Summary
Accumulation of α-synuclein (α-syn) into insoluble aggregates occurs in several related disorders collectively referred to as synucleinopathies. To date, studies have used neural stem cells (NSCs) to examine questions about α-syn propagation, but have overlooked the therapeutic potential of NSC transplantation to modulate cognition in disorders such as dementia with Lewy bodies or Parkinson’s disease dementia. Here, we show that striatal transplantation of NSCs into aged α-syn transgenic mice significantly improves performance in multiple cognitive and motor domains. This recovery is associated with NSC expression of brain-derived neurotrophic factor (BDNF), which restores depleted levels and modulates dopaminergic and glutamatergic systems. Most importantly, transplantation of BDNF-depleted NSCs fails to improve behavior, whereas AAV-mediated BDNF delivery mimics the benefits of NSC transplantation, supporting a critical role for this neurotrophin in functional improvement. Thus, NSC transplantation could offer a promising approach to treat the understudied yet devastating cognitive components of many synucleinopathies.
Highlights
-
•
α-Synuclein mice exhibit significant DLB/PDD-associated cognitive and motor deficits
-
•
Striatal NSC transplantation dramatically improves cognitive and motor function
-
•
BDNF is necessary for NSC-mediated behavioral improvements
-
•
NSC-BDNF promotes recovery by regulating dopaminergic and glutamatergic systems
Blurton-Jones and colleagues examine the effects of neural stem cell (NSC) transplantation in a transgenic model of dementia with Lewy bodies, demonstrating robust improvements in cognitive and motor function. Genetic and pharmacological manipulations reveal that stem-cell-derived BDNF is critical for these improvements and that NSC-mediated modulation of glutamatergic and dopaminergic systems could have broad implications for many synucleinopathies.
Introduction
Dementia with Lewy bodies (DLB) is the second most common cause of age-related dementia, affecting over 1.3 million people in the United States alone (Vann Jones and O’Brien, 2014). DLB is associated with the accumulation of insoluble aggregates of the presynaptic protein α-syn within the cortex, hippocampus, and brainstem that leads to progressive neurodegeneration and impairments in cognition and spontaneous mild parkinsonism (Mayo and Bordelon, 2014). DLB is closely related to a second disorder, Parkinson’s disease (PD) dementia (PDD) that develops in up to 70% of PD patients (Dubois and Pillon, 1997, Marsh and Blurton-Jones, 2012). Current treatments for DLB and PDD are limited and provide only modest symptomatic relief; thus, there is a pressing need to identify new and effective therapies.
Unfortunately, the impact of stem cell transplantation in models that develop α-syn pathology has thus far only been examined in terms of cell-to-cell transmission of pathology (Desplats et al., 2009, Hansen et al., 2011). In contrast, many studies have demonstrated promising improvements in motor function by transplanting dopaminergic (DAergic) precursors in neurotoxin models of PD (Lees and Smith, 1983, Docherty and Burn, 2010). However, cognitive deficits in DLB/PDD are strongly associated with α-syn and neurotoxin models fail to mimic this important phenotype. It therefore remains critical to examine the therapeutic potential of stem cell transplantation in the presence of α-syn pathology and to better understand the impact of cell transplantation in models of DLB and PDD.
In this context, neural stem cells (NSCs), which can migrate and produce high levels of neurotrophic factors, may offer a promising alternative to DA precursor transplantation. Here, we utilized a human α-syn-expressing mouse model that recapitulates many of the salient features of DLB/PDD, including the progressive development of Lewy body pathology and significant cognitive dysfunction (Masliah et al., 2000, Amschl et al., 2013). Using these mice, we investigated the migration and differentiation of transplanted NSCs within the striatum and their impact on behavior, α-syn pathology, and DAergic and glutamatergic regulation. Our results reveal that NSC transplantation can dramatically improve both motor and cognitive function by elevating levels of brain-derived neurotrophic factor (BDNF), a protein implicated in DLB/PDD cognitive impairments (Leverenz et al., 2011). Pharmacological manipulations implicate both DAergic and glutamatergic circuits downstream of BDNF in this recovery. Furthermore, shRNA-mediated loss-of-function studies confirm the necessity of BDNF in this process, and AAV-mediated gain-of-function experiments demonstrate that BDNF can mimic the benefits of NSC transplantation. Collectively, our studies reveal that NSCs can improve both the motor and cognitive symptoms of DLB/PDD in a progressive transgenic model by elevating levels of BDNF and enhancing DAergic and glutamatergic function.
Results
NSC Transplantation Rescues Both Motor and Cognitive Deficits in α-Synuclein Transgenic Mice
In order to assess the impact of NSC transplantation on h-α-syn-associated cognitive and motor dysfunction, we stereotactically injected haplotype-matched murine NSCs or vehicle bilaterally into 12-month-old h-α-syn overexpressing (ASO) mice or WT littermates (100,000 cells per side). At this age, ASO mice exhibit widespread Lewy body-like pathology as well as substantial motor and cognitive impairments (Masliah et al., 2000, Amschl et al., 2013). In order to achieve the greatest impact of transplantation on both cognitive and motor systems that are altered by α-syn accumulation, we targeted the dorsal striatum (Figure 1A; see Supplemental Experimental Procedures), widely known to be involved in learning and memory and highly interconnected with multiple cortical regions. We used previously characterized GFP-expressing NSCs (Mizumoto et al., 2003, Blurton-Jones et al., 2009). At 1 month after transplantation, mice were habituated, trained, and tested on three motor tasks and two cognitive tasks, and engraftment was assessed.
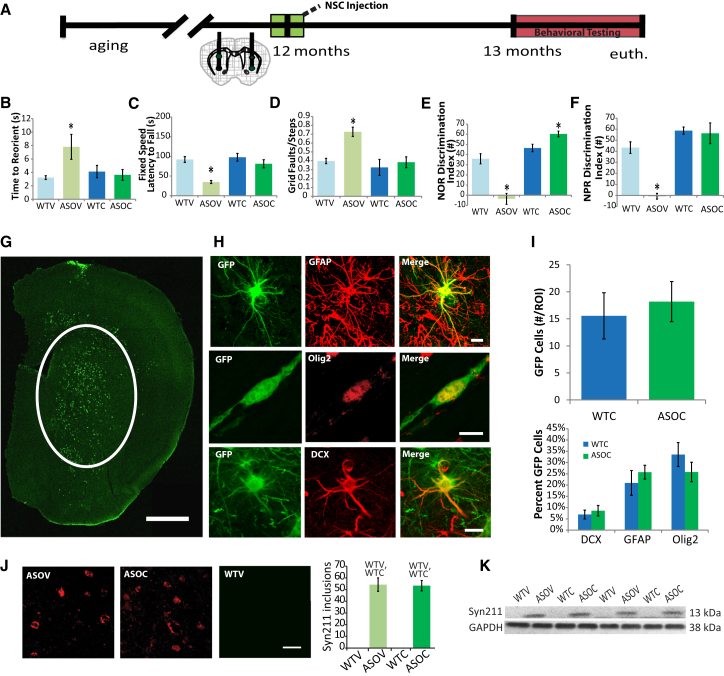
Figure 1.
NSC Transplantation Improves Both Motor and Cognitive Function in α-Synuclein Transgenic Mice
(A) α-Synuclein-overexpressing (ASO) and WT mice were aged to 12 months, and then syngeneic GFP-NSCs were transplanted bilaterally into the striatum; 1 month later, motor and cognitive behavior were assessed.
(B–D) ASO vehicle treated controls (ASOV) show deficits in Pole reorientation (B), Rotarod performance (C), and beam traversal (D) tasks, which were significantly ameliorated by NSC transplantation (ASOC).
(E and F) ASOV mice also showed significant deficits in cortical-dependent NOR (E) and hippocampal-dependent NPR (F), which were improved by NSC transplantation (ASOC).
(G) GFP-NSCs (green) migrated from their medial striatal injection sites and engrafted predominantly within the striatum.
(H) The majority of NSCs differentiated into astrocytes (GFAP, red) and immature oligodendrocytes (Olig2, red). A smaller proportion of NSCs adopted an early neuronal phenotype (DCX, red).
