the iron(III) compound described in this paper.
To investigate a possible mode of action of this iron(III) compound, we evaluated whether the stressful conditions induced by the treatment were associated with an oxidative insult. We found that both infected and uninfected host cells significantly increased the production of ROS only when treated with the compound. This treatment may have caused alterations in the intracellular redox balance of the parasites, leading to antioxidant defense dysfunction and parasite death. Interestingly, the treatment appeared to specifically affect the parasite, with no effect on host cell viability. As previously described in the literature (49), iron may induce the production of free radicals, such as hydroxyl radicals, in biological systems; free radicals are often associated with DNA damage, lipid peroxidation, protein modifications, and other related effects that are characteristic of oxidative stress (50). Other parasites were recently described to be susceptible to chemical compounds that induce oxidative stress, such as miltefosine, which is used against Leishmania donovani (51); glabridin, which is used against Plasmodium falciparum and causes death, as evidenced by the depolarization of mitochondrial membrane potential, the activation of caspase-like proteases, and DNA fragmentation (52); and the compound elatol, which increases ROS production in treated T. cruzi trypomastigotes, leading to autophagy and apoptosis (53). T. gondii has evolved a robust antioxidant defense system (27), which is primarily based on the activity of the SOD (54) and CAT (53, 55, 56) antioxidant enzymes, enabling the parasite to deal with ROS produced by immune cells. However, the parasite was unable to handle the increased intracellular oxidation that was generated in the host by the iron(III) compound. To understand why the parasite was unable to counteract the increase in host intracellular oxidation that was induced by the iron(III) compound, the T. gondii antioxidant enzymes SOD and CAT were quantified. The susceptibility of the parasite to oxidative stress likely occurred due to a reduction in activity of the enzymatic antioxidant protection system, which is represented by the SOD and CAT enzymes. Interestingly, the SOD and CAT enzymes may be inactivated by increased ROS in different in vitro (57) and in vivo (58) models.
In conclusion, we found that an increase in ROS production after treatment with the iron(III) compound was the main factor responsible for the cytotoxicity of the compound, which killed 60% of the parasite population via different cell death pathways after 6 days of continuous treatment. The compound investigated in this study has high efficacy in vitro and may be considered a promising chemotherapeutic agent against T. gondii, due to its lack of toxicity to the host cell and its ability to reduce the levels of metalloenzymes that are important for the antioxidant protection of the parasite by generating oxidative stress. In vivo studies will be performed in the future to validate the efficacy of the compound.
ACKNOWLEDGMENTS
We thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) in Brazil for their financial support.
We also thank Eliandro Lima for technical assistance.
J. A. Portes was responsible for carrying out the experimental part of the paper and helped in drafting the text of the article, T. G. Souza was responsible for counting parasite interactions of the host cells, T. A. T. dos Santos helped structure the experimental part of the paper, L. L. R. da Silva was responsible for analyzing the viability of host cells in the presence of the drug tested in the experiments, T. P. Ribeiro was responsible for the experimental part of the enzymatic activity of superoxide dismutase and catalase tests, M. D. Pereira was responsible for descriptions of the results of enzymatic activities and helped in drafting the text of the paper, A. Horn, Jr., was responsible for the synthesis of the drug used in the paper and assisted in drafting the text of the article, C. Fernandes was responsible for obtaining the crystallography analysis of the drug used in the paper and helped in drafting the text of the article, R. A. DaMatta was responsible for the preparation and construction of ideas for the paper and helped in drafting the text of the article, W. de Souza was responsible for the preparation and construction of ideas for the paper and helped in drafting the text of the article, and S. H. Seabra participated in the experimental procedures and was responsible for the preparation and construction of ideas for the paper and helped in drafting the text of the article.