(I) No differences in NSC engraftment or differentiation were detected between WTC and ASOC groups.
(J) Immunohistochemical analysis also revealed no differences in either striatal h-α-syn inclusion number between ASOV and ASOC mice, and no differences were detected in WT mice; Fisher’s PLSD post hoc WTV,WTCp < 0.003.
(K) Western blot analysis of striatal monomeric h-α-syn (Syn211) was not significantly altered by NSCs. Data are presented as mean ± SEM; ANOVA p < 0.05 and Fisher’s PLSD post hoc ∗p < 0.0001 compared with all other groups (n = 6–8 mice/group).
Scale bar represents 100 μm (B) and 10 μm (C and D). See also Figures S1 and S2.
As expected, vehicle-treated ASO (ASOV) mice showed significant impairments in all three motor tasks compared with vehicle-injected WTs (WTV). The ASOV mice took twice as long to reorient before descending on the pole task (Figure 1B; F (3, 18) = 3.9; ANOVA p = 0.03). Likewise, ASOV mice fell of the Rotarod in half the time of WTV controls (Figure 1C; F(3, 18) = 9.5; ANOVA p = 0.0006) and exhibited an 83% increase in foot faults in the beam traversal task (Figure 1D; F(3, 18) = 12, ANOVA p = 0.0002). In contrast, ASO mice that received NSC transplants (ASOC) performed similarly to both WTV and NSC-injected WT mice (WTC) in all three motor tasks, demonstrating that NSC transplantation can dramatically improve motor function in transgenic α-synuclein mice.
To examine cognition in a model with motor impairments, it is critical to use tasks that are not heavily influenced by motor function. We therefore utilized Novel Object Recognition (NOR) and Novel Place Recognition (NPR) tasks: low-stress paradigms that quantify the proportion of time spent examining a novel object and provide data on cortical-dependent and hippocampal-dependent memory, respectively. All four groups were habituated, trained, and tested following standard protocols (Supplemental Experimental Procedures). Twenty-four hours after training, mice were exposed to a novel object or novel object placement, and the discrimination ratio between exploration time of old and new objects was calculated. As shown, ASOV mice exhibited significant impairments in NOR compared with WTV and WTC groups, demonstrating that h-α-syn overexpression can model important aspects of DLB/PDD-related cognitive impairment (Figure 1E; F(3, 18) = 19.3; ANOVA p = 0.0001). Striatal transplantation of NSCs dramatically improved performance in this cortical-dependent task, as ASOC mice discriminated between novel and familiar objects to a greater degree than either WTV or WTC mice, indicating not only a benefit, but enhancement in this task. In the hippocampal-dependent NPR task, ASOV mice again showed significant impairments that were also rescued by NSC transplantation (Figure 1F; F(3, 18) = 21; ANOVA p = 0.0001). Because we found no differences in activity during training (data not shown), this confirmed that h-α-syn expression and NSC transplantation alter memory performance independent of effects on motor function. Thus, we demonstrate that NSC transplantation can rescue both motor and cognitive deficits in a transgenic model of DLB/PDD.
NSCs Migrate throughout the Striatum and into the Cortex but Do Not Reach the Hippocampus
To begin to decipher the mechanism by which GFP-NSC transplantation ameliorates behavioral deficits, we examined the migration and differentiation of engrafted cells. Confocal microscopy demonstrated that at 6 weeks following transplantation GFP-NSCs had migrated throughout the striatum (Figure 1G). A modest number of cells were also observed within the motor and perirhinal cortices and amygdala, and in a few animals, some cells even reached the substantia nigra (data not shown). However, no GFP-NSCs were detected within the hippocampus of any animals. Engrafted NSCs differentiated primarily into astrocytes and immature oligodendrocytes, with only a few cells exhibiting an early neuronal fate (Figures 1H, 1I, and S1). No examples of fully matured GFP/NeuN double-labeled neurons were detected (Figure S1). Taken together, these findings are consistent with previous reports that murine NSCs predominantly acquire gliogenic phenotypes when transplanted into non-neurogenic regions and with other studies that utilized these postnatal day 1-derived cells (Herrera et al., 1999, Yamasaki et al., 2007, Blurton-Jones et al., 2009). Interestingly, no significant differences in NSC differentiation were detected between ASO and WT transplanted mice (Figure 1I), and each cell type migrated with similar distribution and distance from the injection site (Figure S1A). Finally, we examined markers of undifferentiated and proliferating cell types, finding that a small number of NSCs that had migrated beyond the dorsal striatum still expressed the mitotic marker Ki67, or immature NSC markers Nestin or Vimentin (Figures S2A–S2C).
Human α-Synuclein Pathology Is Not Altered by NSC Transplantation
Previous studies have shown that reduction of α-syn can improve motor function in α-syn transgenic mice (Masliah et al., 2011). We therefore examined the effects of striatal NSC transplantation on α -syn pathology in multiple brain regions. Using a human-specific antibody, we first confirmed that h-α-syn protein was expressed exclusively in ASO mice. Interestingly, we found no effect of NSC transplantation on h-α-syn, as both ASOV and ASOC groups exhibited equivalent numbers of Lewy-body-like inclusions within the dorsal striatum (Figure 1J). Further quantification of both detergent-soluble and insoluble h-α-syn by western blot in the striatum (Figure 1K), hippocampus, perirhinal, motor and prefrontal cortices, and the substantia nigra also confirmed that NSCs had no effect on monomeric h-α-syn (Figures S3A–S3F). Likewise, monomeric α-syn phosphorylated at serine 129 (pS129), a pathological associated epitope, was also equivalent between NSC and vehicle-injected ASO mice (post hoc Fishers’s PLSD, p = 0.14; data not shown).
NSC-Induced Cognitive and Motor Improvements Are Accompanied by Increased BDNF
Having found that NSCs had no impact on α-synuclein pathology, we began to explore other potential mechanisms that could be involved in NSC-mediated motor and cognitive improvements. We previously showed that these GFP-NSCs can produce high levels of specific growth factors including BDNF (Blurton-Jones et al., 2009). Western blot analysis revealed that striatal BDNF expression in ASOV mice was half of that in WTV controls, but was fully restored to WT levels in ASOC mice by NSC transplantation (Figures 2A and 2B; F(3, 18) = 3.8; ANOVA p = 0.03). NSCs did not increase BDNF in WTC mice, likely due to neurotrophic self-regulatory mechanisms (Canossa et al., 1997, Bambah-Mukku et al., 2014). Importantly, the BDNF receptor TrkB was unaltered by either genotype or treatment (Figures S3G and S3H), suggesting that increased BDNF could readily influence behavior via the existing TrkB receptor population. In contrast, no difference in expression of glial-derived neurotrophic factor (GDNF; Figures S3G–S3J) or its phosphorylated receptor RET were observed between any treatment groups, indicating that changes in BDNF specifically play an important role in α-syn transgenic mice. In addition to the detected changes in BDNF, phosphorylation of Erk1 (44 kDa), a major downstream effector of BDNF signaling, was also increased by NSC transplantation in both ASOC and WTC mice (Figures 2A and 2C; F(3, 18) = 4.2; ANOVA p = 0.02). We confirmed that transplanted NSCs continue to express BDNF primarily in cells co-expressing GFAP or vimentin (Figure 2D), suggesting that glial-fated NSCs may contribute substantially to increasing BDNF levels. Thus, NSC-mediated changes in BDNF appear to influence growth and plasticity-associated signal transduction within the striatum.
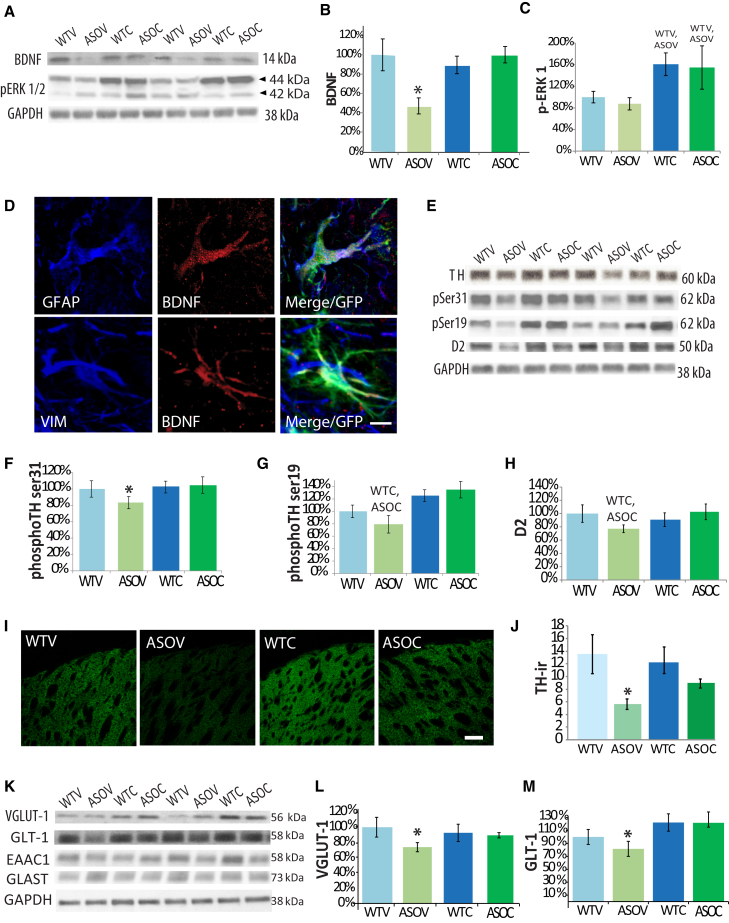
Figure 2.
NSC Transplantation Enhances Striatal BDNF Signaling with Downstream Effects on Dopamine and Glutamate Systems
(A and B) Western blots revealed that striatal BDNF was significantly decreased in ASOV mice but restored to WT levels by NSC transplantation.
(C) Downstream of BDNF, phosphorylated Erk1was significantly elevated by transplantation (A and C).
(D) Transplanted cells continue to express BDNF, primarily within GFAP or Vimentin co-labeled astrocytic cells.
(E–G) Activated forms of TH (p-ser31 and p-ser19) were significantly decreased in ASOV mice and elevated by transplantation.
(H) The DA receptor type D2 was also decreased in ASOV mice and elevated in ASOC mice (E and H).
(I and J) Confocal optical densitometry confirmed significantly reduced expression of TH in ASOV striatum that was restored toward WT levels in ASOC mice.
(K and L) Striatal VGLUT-1 was also significantly decreased in ASOV mice and restored to WT levels by NSCs.
(M) NSC transplantation also elevated striatal levels of GLT-1, but not EAAC1 and GLAST (K and M). Data are presented as mean ± SEM. All western blot graphs are presented as a percentage of WTV group. Fisher’s PLSD post hoc ∗p < 0.03 compared with all other groups, WTV, ASOVp < 0.01, WTC, ASOCp < 0.004 (n = 6–8 mice per group).
Scale bar represents 10 μm (D) and 30 μm (I).
NSC Transplantation Alters Dopaminergic and Glutamatergic Systems
To further understand the mechanism by which NSC-derived BDNF ameliorates motor and cognitive behaviors, we examined changes in expression of the rate-limiting enzyme in DA synthesis, tyrosine hydroxylase (TH), as well as markers of glutamate transport and regulation. First, we utilized a well-established optical signal intensity analysis to examine TH terminals within the striatum (Fernagut et al., 2007). As expected, we detected a significant reduction in TH-immunoreactive (TH-ir) terminals within the dorsal striatum of ASOV mice relative to WTV controls (Figures 2I, 2J, and S3K). More importantly, there was a significant partial restoration of TH density in ASO mice that received NSCs. In contrast, we confirmed as previously reported that TH-ir neurons in the substantia nigra pars compacta (SNpc) were not altered by h-α-syn in this model or by NSC transplantation (Rockenstein et al., 2002) (Figure S3L). To follow up on these findings, we performed biochemical analysis of TH as well as two phospho-epitopes of TH (serine-19 and serine-31) that are associated with increased activity and dopamine production. Consistent with our immunohistochemical data, TH phosphorylated at either site was significantly increased in ASOC mice, suggesting that NSCs elevate TH activity (Figures 2E–2G; F(3, 18) = 5.2; ANOVA p = 0.01). In further support of this effect, expression of the DA receptor D2 was also significantly increased with NSCs (Figures 2E and 2H; F(3, 18) = 6.4; ANOVA p = 0.03). These data strongly suggest that NSC transplantation enhances the function and signaling of existing nigrostriatal DAergic neurons.
We next examined the expression of glutamate transporters within the striatum to determine whether α-syn expression or NSC transplantation might influence corticostriatal and hippocampal-striatal glutamatergic projections. BDNF is known to impact glutamate signaling and activity to enhance plasticity (Gottmann et al., 2009), and previous studies have strongly implicated glutamatergic dysfunction in PD (Greenamyre, 1993, Albin et al., 1995); nevertheless, the potential therapeutic relevance of glutamatergic systems to synucleinopathies and especially DLB/PDD-associated cognitive dysfunction has been largely unexplored. Corticostriatal glutamatergic projections can be readily identified and examined via expression of vesicular glutamate transporter 1 (VGLUT-1). When levels of VGLUT-1 within the dorsal striatum were quantified, we found a significant 35% decrease in ASOV mice versus WTV controls. More importantly, this reduction was restored to normal levels by NSC transplantation (Figures 2K and 2L; F(3,18) = 3.3; ANOVA p = 0.04). In contrast, thalamostriatal specific VGLUT-2 expression was unchanged, suggesting NSCs have specific effect on corticostriatal glutamatergic systems (post hoc Fishers’s PLSD p = 0.1, data not shown).
Another glutamate transporter implicated in PD is the glial-specific transporter GLT-1 (Massie et al., 2010, Salvatore et al., 2012), which plays an important role in multiple aspects of neuronal plasticity and can be upregulated by BDNF (Rodriguez-Kern et al., 2003, Pita-Almenar et al., 2012). Although GLT-1 levels were unaltered between WTV and ASOV groups, we found that NSC transplantation significantly increased GLT-1 expression in both ASOC and WTC groups (Figures 2K and 2M; F(3,18) = 7.6; ANOVA p = 0.002). By comparison, no changes were detected in the two other glutamate transporters expressed by both neurons and glia, EAAC1 or GLAST (Figure 2K). Together these data show that corticostriatal glutamatergic systems are indeed altered in α-syn transgenic mice and that NSC transplantation can modulate astrocyte-dependent corticostriatal glutamatergic systems.