REFERENCES
- 1.Levine ND, Corliss JO, Cox FEG, Deroux G, Grain J, Honigberg BM, Leedale GF, Loeblich AR, Lom J, Lynn D, Merinfeld EG, Page FC, Poljansky G, Sprague V, Vavra J, Wallace FG. 1980. A newly revised classification of the Protozoa. J Protozool 27:37–58. doi: 10.1111/j.1550-7408.1980.tb04228.x. [DOI] [PubMed] [Google Scholar]
- 2.Hill DE, Chirukandoth S, Dubey JP. 2005. Biology and epidemiology of Toxoplasma gondii in man and animals. Anim Health Res Rev 6:41–61. doi: 10.1079/AHR2005100. [DOI] [PubMed] [Google Scholar]
- 3.Sullivan WJ Jr, Smith AT, Joyce BR. 2009. Understanding mechanisms and the role of differentiation in pathogenesis of Toxoplasma gondii: a review. Mem Inst Oswaldo Cruz 104:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fergurson DJP, Hutchison WM, Pettersen E. 1989. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. An immunocytochemical and ultrastructural study. Parasitol Res 75:599–603. doi: 10.1007/BF00930955. [DOI] [PubMed] [Google Scholar]
- 5.Tenter AM, Heckeroth AR, Weiss LM. 2000. Toxoplasma gondii: from animals to humans. Int J Parasitol 30:1217–1258. doi: 10.1016/S0020-7519(00)00124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAuley J, Boyer KM, Patel D, Mets M, Swisher C, Roizen N, Wolters C, Stein L, Stein M, Schey W, Remington J, Meier P, Johnson D, Heydeman P, Holfels E, Withers S, Mack D, Brown C, Patton D, McLeod R. 1994. Early and longitudinal evaluations of treated infants and children and untreated historical patients with congenital toxoplasmosis: the Chicago Collaborative Treatment Trial. Clin Infect Dis 18:38–72. doi: 10.1093/clinids/18.1.38. [DOI] [PubMed] [Google Scholar]
- 7.Kamerkar S, Davis PH. 2012. Toxoplasma on the brain: understanding host-pathogen interactions in chronic CNS infection J Parasitol Res 2012:589295. doi: 10.1155/2012/589295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haverkos HW. 1987. Assessment of therapy for toxoplasma encephalitis, The TE Study Group. Am J Med 82:907–914. doi: 10.1016/0002-9343(87)90151-3. [DOI] [PubMed] [Google Scholar]
- 9.Leport C, Raffi F, Matheron S, Katlama C, Regnier B, Saimot AG, Marche C, Vedrenne C, Vilde JL. 1988. Treatment of central nervous system toxoplasmosis with pyrimethamine/sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am J Med 84:94–100. doi: 10.1016/0002-9343(88)90014-9. [DOI] [PubMed] [Google Scholar]
- 10.Georgiev VS. 1994. Management of toxoplasmosis. Drugs 48:179–188. doi: 10.2165/00003495-199448020-00005. [DOI] [PubMed] [Google Scholar]
- 11.Ramírez-Macías I, Marín C, Salas JM, Caballero A, Rosales MJ, Villegas N, Rodríguez-Dieguez A, Barea E, Sánchez-Moreno M. 2011. Biological activity of three novel complexes with the ligand 5-methyl-1,2,4-triazolo[1,5-a]pyrimidin-7(4H)-one against Leishmania spp. J Antimicrob Chemother 66:813–819. doi: 10.1093/jac/dkq537. [DOI] [PubMed] [Google Scholar]
- 12.Caballero AB, Marín C, Rodríguez-Diéguez A, Ramírez-Macías I, Barea E, Sánchez-Moreno M, Salas JM. 2011. In vitro and in vivo antiparasital activity against Trypanosoma cruzi of three novel 5-methyl-1,2,4-triazolo[1,5-a]pyrimidin-7(4H)-one-based complexes. J Inorg Biochem 105:770–776. doi: 10.1016/j.jinorgbio.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 13.Horn A Jr, Fernandes C, Bortoluzzi AJ, Vugman NV, Herbst MH. 2005. Coordination chemistry of the new ligand 1-(bis-pyridin-2-ylmethyl-amino)-3-chloropropan-2-ol (HPCINOL) with copper(II). X-ray crystal structure, spectroscopic and electrochemical properties of the complex [Cu(HPCINOL)(CH3CN)](ClO4)2. J Mol Struct 749:96–102. doi: 10.1016/j.molstruc.2005.03.045. [DOI] [Google Scholar]
- 14.Fernandes C, Parrilha GL, Lessa JA, Santiago LJM, Kanashiro MM, Boniolo FS, Bortoluzzi AJ, Vugman NV, Herbst MH, Horn A Jr. 2006. Synthesis, crystal structure, nuclease and in vitro antitumor activities of a new mononuclear copper(II) complex containing a tripodal N3O ligand. Inorg Chim Acta 359: 3167–3176. doi: 10.1016/j.ica.2006.04.007. [DOI] [Google Scholar]