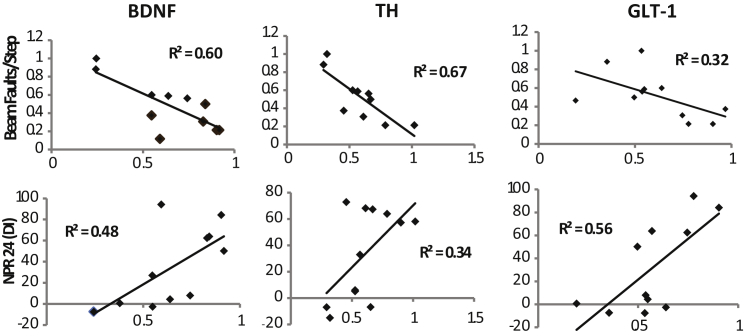
Changes in BDNF, TH, and GLT-1 Correlate with Distinct Improvements in Motor and Cognitive Tasks
The dramatic reduction in BDNF induced by h-α-syn expression and the corresponding elevation of BDNF following NSC transplantation suggest that this neurotrophin plays an important role in NSC-mediated behavioral improvements. Supporting this hypothesis, striatal BDNF expression in individual mice correlates well with NPR performance (R2 = 0.48; p = 0.005) and inversely to beam traversal faults (Figure 3; R2 = 0.60; p = 0.0001). Potentially downstream of BDNF, TH expression is also correlated strongly with beam traversal performance, but less so with hippocampal-dependent NPR. Conversely, GLT-1 was significantly associated with NPR but showed a less consistent association with beam traversal performance (Figure 3). To further examine the potential divergent role of these systems in motor and cognitive recovery we employed pharmacological loss-of function approaches using the DA synthesis inhibitor α-methyl-p-tyrosine (αMT) and GLT-1 inhibitor dihydrokainic acid (DHK). Safe and optimal doses were determined by dose response studies (Figure S4). A new set of ASO mice were then transplanted with NSCs with or without co-treatment with αMT (ASOC-αMT) or DHK (ASOC-DHK) (Figure 4A). At 1 month after transplantation, behavioral testing was again performed. In Rotarod and beam traversal motor tasks, αMT prevented NSC-induced recovery; however, ASOC-DHK mice still exhibited improved performance (Figures 4B and 4C; F(3, 20) = 9.6; ANOVA p = 0.0004; F(3, 20) = 3.9; ANOVA p = 0.03). These data further support the notion that while TH phosphorylation is clearly necessary for NSC-mediated motor improvements, changes in striatal glutamate reuptake via GLT-1 are not. Intriguingly, NSC-induced recovery of NOR was blocked by both αMT and DHK treatments in ASOC mice (Figure 4D; F(3, 20) = 13.3; ANOVA p = 0.0001), indicating that both GLT-1 and TH activity are important for NSC-mediated improvements in cortical-dependent memory. In contrast, while DHK prevented NSC-mediated improvements in NPR, αMT co-treatment had no effect on this hippocampal-dependent task, as ASOC-αMT mice performed equivalently to saline-injected ASOC mice (Figure 4E; F(3, 20) = 8.2; ANOVA p = 0.009). Taken together, these findings indicate that an NSC-induced increase in GLT-1 is critical for both NOR and NPR, but that NSC-mediated DA regulation contributes primarily to motor improvements and NOR but not to hippocampal-dependent NPR.
Figure 3.
BDNF Expression Correlates with Both Motor and Cognitive Function, whereas TH and GLT-1 Show Distinct Associations with Motor and Cognitive Function
BDNF, TH, and GLT-1 were compared with cognitive (NPR) and motor (beam faults/step) performance in ASO and ASOC mice. BDNF was highly correlated with improvements in both behaviors. However, while TH was more closely correlated with motor behavior, GLT-1 was more significantly associated with cognitive function, suggesting that dopaminergic and glutamatergic systems play key roles in motor and cognitive recovery respectively. Data points represent within-subject means of individual animals p < 0.05. X axis, protein levels are represented as the percentage of WTV; y axis, beam faults/step are numerical ratios. NPR DI values are discrimination index ratios (see Supplemental Experimental Procedures; n = 7 mice per group). See also Figure S3.
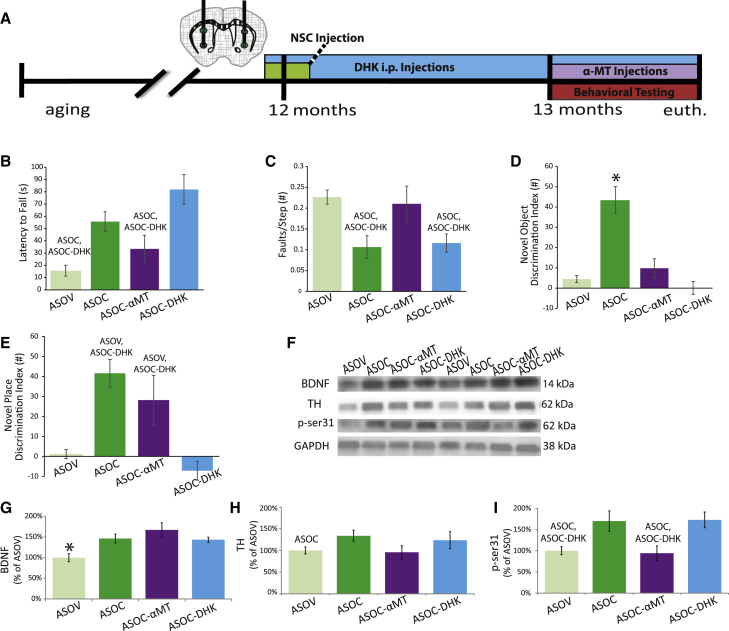
Figure 4.
TH and GLT-1 Function Are Necessary for NSC-Mediated Behavioral Improvements
(A) One group of ASOC mice received intraperitoneal DHK injections daily following a post-NSC-transplantation recovery period of 2 days and continuing through behavioral testing. Another group of ASOC mice received the TH inhibitor α-methyl-p-tyrosine (ASOC-αMT) over the ten days prior to and during behavioral assessment.
(B and C) αMT prevented NSC-mediated Rotarod (B) and beam traversal (C) recovery in ASOC mice; however, ASOC-DHK treated mice displayed similar recovery of motor function to the ASOC group in both tasks.
(D) Conversely, in novel object recognition, NSC-mediated improvement was abolished by both αMT and DHK.
(E) NSC-mediated recovery in NOR was not inhibited by αMT, but was prevented by DHK.
(F–I) Striatal BDNF was increased by NSCs regardless of αMT or DHK treatment. TH levels were not significantly altered by NSCs and αMT co-treatment; however, the activated form of TH (ser31) was reduced to ASOV levels by αMT despite NSC transplantation (F, H, and I). Data presented as mean ± SEM. Western blot graphs presented as the percentage of ASOV group. Fisher’s PLSD post hoc ASOC, ASOC-DHKp < 0.02, ∗p < 0.0004 compared with all other groups (n = 6–8 mice per group).
See also Figure S4.
Importantly, BDNF expression was unaltered by either αMT or DHK treatments, remaining elevated in all three NSC-transplanted groups (Figures 4F and 4G; F(3, 20) = 6.3; ANOVA p = 0.004), suggesting that the observed changes in DA and glutamate systems occur downstream of BDNF signaling. In support of this, TH ser31 phosphorylation was decreased similarly in both ASOV and ASOC-αMT mice but remained elevated in saline- or DHK-injected ASOC mice (Figures 4F, 4H, and 4I; F(3, 20) = 5.4; ANOVA p = 0.007). These experiments further confirmed the importance of NSC-induced changes in both DA and glutamate systems and their role in NSC-mediated motor and cognitive improvements. They also suggest that motor dysfunction and NSC-mediated recovery are more closely tied to DAergic systems, whereas cognitive tasks are more influenced by NSC-mediated modulation of glutamatergic systems, and that both of these behaviors are strongly influenced by BDNF.
NSC-Derived BDNF Is Essential for Stem Cell-Induced Cognitive and Motor Benefits
Given the strong correlations between striatal BDNF levels and both motor and cognitive function, we hypothesized that NSC-derived BDNF may be critical for NSC-mediated improvements. We therefore employed a loss-of-function approach to elucidate the role of BDNF in behavioral recovery (Figure 5A). NSCs were stably modified to express a shRNA targeting BDNF, leading to an 84% knockdown of BDNF protein (Figure 5B). Importantly, the resulting cells maintained multipotency as evidenced by unaltered expression of the NSC markers Sox 2 and Musashi (Figure 5B). The effects of transplantation with BDNFshRNA-NSCs (BKCs) or unaltered NSCs (NSCs) were then examined in a new cohort of 12-month-old mice.
Figure 5.
BDNF Is Necessary for NSC-Induced Motor and Cognitive Rescue
(A and B) A new cohort of ASO and WT mice aged to 12 months were injected bilaterally with either NSCs or BDNF shRNA knockdown NSCs (BKCs); behavior was assessed as previously described. NSC BDNF expression was reduced 84% by shRNA in BKCs (p < 0.0001), and multipotency was not affected by BDNF knockdown as measured by Sox2 and Musashi expression (B; n = 6 biological replicates).
(C and D) After four weeks, transplantation of BDNFshRNA -NSCs into ASO mice failed to improve either Rotarod (C) or beam traversal performance (D).
(E and F) Similarly, NSC-derived BDNF was critical for NSC-mediated improvements in cognitive function in NOR and NPR tasks in ASO mice (D–F).
(G–J) As in the ASOV group, striatal BDNF was not increased in ASO-BKC mice (G and H). BDNFshRNA -NSCs were also unable to significantly increase striatal TH ser31 or GLT-1 expression (G, I, and J).
(K) In ASO mice, transplanted NSCs express BDNF (red), whereas BDNF is not detected in transplanted, BDNFshRNA-NSCs. Data presented as mean ± SEM. Western blot graphs presented as % of WTV group. Fisher’s PLSD post hoc WTV, ASOCp < 0.04, WTVp < 0.02, ASOCp < 0.04 (n = 6–8 mice per group).
As in our initial experiments, transplantation of NSCs increased the amount of time that ASO mice could stay on the Rotarod in comparison to vehicle-injected ASO mice. In contrast, transplantation with BKCs failed to improve performance in ASO mice (Figure 5C; F(3,16) = 8.3, 5.6; ANOVA p = 0.006), indicating that NSC-derived BDNF is essential for NSC-mediated motor recovery. Performance on the beam traversal task further confirmed these exciting findings, showing that BKCs could not reduce the number of errors made by ASO mice (Figure 5D; F(3,16) = 6.2; ANOVA p = 0.004). In NOR and NPR cognitive tasks, both WTV and ASOC mice again showed significant preference for the novel object or novel location equivalent to that observed in our initial studies; nevertheless, transplantation with BKCs failed to improve memory for either the familiar object or location (Figures 5E and 5F; F(3,16) = 18.4; ANOVA p = 0.0001). These results clearly demonstrate that NSC-derived BDNF is necessary for both the motor and cognitive benefits of NSC transplantation.
We next performed biochemical analysis confirming that striatal BDNF levels were indeed increased in ASOC mice but not in ASO-BKC mice (Figures 5G and 5H; F(3,20) = 4.6; ANOVA p = 0.01). Likewise, striatal TH ser31 (F(3,20) = 4.4; ANOVA p = 0.02) and GLT-1 (F(3,20) = 1.7; ANOVA p = 0.04) were elevated by transplantation of NSCs in ASO mice, but were unchanged between ASOV and ASO-BKC mice, demonstrating that NSC-derived BDNF plays an important role in regulating DAergic and glutamatergic neurotransmitter systems (Figures 5G, 5I, and 5J). Finally, we confirmed that at 1 month following transplantation NSCs continue to produce BDNF, whereas BKCs produce far lower levels (Figure 5K), providing further evidence that NSC-derived BDNF is necessary to elevate striatal BDNF to behaviorally relevant levels. Together these results strongly implicate the necessity of NSC-derived BDNF for both the motor and cognitive benefits of NSC transplantation and the observed changes in DAergic and glutamatergic systems.
Viral Delivery of BDNF Mimics the Effects of NSCs
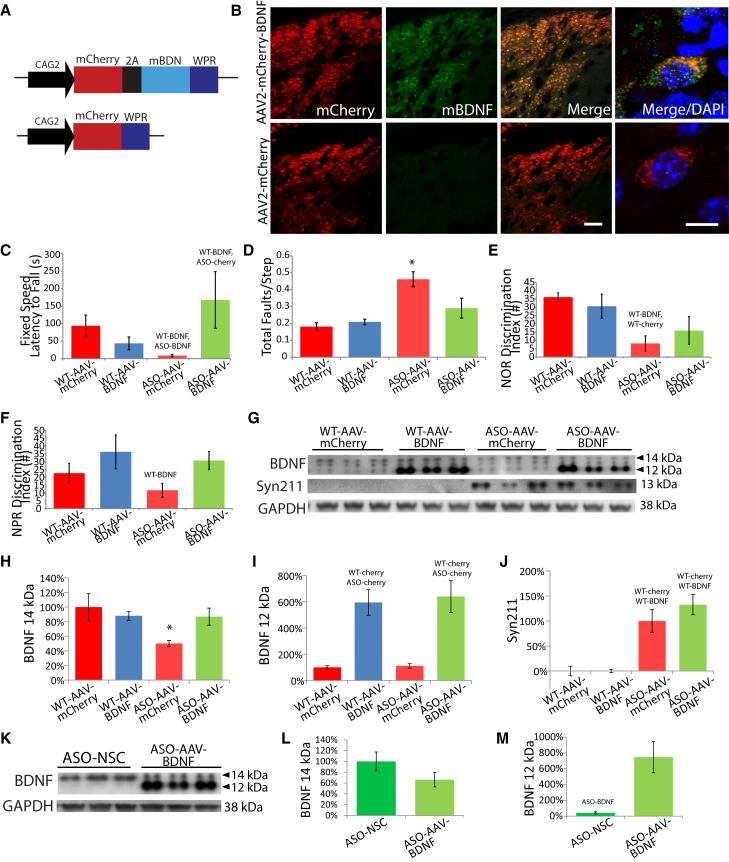
Having shown that NSC-derived BDNF expression is essential for both motor and cognitive behavioral recovery, we next asked whether BDNF alone could provide a similar benefit. Both ASO and WT mice received bilateral striatal injections of either AAV2-mCherry or AAV2-mCherry-BDNF (1 μl of 1.5 × 1013 viral particles/ml; Figures 6A and 6B). Four weeks later, mice were subjected to the same set of behavioral tasks (Figures 6C–6F). Similar to NSC transplantation, AAV2-BDNF transduction improved both Rotarod (Figure 6C; F(3,27) = 7.3, ANOVA p = 0.01) and beam traversal performance (Figure 6D; F(3,27) = 7.8, ANOVA p = 0.009) in ASO-BDNF mice compared with ASO-mCherry mice, but had no effect in WT groups. In the cognitive tasks, although AAV2-BDNF increased preference for novel object and place in ASO-BDNF mice compared with ASO-mCherry mice, overall differences between all four groups via ANOVA were not significant (Figures 6E and 6F; F(3,27) = 1.2, ANOVA p = 0.06). This suggests that AAV-BDNF leads to similar motor behavioral recovery to NSC-induced BDNF; however, cognitive function is not as effectively restored.
Figure 6.
Viral Delivery of BDNF Partially Mimics the Effects of NSCs on Motor and Cognitive Function
(A) A new cohort of ASO and WT mice aged 12 months were bilaterally injected with either AAV2-mCherry-2A-BDNF or AAV2-mCherry.
(B) AAV2-BDNF expression at the injection site (scale bar represents 100 μm) and subcellular localization of BDNF (scale bar represents 10 μm) were verified by immunohistochemistry (B).
(C and D) AAV2-BDNF reversed motor deficits in ASO mice for both Rotarod (C) and beam traversal (D) tasks.
(E and F) In novel object and place recognition tasks, AAV2-BDNF modestly improved performance, although these effects were not significant (E and F).
(G–J) Two bands representing mature BDNF were observed by western blot in AAV2-BDNF-treated mice (G). The 14 kDa band, which was decreased in AAV2-mCherry-treated ASO mice, was significantly increased by AAV2-BDNF (G and H). The protein at 12 kDa was dramatically increased by AAV2-BDNF expression in both WT and ASO mice (G and I). Monomeric h-α-syn (Syn211) was unaltered by AAV2-BDNF expression (G and J).
(K–M) Finally, BDNF expression levels were compared between ASO-AAV-BDNF and ASO-NSC mice (K). AAV2-BDNF resulted in slightly although not significantly lower levels of 14 kDa BDNF compared with NSC transplantation (K and L) but a 500% increase in 12 kDa BDNF relative to NSCs (K and M). Data presented as mean ± SEM. Western blot graphs presented as a percentage of the WTV group (H–J) or ASO-NSC (L and M). Fisher’s PLSD post hoc WT-BDNFp < 0.04, WT-BDNF,ASO-Cherryp < 0.03, WT-BDNF,WT-Cherryp < 0.01, WT-Cherry, ASO-Cherryp < 0.005, WT- Cherry, WT-BDNFp < 0.0004, ASO-BDNFp < 0.005, ∗p < 0.05 compared to all other groups (n = 7–9 mice per group).