- 15.Horn A Jr, Parrilha GL, Melo KV, Fernandes C, Horner M, Visentin Ldo C, Santos JAS, Santos MS, Eleutherio ECA, Pereira MD. 2010. An iron-based cytosolic catalase and superoxide dismutase mimic complex. Inorg Chem 49:1274–1276. doi: 10.1021/ic901904b. [DOI] [PubMed] [Google Scholar]
- 16.Horn A Jr, Fernandes C, Parrilha GL, Kanashiro MM, Borges FV, de Melo EJT, Schenk G, Terenzi H, Pich CT. 2013. Highly efficient synthetic iron-dependent nucleases activate both intrinsic and extrinsic apoptotic death pathways in leukemia cancer cells. J Inorg Biochem 128:38–47. doi: 10.1016/j.jinorgbio.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 17.Parrilha GL, Fernandes C, Bortoluzzi AJ, Szpoganicz B, Silva MdS, Pich CT, Terenzi H, Horn A Jr. 2008. A new μ-oxo di-iron complex with suitable features to mimic metallohydrolase activity: X-ray molecular structure, aqua solution behavior and nuclease activity of the complex [Fe(HPCINOL)(SO4)]2-μ-oxo. Inorg Chem Commun 11:643–647. doi: 10.1016/j.inoche.2008.02.019. [DOI] [Google Scholar]
- 18.Koh JY, Choi DW. 1987. Quantitative determination of glutamate mediated cortical neuronal injury in cell culture by lactate dehydrogenase efflux assay. J Neurosci Methods 20:83–90. doi: 10.1016/0165-0270(87)90041-0. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Zhang J. 1997. Inhibition of apoptosis by ginsenoside Rg1 in cultured cortical neurons. Chin Med J (Engl) 110:535–539. [PubMed] [Google Scholar]
- 20.dos Santos TAT, Portes JA, Damasceno-Sá JC, Caldas LA, de Souza W, DaMatta RA, Seabra SH. 2011. Phosphatidylserine exposure by Toxoplasma gondii is fundamental to balance the immune response granting survival of the parasite and of the host. PLoS One 6:e27867. doi: 10.1371/journal.pone.0027867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magno R, Lemgruber L, Vommaro RC, De Souza W, Attias M. 2005. Intravacuolar network may act as a mechanical support for Toxoplasma gondii inside the parasitophorous vacuole. Microsc Res Tech 67:45–52. doi: 10.1002/jemt.20182. [DOI] [PubMed] [Google Scholar]
- 22.Jakubowski W, Bartosz G. 2000. 2,7-Dichlorofluorescin oxidation and reactive oxygen species: what does it measure? Cell Biol Int 24:757–760. doi: 10.1006/cbir.2000.0556. [DOI] [PubMed] [Google Scholar]
- 23.Murray HW, Cohn ZA. 1979. Macrophage oxygen-dependent antimicrobial activity. I. Susceptibility of Toxoplasma gondii to oxygen intermediates. J Exp Med 150:938–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Aebi H. 1964. Catalase in vitro. Methods Enzymol 105:114–118. [DOI] [PubMed] [Google Scholar]
- 26.Zhang YM, Halonen SK, Ma YF, Wittner M, Weiss LM. 2001. Initial characterization of CST1, a Toxoplasma gondii cyst wall glycoprotein. Infect Immun 69:501–507. doi: 10.1128/IAI.69.1.501-507.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RA, Britigan BE. 1997. Role of oxidants in microbial pathophysiology. Clin Microbiol Rev 10:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Oliveira TC, Silva DAO, Rostkowska C, Béla SR, Ferro EAV, Magalhães PM, Mineo JR. 2009. Toxoplasma gondii: effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Exp Parasitol 122:233–241. doi: 10.1016/j.exppara.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Meneceur P, Bouldouyre MA, Aubert D, Villena I, Menotti J, Sauvage V, Garin JF, Derouin F. 2008. In vitro susceptibility of various genotypic strains of Toxoplasma gondii to pyrimethamine, sulfadiazine, and atovaquone. Antimicrob Agents Chemother 52:1269–1277. doi: 10.1128/AAC.01203-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin C, Kaewintajuk K, Jiang J, Jeong W, Kamata M, KIM H, Wataya Y, Park H. 2009. Toxoplasma gondii: a simple high-throughput assay for drug screening in vitro. Exp Parasitol 121:132–136. doi: 10.1016/j.exppara.