To understand why AAV2-BDNF leads to similar motor recovery as NSC-derived BDNF, but a diminished effect on cognition, we examined striatal BDNF expression. BDNF can undergo various forms of post-translational modification, including phosphorylation and ATP-binding, which can in turn increase its biological activity (König et al., 2008, Ferenz et al., 2012). Intriguingly, in addition to the typical mature 14 kDA BDNF isoform observed in mouse brain lysates, we also detected a second 12 kDa BDNF band in AAV2-BDNF groups. As this lower band may represent an alternatively processed or perhaps unphosphorylated form of BDNF, we analyzed both bands individually. As expected, the 14 kDa BDNF band was decreased by 50% in ASO- mCherry mice compared with the WT-mCherry group (Figures 6G and 6H; F(3,27) = 6.8, ANOVA p = 0.02). Furthermore, AAV2-BDNF resulted in a significant 37% increase in 14kDA BDNF levels in ASO-AAV-BDNF mice. In contrast, analysis of the 12 kDa band revealed a different pattern of expression, exhibiting an over 5-fold increase in both AAV2-BDNF groups versus AAV2-mCherry groups (Figures 6G and 6I; F(3,27) = 42.5, ANOVA p < 0.0001). Despite this high elevation of BDNF by AAV transduction, there was no effect on monomeric h-α-syn expression (Figures 6G and 6J). In order to determine why AAV2-BDNF was not able to significantly restore cognitive function despite very high levels of the 12 kDa BDNF, we compared expression levels directly to NSC-transplanted ASO mice whose behavior was fully rescued. Interestingly, we find that in contrast to ASO-BDNF mice, NSC transplantation primarily increases levels of the 14kDa BDNF protein (Figure 6K). Furthermore, ASO-BDNF mice express modestly lower levels of the 14 kDa form and much higher levels of the 12kDa isoform compared with ASO-NSC mice (Figures 6L and 6M; F(3,27) = 12.5, ANOVA p < 0.005).
Taken together, these data suggest that differences in post-translational modification of BDNF provided by NSCs versus AAV likely explain the shift from the typical 14 kDA endogenous BDNF signal to a predominant 12 kDa BDNF band in AAV-BDNF samples. The addition of a single phosphorylation could for example readily shift the electrophoretic mobility of a given protein by the observed 2 kDa. These intriguing results could provide a potential explanation for why AAV-mediated BDNF delivery leads to a less robust effect on cognition, although future studies will clearly be needed to better understand the influence of post-translational modifications on BDNF-induced behavioral effects.
Discussion
Here we demonstrate that transplantation of NSCs can dramatically improve both cognitive and motor function in a progressive transgenic model of DLB/PDD. Interestingly, the benefits occur without altering α-syn levels or inclusion number, instead revealing a critical role for BDNF-induced modulation of DAergic and glutamatergic systems in both the disease process and NSC-mediated recovery. We also show that although viral delivery of BDNF can mimic several outcomes of NSC transplantation, NSCs provide a greater benefit to cognitive function, suggesting that NSCs may be a more effective vehicle for BDNF-dependent recovery. Together, these studies demonstrate that transplantation of preferentially gliogenic NSCs could offer a promising new therapeutic approach to treat both the motor and understudied cognitive components of synucleinopathies.
Currently approved therapies for DLB/PDD primarily focus on strategies to replace or compensate for missing DA via L-DOPA therapy or electrical modulation of DA pathways (Bonelli et al., 2004, McKeith et al., 2005). While these approaches can provide meaningful relief of motor dysfunction, their effect on cognitive symptoms appears to be marginal and inconsistent (Aarts et al., 2014, Robbins and Cools, 2014) and may even accelerate global cognitive decline (Kim et al., 2014). Although DLB can include DA loss in the nigrostriatal pathway, it is modest (Colloby et al., 2012), and DA transporter binding does not correlate to cognitive decline in DLB (Ziebell et al., 2013). This collective evidence strongly argues for an alternative to DA therapy for DLB that is catered toward the specific consequences of synucleinopathy. The importance of α-synuclein in DLB/PDD is highlighted by the strong correlation between α-syn accumulation and cognitive dysfunction in patients (Halliday et al., 2014). Indeed, a recent report confirmed that cerebrospinal fluid levels of α-syn correlates well with cognitive decline, but not with motor dysfunction (Stewart et al., 2014). The testing and development of treatments that could relieve the cognitive symptoms of DLB/PDD therefore likely requires the use of models that exhibit robust α-syn pathology with cognitive deficits, such as the ASO model employed here.
Interestingly, BDNF has also previously been implicated in the development of synucleinopathies. Specifically, haplosufficiency for the TrkB receptor results in nigral cell loss and accumulation of α-syn in aged mice (von Bohlen und Halbach et al., 2005). Changes in α-syn can in turn influence BDNF, as overexpression of WT α-syn in primary neurons reduces BDNF production by suppressing the transactivation of CREB and NFAT, two transcription factors that can regulate BDNF expression (Saha et al., 2000, Yuan et al., 2010). It is likely that introduction of supplemental NSC or AAV2 derived BDNF was able to overcome this deficiency despite the persistence of α-syn expression.
Although the ASO model does not exhibit substantial neuronal loss, the correlations between DA and glutamatergic proteins and behavioral function suggest that ASO mice nicely model the detrimental effects of α-syn accumulation on these transmitter systems. Perhaps more remarkable are the distinct associations of NSC-induced DA changes with motor improvement and glutamatergic changes with cognitive improvement (Figures 3 and 4). In support of our findings, it was recently shown that striatal DA transmission is disrupted in BDNF-deficient mice (Bosse et al., 2012); thus, the reductions in TH and phospho-TH that we observe in ASO mice are likely influenced by the diminished levels of BDNF in these mice. In the ASO model, changes in TH and phospho-TH induced by NSC transplantation likely reflect an enhancement of activity and function of existing nigrostriatal projections that in turn improves behavior (Hyman et al., 1994, Siuciak et al., 1996). Supporting this notion, pharmacological inhibition of TH in the presence of NSC transplants prevented NSC-induced motor and cortical-dependent cognitive benefits (Figure 4). Fascinatingly, NSC-mediated changes in GLT-1 function were necessary for improvement in both cortical- and hippocampal-dependent cognitive tasks, but not motor performance. Although GLT-1 expression is altered by NSC transplantation regardless of α-syn overexpression, this marker may indicate an increase in NSC-derived astrocytes, which are in turn stimulated to produce BDNF in ASO mice (Figure 2D). Deficiency of striatal VGLUT-1 (Granseth et al., 2015) or GLT-1 (Hsu et al., 2015) can both lead to cognitive deficits, further supporting the hypothesis that NSC-induced increases in these transporters improves cognition. Finally, expression and distribution of both VGLUT-1 (Melo et al., 2013) and GLT-1 (Rodriguez-Kern et al., 2003) can be influenced by BDNF, further substantiating an upstream effector role for this neurotrophin in restoring balance to glutamate neurotransmission. The dissociation of the effects of NSCs on DAergic and glutamatergic systems and motor and cognitive function suggests that a balanced regulation of both striatal DA and corticostriatal glutamate could be key to rescuing α-syn-related deficits.
The necessity of BDNF in NSC-mediated behavioral improvement in ASO mice is further confirmed by the inability of BDNFshRNA-NSCs to rescue either motor or cognitive function. In contrast, AAV2-BDNF mimics the effects of NSC transplantation on motor function and partially improves cognitive function. The less striking impact of AAV2-BDNF compared with NSC delivery may be explained by differences in the post-translational modification of BDNF (Mowla et al., 2001, König et al., 2008, Ferenz et al., 2012). Alternatively, it is plausible that additional NSC-derived factors might enhance the efficacy of NSC transplantation (Redmond et al., 2007). In conclusion, our data strongly suggest that striatal NSC transplantation and the resulting elevation of BDNF could provide a promising therapeutic approach to restore dopaminergic and glutamatergic neurotransmission and motor and cognitive function in in DLB/PDD.
Experimental Procedures
Animals, NSC Transplantation, AAV-BDNF Injection, and Behavior
All procedures were performed in strict accordance with the UC Irvine and NIH animal use regulations and were approved by an institutional review board. Hemizygous ASO mice have been previously characterized and are maintained on a purebred C57B/6 background (Masliah et al., 2000). Hippocampal/cortical GFP-NSCs were microdissected from syngeneic GFP-transgenic mice at postnatal day 1, grown as adherent monolayers, and transplanted at passage 15 as previously described (Mizumoto et al., 2003, Blurton-Jones et al., 2009). BDNFshRNA GFP-NSCs were also generated via lentiviral delivery and stable selection of a shRNA construct targeting murine BDNF (Blurton-Jones et al., 2009). Twelve-month-old ASO and WT mice were randomly assigned to a treatment group and either vehicle, or 50,000 NSCs per site (1 μl volume, two sites per side) were transplanted bilaterally into the dorsal striatum (Bregma +0.03 AP, ±2.0 ML, −3.0 and −3.5 DV; Supplemental Experimental Procedures). For AAV experiments, AAV2-mCherry or AAV-mCherry-2A-BDNF (Vector Biolabs) were injected bilaterally at the same striatal coordinates (1 μl of 1.5 × 1013 viral particles/ml). Behavioral studies were performed and analyzed blinded and examined motor function using Rotarod, pole, and beam traversal tests, whereas cortical- and hippocampal-dependent learning and memory were examined using novel object and NPR, respectively (Supplemental Experimental Procedures).
Biochemical, Histological, and Statistical Analyses
At 6 weeks after transplantation, mice were sacrificed by Euthasol and transcardial perfusion (Supplemental Experimental Procedures). For biochemical assessment, tissue was microdissected from flash-frozen half brains; 40-μm sections of post-fixed half brains were processed for immunohistochemistry and imaged via confocal microscopy (Supplemental Experimental Procedures). All animals were randomly assigned to treatment groups with randomly generated identification codes to keep the researcher blind throughout testing and analysis. Comparisons between multiple groups utilized two-way ANOVA followed by Fisher’s PLSD post hoc tests (Supplemental Experimental Procedures). Figure asterisks denote significance difference from all other groups; a significant difference from specific groups is denoted with the name of that group in place of asterisks.
Author Contributions
Conception and design, transplantation studies, pharmacological studies, data collection and analysis, manuscript writing, N.R.S.G.; Protein isolation, data collection and analysis, J.C.; Genotyping, western blots, immunohistochemistry, A.P., S.S., and G.F.; Establishment of ASO colony, J.D.; Donation of ASO founder mice, conception, E.M.; Conception and design, confocal microscopy, generation of BDNFshRNA-NSC line, financial support, and manuscript writing, M.B.J. All authors read and approved the final manuscript.
Acknowledgments
This work was supported by an NSF fellowship (N.R.S.G.) and departmental startup funds AG029378, AG048099, AG16573 (M.B.J.), AG022074, AG18440, and AG10435 (E.M.).
Published: October 15, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental Information includes Supplemental Experimental Procedures and Figures S1–S4 and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2015.09.008.
Supplemental Information
References
- Aarts E., Nusselein A.A., Smittenaar P., Helmich R.C., Bloem B.R., Cools R. Greater striatal responses to medication in Parkinson's disease are associated with better task-switching but worse reward performance. Neuropsychologia. 2014;62:390–397. doi: 10.1016/j.neuropsychologia.2014.05.023. [DOI] [PubMed] [Google Scholar]
- Albin R.L., Young A.B., Penney J.B. The functional anatomy of disorders of the basal ganglia. Trends Neurosci. 1995;18:63–64. [PubMed] [Google Scholar]
- Amschl D., Neddens J., Havas D., Flunkert S., Rabl R., Römer H., Rockenstein E., Masliah E., Windisch M., Hutter-Paier B. Time course and progression of wild type α-synuclein accumulation in a transgenic mouse model. BMC Neurosci. 2013;14:6. doi: 10.1186/1471-2202-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambah-Mukku D., Travaglia A., Chen D.Y., Pollonini G., Alberini C.M. A positive autoregulatory BDNF feedback loop via C/EBPβ mediates hippocampal memory consolidation. J. Neurosci. 2014;34:12547–12559. doi: 10.1523/JNEUROSCI.0324-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blurton-Jones M., Kitazawa M., Martinez-Coria H., Castello N.A., Müller F.J., Loring J.F., Yamasaki T.R., Poon W.W., Green K.N., LaFerla F.M. Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease. Proc. Natl. Acad. Sci. USA. 2009;106:13594–13599. doi: 10.1073/pnas.0901402106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonelli S.B., Ransmayr G., Steffelbauer M., Lukas T., Lampl C., Deibl M. L-dopa responsiveness in dementia with Lewy bodies, Parkinson disease with and without dementia. Neurology. 2004;63:376–378. doi: 10.1212/01.wnl.0000130194.84594.96. [DOI] [PubMed] [Google Scholar]
- Bosse K.E., Maina F.K., Birbeck J.A., France M.M., Roberts J.J., Colombo M.L., Mathews T.A. Aberrant striatal dopamine transmitter dynamics in brain-derived neurotrophic factor-deficient mice. J. Neurochem. 2012;120:385–395. doi: 10.1111/j.1471-4159.2011.07531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canossa M., Griesbeck O., Berninger B., Campana G., Kolbeck R., Thoenen H. Neurotrophin release by neurotrophins: implications for activity-dependent neuronal plasticity. Proc. Natl. Acad. Sci. USA. 1997;94:13279–13286. doi: 10.1073/pnas.94.24.13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloby S.J., McParland S., O’Brien J.T., Attems J. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain. 2012;135:2798–2808. doi: 10.1093/brain/aws211. [DOI] [PubMed] [Google Scholar]
- Desplats P., Lee H.J., Bae E.J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty M.J., Burn D.J. Parkinson’s disease dementia. Curr. Neurol. Neurosci. Rep. 2010;10:292–298. doi: 10.1007/s11910-010-0113-7. [DOI] [PubMed] [Google Scholar]
- Dubois B., Pillon B. Cognitive deficits in Parkinson’s disease. J. Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Ferenz K.B., Gast R.E., Rose K., Finger I.E., Hasche A., Krieglstein J. Nerve growth factor and brain-derived neurotrophic factor but not granulocyte colony-stimulating factor, nimodipine and dizocilpine, require ATP for neuroprotective activity after oxygen-glucose deprivation of primary neurons. Brain Res. 2012;1448:20–26. doi: 10.1016/j.brainres.2012.02.016. [DOI] [PubMed] [Google Scholar]
- Fernagut P.O., Hutson C.B., Fleming S.M., Tetreaut N.A., Salcedo J., Masliah E., Chesselet M.F. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottmann K., Mittmann T., Lessmann V. BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses. Exp. Brain Res. 2009;199:203–234. doi: 10.1007/s00221-009-1994-z. [DOI] [PubMed] [Google Scholar]
- Granseth B., Andersson F.K., Lindström S.H. The initial stage of reversal learning is impaired in mice hemizygous for the vesicular glutamate transporter (VGluT1) Genes Brain Behav. 2015;14:477–485. doi: 10.1111/gbb.12230. [DOI] [PubMed] [Google Scholar]
- Greenamyre J.T. Glutamate-dopamine interactions in the basal ganglia: relationship to Parkinson’s disease. J. Neural Transm. 1993;91:255–269. doi: 10.1007/BF01245235. [DOI] [PubMed] [Google Scholar]