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 31.Doliwa C, Escotte-Binet S, Aubert D, Velard F, Schmid A, Geers R, Villena I. 2013. Induction of sulfadiazine resistance in vitro in Toxoplasma gondii. Exp Parasitol 133:131–136. doi: 10.1016/j.exppara.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 32.van der Ven AJ, Schoondermark-van de Ven EM, Camps W, Melchers WJ, Koopmans PP, van der Meer JW, Galama JM. 1996. Anti-toxoplasma effect of pyrimethamine, trimethoprim and sulphonamides alone and in combination: implications for therapy. J Antimicrob Chemother 38:75–80. doi: 10.1093/jac/38.1.75. [DOI] [PubMed] [Google Scholar]
- 33.Portes Jde A, Netto CD, da Silva AJM, Costa PRR, DaMatta RA, dos Santos TAT, De Souza W, Seabra SH. 2012. A new type of pterocarpanquinone that affects Toxoplasma gondii tachyzoites in vitro. Vet Parasitol 186:261–269. doi: 10.1016/j.vetpar.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Martins-Duarte ES, Lemgruber L, Lorente SO, Gros L, Magaraci F, Gilbert IH, de Souza W, Vommaro RC. 2010. Evaluation of three novel azasterols against Toxoplasma gondii. Vet Parasitol 177: 157–161. doi: 10.1016/j.vetpar.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 35.Mavin S, Joss AWL, Ball J, Ho-Yen DO. 2004. Do Toxoplasma gondii RH strain tachyzoites evolve during continuous passage? J Clin Pathol 57:609–611. doi: 10.1136/jcp.2003.013763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Howe DK, Sibley LD. 1994. Toxoplasma gondii: analysis of different laboratory stocks of the RH strain reveals genetic heterogeneity. Exp Parasitol 78:242–245. doi: 10.1006/expr.1994.1024. [DOI] [PubMed] [Google Scholar]
- 37.Howe DK, Sibley LD. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172:1561–66. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira-da-Silva Mda F, Takacs AC, Barbosa HS, Gross U, Lüder CG. 2009. Primary skeletal muscle cells trigger spontaneous Toxoplasma gondii tachyzoite to bradyzoite conversion at higher rates than fibroblasts. Int J Med Microbiol 299:381–388. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira-da-Silva Mda F, Rodrigues RM, Andrade EF, Carvalho Ld, Gross U, Lüder CG, Barbosa HS. 2009. Spontaneous stage differentiation of mouse-virulent Toxoplasma gondii RH parasites in skeletal muscle cells: an ultrastructural evaluation. Mem Inst Oswaldo Cruz 104:196–200. [DOI] [PubMed] [Google Scholar]
- 40.Djurković-Djaković O, Nikolić A, Bobić B, Klun I, Aleksić A. 2005. Stage conversion of Toxoplasma gondii RH parasites in mice by treatment with atovaquone and pyrrolidine dithiocarbamate. Microbes Infect 7:49–54. doi: 10.1016/j.micinf.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 41.Villard O, Candolfi E, Ferguson DJP, Marcellin L, Kien T. 1997. Loss of oral infectivity of tissue cysts of Toxoplasma gondii RH strain to outbred Swiss Webster mice. Int J Parasitol 27:1555–1559. doi: 10.1016/S0020-7519(97)00144-6. [DOI] [PubMed] [Google Scholar]
- 42.Gurnett AM, Liberator PA, Dulski PM, Salowe SP, Donald RG, Anderson JW, Wiltsie J, Diaz CA, Harris G, Chang B, Darkin-Rattray SJ, Nare B, Crumley T, Blum PS, Misura AS, Tamas T, Sardana MK, Yuan J, Biftu T, Schmatz DM. 2002. Purification and molecular characterization of cGMP-dependent protein kinase from apicomplexan parasites. A novel chemotherapeutic target. J Biol Chem 277:15913–15922. [DOI] [PubMed] [Google Scholar]
- 43.Nare B, Allocco JJ, Liberator PA, Donald RG. 2002. Evaluation of a cyclic GMP-dependent protein kinase inhibitor in treatment of murine toxoplasmosis: gamma interferon is required for efficacy. Antimicrob Agents Chemother 46:300–307. doi: 10.1128/AAC.46.2.300-307.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black MW, Boothroyd JC. 2000. Lytic cycle of Toxoplasma gondii. Microbiol Mol Biol Rev 64:607–623. doi: 10.1128/MMBR.64.3.607-623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menna-Barreto RF, Laranja GA, Silva MC, Coelho MG, Paes MC, Oliveira MM, de Castro SL. 2008. Anti-Trypanosoma cruzi activity of Pterodon pubescens seed oil: geranylgeraniol as the major bioactive component. Parasitol Res 103:111–117. doi: 10.1007/s00436-008-0937-0. [DOI] [PubMed] [Google Scholar]
- 46.Lavine MD, Arrizabalaga G. 2012. Analysis of monensin sensitivity in Toxoplasma gondii reveals autophagy as a mechanism for drug induced death. PLoS One 7:e42107. doi: 10.1371/journal.pone.0042107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menna-Barreto RF, Corrêa JR, Cascabulho CM, Fernandes MC, Pinto AV, Soares MJ, De Castro SL. 2009. Naphthoimidazoles promote different death phenotypes in Trypanosoma cruzi. Parasitology 136:499–510. doi: 10.1017/S0031182009005745. [DOI] [PubMed] [Google Scholar]
- 48.Menna-Barreto RF, Salomão K, Dantas AP, Santa-Rita RM, Soares MJ, Barbosa HS, de Castro SL. 2009. Different cell death pathways induced by drugs in Trypanosoma cruzi: an ultrastructural study. Micron 40:157–168. doi: 10.1016/j.micron.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 49.Jones-Lee A, Lee GF. 2005. Role of iron chemistry in controlling the release of pollutants from resuspended sediments. Remediation 16:33–41. doi: 10.1002/rem.20068. [DOI] [Google Scholar]
- 50.Jomova K, Valko M. 2011. Advances in metal-induced oxidative stress and human disease. Toxicology 283:65–87. doi: 10.1016/j.tox.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 51.Mishra J, Singh S. 2013. Miltefosine resistance in Leishmania donovani involves suppression of oxidative stress-induced programmed cell death. Exp Parasitol 135:397–406. doi: 10.1016/j.exppara.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Cheema HS, Prakash O, Pal A, Khan F, Bawankule DU, Darokar MP. 2014. Glabridin induces oxidative stress mediated apoptosis like cell death of malaria parasite Plasmodium falciparum. Parasitol Int 63:349–358. doi: 10.1016/j.parint.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Desoti VC, Lazarin-Bidóia D, Sudatti DB, Pereira RC, Alonso A, Ueda-Nakamura T, Filho BPD, Nakamura CV, Silva SO. 2012. Trypanocidal action of (−)-elatol involves an oxidative stress triggered by mitochondria dysfunction. Mar Drugs 10:1631–1646. doi: 10.3390/md10081631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ödberg-Ferragut C, Renault JP, Viscogliosi E, Toursel C, Briche I, Engels A, Lepage G, Morgenstern-Badarau I, Camus D, Tomavo S, Dive D. 2000. Molecular cloning, expression analysis and iron metal cofactor characterization of a superoxide dismutase from Toxoplasma gondii. Mol Biochem Parasitol 106:121–129. doi: 10.1016/S0166-6851(99)00211-X. [DOI] [PubMed] [Google Scholar]
- 55.Ding M, Clayton C, Soldati D. 2000. Toxoplasma gondii catalase: are there peroxisomes in toxoplasma? J Cell Sci 113:2409–2419. [DOI] [PubMed] [Google Scholar]
- 56.Kaasch AJ, Joiner KA. 2000. Targeting and subcellular localization of Toxoplasma gondii catalase. Identification of peroxisomes in an apicomplexan parasite; J Biol Chem 275: 1112–1118. doi: 10.1074/jbc.275.2.1112. [DOI] [PubMed] [Google Scholar]
- 57.Kim SY, Kwon OJ, Park JW. 2001. Inactivation of catalase and superoxide dismutase by singlet oxygen derived from photoactivated dye. Biochimie 83:437–444. doi: 10.1016/S0300-9084(01)01258-5. [DOI] [PubMed] [Google Scholar]
- 58.Costa VM, Amorim MA, Quintanilha A, Moradas-Ferreira P. 2002. Hydrogen peroxide-induced carbonylation of key metabolic enzymes in Saccharomyces cerevisiae: the involvement of the oxidative stress response regulators Yap1 and Skn7. Free Radic Biol Med 33:1507–1515. doi: 10.1016/S0891-5849(02)01086-9. [DOI] [PubMed] [Google Scholar]