- Halliday G.M., Leverenz J.B., Schneider J.S., Adler C.H. The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov. Disord. 2014;29:634–650. doi: 10.1002/mds.25857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C., Angot E., Bergström A.L., Steiner J.A., Pieri L., Paul G., Outeiro T.F., Melki R., Kallunki P., Fog K. α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J. Clin. Invest. 2011;121:715–725. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera D.G., Garcia-Verdugo J.M., Alvarez-Buylla A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann. Neurol. 1999;46:867–877. doi: 10.1002/1531-8249(199912)46:6<867::aid-ana9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hsu C.Y., Hung C.S., Chang H.M., Liao W.C., Ho S.C., Ho Y.J. Ceftriaxone prevents and reverses behavioral and neuronal deficits in an MPTP-induced animal model of Parkinson’s disease dementia. Neuropharmacology. 2015;91:43–56. doi: 10.1016/j.neuropharm.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Hyman C., Juhasz M., Jackson C., Wright P., Ip N.Y., Lindsay R.M. Overlapping and distinct actions of the neurotrophins BDNF, NT-3, and NT-4/5 on cultured dopaminergic and GABAergic neurons of the ventral mesencephalon. J. Neurosci. 1994;14:335–347. doi: 10.1523/JNEUROSCI.14-01-00335.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.J., Jeon B.S., Paek S.H., Lee K.M., Kim J.Y., Lee J.Y., Kim H.J., Yun J.Y., Kim Y.E., Yang H.J., Ehm G. Long-term cognitive outcome of bilateral subthalamic deep brain stimulation in Parkinson’s disease. J. Neurol. 2014;261:1090–1096. doi: 10.1007/s00415-014-7321-z. [DOI] [PubMed] [Google Scholar]
- König S., Hasche A., Pallast S., Krieglstein J., Klumpp S. Detection of ATP-binding to growth factors. J. Am. Soc. Mass Spectrom. 2008;19:91–95. doi: 10.1016/j.jasms.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Lees A.J., Smith E. Cognitive deficits in the early stages of Parkinson’s disease. Brain. 1983;106:257–270. doi: 10.1093/brain/106.2.257. [DOI] [PubMed] [Google Scholar]
- Leverenz J.B., Watson G.S., Shofer J., Zabetian C.P., Zhang J., Montine T.J. Cerebrospinal fluid biomarkers and cognitive performance in non-demented patients with Parkinson’s disease. Parkinsonism Relat. Disord. 2011;17:61–64. doi: 10.1016/j.parkreldis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh S.E., Blurton-Jones M. Examining the mechanisms that link β-amyloid and α-synuclein pathologies. Alzheimers Res. Ther. 2012;4:11. doi: 10.1186/alzrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- Masliah E., Rockenstein E., Mante M., Crews L., Spencer B., Adame A., Patrick C., Trejo M., Ubhi K., Rohn T.T. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Massie A., Goursaud S., Schallier A., Vermoesen K., Meshul C.K., Hermans E., Michotte Y. Time-dependent changes in GLT-1 functioning in striatum of hemi-Parkinson rats. Neurochem. Int. 2010;57:572–578. doi: 10.1016/j.neuint.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Mayo M.C., Bordelon Y. Dementia with Lewy bodies. Semin. Neurol. 2014;34:182–188. doi: 10.1055/s-0034-1381741. [DOI] [PubMed] [Google Scholar]
- McKeith I.G., Dickson D.W., Lowe J., Emre M., O’Brien J.T., Feldman H., Cummings J., Duda J.E., Lippa C., Perry E.K., Consortium on DLB Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- Melo C.V., Mele M., Curcio M., Comprido D., Silva C.G., Duarte C.B. BDNF regulates the expression and distribution of vesicular glutamate transporters in cultured hippocampal neurons. PLoS ONE. 2013;8:e53793. doi: 10.1371/journal.pone.0053793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto H., Mizumoto K., Shatos M.A., Klassen H., Young M.J. Retinal transplantation of neural progenitor cells derived from the brain of GFP transgenic mice. Vision Res. 2003;43:1699–1708. doi: 10.1016/s0042-6989(03)00235-9. [DOI] [PubMed] [Google Scholar]
- Mowla S.J., Farhadi H.F., Pareek S., Atwal J.K., Morris S.J., Seidah N.G., Murphy R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001;276:12660–12666. doi: 10.1074/jbc.M008104200. [DOI] [PubMed] [Google Scholar]
- Pita-Almenar J.D., Zou S., Colbert C.M., Eskin A. Relationship between increase in astrocytic GLT-1 glutamate transport and late-LTP. Learn. Mem. 2012;19:615–626. doi: 10.1101/lm.023259.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond D.E., Jr., Bjugstad K.B., Teng Y.D., Ourednik V., Ourednik J., Wakeman D.R., Parsons X.H., Gonzalez R., Blanchard B.C., Kim S.U. Behavioral improvement in a primate Parkinson’s model is associated with multiple homeostatic effects of human neural stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:12175–12180. doi: 10.1073/pnas.0704091104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T.W., Cools R. Cognitive deficits in Parkinson’s disease: a cognitive neuroscience perspective. Mov. Disord. 2014;29:597–607. doi: 10.1002/mds.25853. [DOI] [PubMed] [Google Scholar]
- Rockenstein E., Mallory M., Hashimoto M., Song D., Shults C.W., Lang I., Masliah E. Differential neuropathological alterations in transgenic mice expressing alpha-synuclein from the platelet-derived growth factor and Thy-1 promoters. J. Neurosci. Res. 2002;68:568–578. doi: 10.1002/jnr.10231. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Kern A., Gegelashvili M., Schousboe A., Zhang J., Sung L., Gegelashvili G. Beta-amyloid and brain-derived neurotrophic factor, BDNF, up-regulate the expression of glutamate transporter GLT-1/EAAT2 via different signaling pathways utilizing transcription factor NF-kappaB. Neurochem. Int. 2003;43:363–370. doi: 10.1016/s0197-0186(03)00023-8. [DOI] [PubMed] [Google Scholar]
- Saha A.R., Ninkina N.N., Hanger D.P., Anderton B.H., Davies A.M., Buchman V.L. Induction of neuronal death by alpha-synuclein. Eur. J. Neurosci. 2000;12:3073–3077. doi: 10.1046/j.1460-9568.2000.00210.x. [DOI] [PubMed] [Google Scholar]
- Salvatore M.F., Davis R.W., Arnold J.C., Chotibut T. Transient striatal GLT-1 blockade increases EAAC1 expression, glutamate reuptake, and decreases tyrosine hydroxylase phosphorylation at ser(19) Exp. Neurol. 2012;234:428–436. doi: 10.1016/j.expneurol.2012.01.012. [DOI] [PubMed] [Google Scholar]
- Siuciak J.A., Boylan C., Fritsche M., Altar C.A., Lindsay R.M. BDNF increases monoaminergic activity in rat brain following intracerebroventricular or intraparenchymal administration. Brain Res. 1996;710:11–20. doi: 10.1016/0006-8993(95)01289-3. [DOI] [PubMed] [Google Scholar]
- Stewart T., Liu C., Ginghina C., Cain K.C., Auinger P., Cholerton B., Shi M., Zhang J., Parkinson Study Group DATATOP Investigators Cerebrospinal fluid α-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am. J. Pathol. 2014;184:966–975. doi: 10.1016/j.ajpath.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann Jones S.A., O’Brien J.T. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol. Med. 2014;44:673–683. doi: 10.1017/S0033291713000494. [DOI] [PubMed] [Google Scholar]
- von Bohlen und Halbach O., Minichiello L., Unsicker K. Haploinsufficiency for trkB and trkC receptors induces cell loss and accumulation of alpha-synuclein in the substantia nigra. FASEB J. 2005;19:1740–1742. doi: 10.1096/fj.05-3845fje. [DOI] [PubMed] [Google Scholar]
- Yamasaki T.R., Blurton-Jones M., Morrissette D.A., Kitazawa M., Oddo S., LaFerla F.M. Neural stem cells improve memory in an inducible mouse model of neuronal loss. J. Neurosci. 2007;27:11925–11933. doi: 10.1523/JNEUROSCI.1627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Sun J., Zhao M., Hu J., Wang X., Du G., Chen N.H. Overexpression of alpha-synuclein down-regulates BDNF expression. Cell. Mol. Neurobiol. 2010;30:939–946. doi: 10.1007/s10571-010-9523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebell M., Andersen B.B., Pinborg L.H., Knudsen G.M., Stokholm J., Thomsen G., Karlsborg M., Høgh P., Mørk M.L., Hasselbalch S.G. Striatal dopamine transporter binding does not correlate with clinical severity in dementia with Lewy bodies. J. Nucl. Med. 2013;54:1072–1076. doi: 10.2967/jnumed.112.114025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.