Abstract
Background:
Learning, the process of acquiring memory such as behavioral change due to previous experiences and most intensively studied subjects in the field of neuroscience through various approaches to understand the mechanisms. Brahmi Ghrita (BG) was claimed for the treatment of learning and memory disorders in human being.
Aim:
To assess learning and memory activity of BG in normal rats.
Materials and Methods:
For this study, 24 rats were taken and divided into four groups (six in each). First group served as the control group, second and the third group as BG treated groups (400 and 800 mg/kg, p. o.) and the fourth group as standard drug (piracetam) 500 mg/kg, p.o. treated group. Learning and memory, activity of BG was evaluated in normal rats, using elevated maze plus and passive avoidance test.
Results:
BG and piracetam treated rats demonstrated a significant decrease in transfer latency in modified elevated plus maze test and increase in step through latency in passive avoidance test compared with control rats in a dose dependent manner. It may be due to increase in learning and memory. It was also reported that prolonged use of Bacopa monneri (L.) Pennell causes elevation of cerebral glutamic acid and transient increase in gamma aminobutaric acid, which may be helpful in the process of learning.
Conclusion:
Brahmi Ghrita enhances learning and memory as analogous to standard drug (piracetam) in normal rats.
Keywords: Brahmi Ghrita, modified elevated plus maze, passive avoidance
Introduction
Poor memory, lower retention and slow recall are common problems in today's stressful competitive life. Several medicinal plants, designated as Rasayana (rejuvenation) Drugs in Ayurveda, are supposed to have specific influence on brain functions. Brahmi Ghrita (BG) contains Bacopa monneri (L.) Pennell, Acorus calamus L., Convolvulus pluricaulis Choisy, Saussurea lappa DC. and 10-year-old cows clarified butter. It has been used in Ayurveda to treat memory disorders.[1] B. monneri has been used as a well-known medicine for a number of disorders, particularly those involving anxiety, learning and memory disorders.[2] The roots and rhizomes of A. calamus are used in Ayurvedic medicine on a regular basis for the treatment of insomnia, melancholia, neurosis, loss of memory and remittent fevers. C. pluricaulis significantly improve learning and memory.[3] S. lappa has been reported to possess anti-inflammatory activity.[4] Clarified butter is described in Ayurveda as a memory enhancer, anticonvulsant and anti-inflammatory agent.[5] In spite of considerable literature available on herbal components of BG, there is no known data regarding the pharmacological evaluation of this formulation for the nootropic effect. The present study is focused on the evaluation of the nootropic activity of BG on rats.
Materials and Methods
Procurement and identification of raw material
Fresh Brahmi plants were collected from Ramanagar, Varanasi Dist. (U.P). Vacha, Kushtha, Shankhapushpi collected from local market of Varanasi and Go-Ghrita was procured from Jalan Shop (A Local Market of Varanasi). These raw drugs were identified by the expert of Department of Dravyaguna, IMS, BHU, Varanasi.
Preparation of BG
BG was prepared as described in one of our earlier studies.[6] Briefly, it was prepared by adding paste of B. monneri (40% w/w), A. calamus (20% w/w), C. pluricaulis (20% w/w) and S. lappa (20% w/w) in freshly prepared 3 liter juice of B. monneri in stainless steel vessel having 750 ml clarified butter. Above mixture was heated for 9 h and filtered after acquiring completion test. In this way, BG was prepared.
Animals
Charles Foster rats of either sex weighing between 160 and 180 g were used for experimental study. The animals were obtained from the Central Animal House, Institute of Medical Sciences, Banaras Hindu University, Varanasi. Principles of laboratory animal care (NIH publication number #85-23, revised in 1985) guidelines were always followed and prior approval of Institutional Animal Ethical Committee (No. Dean/10-11/150) of Banaras Hindu University was obtained before commencing experiments.
Grouping and posology
Total 24 rats were divided in 4 groups. 1st group served as a normal control group, 2nd and 3rd group served as test drug group in which BG administered orally at the dose 400 and 800 mg/kg respectively. 4th group served as standard control group and administered Piracetam 500 mg/kg. Test drug and standard drugs were administered once a day for seven consecutive days through oral route. Vehicle was not used in any group of animals, only food and water was given to all group animals.
Learning and memory activity
Following validated animal models were used for evaluation of learning and memory activity of BG in rats and the experiment was conducted according to the procedure standardized in our laboratories.[3]
Modified elevated plus maze test
This test was used to assess the retention of learning and memory.[7] The plus maze consisted of two opposite open arms, 50 cm × 10 cm, crossed with two enclosed arms, of the same dimensions with walls 40 cm high. The arms were connected with a central square (10 cm × 10 cm) to give the apparatus a plus sign appearance. The maze was kept in a dimly light room elevated 50 cm above floor level. On day one, a rat was placed on the far end of one of the open arms, facing away from the center and the time taken by the animal to enter one of the closed arms (transfer latency on day 1) was recorded with the help of a stopwatch. The rat was left in the enclosed arm for 10-15 s and returned to its home cage. On day 2, same procedure was repeated and similarly after an interval of 1 week, on day 9, the transfer latency was again recorded.
Passive avoidance test
This test was widely used in testing drug effects on memory[8] and also used for normal behavior of rats and was developed by Kings and Glasser.[9] The step through passive avoidance behavior will be evaluated by using the light-dark apparatus, which has two walls of wood and the remaining two walls of transparent plexiglass. It was divided into two equal compartments (30 cm × 25 cm × 30 cm) by a plexiglass with a 10 cm × 10 cm. Opening in the center. A guillotine door between the two compartments controlled the opening. The light compartment was painted white and a 15 W lamp illuminated it. The interior of the dark chamber was painted black and had a ceiling. Each compartment had a copper grid floor. To ensure electrical separation, there was a 1.5-cm gap between the two floors in the light-dark box, at the opening between the two chambers. On day 1, a rat will be placed in the white box and the time taken to enter into the dark box was noted. As soon as the rat entered the dark box, the guillotine door was closed and foot electric shock (0.5 mA, 3 s) was delivered. The rat was then replaced to its home cage. On the following day (24 h retention interval) each rat was again placed in the white box and given a 5-min inhibition period. Latency to step through to the dark chamber was recorded. Electric shock was not delivered on day 2. To animals remaining in the white box for a 5-min test period, the maximum score of 300 s was assigned. On day 9 (after a gap of 1 week), latency to step through was again recorded to test the retention of the passive avoidance learning.
Step-through latency to dark chamber during the learning phase is taken as measurement of learning and increase of step-through latency is defined as learning.[10]
Active avoidance test
This test was fundamental learning behavior phenomenon.[11] This is an instrumental conditioning paradigm; the animal learns to control the administration of the Unconditioned Stimulus (UCS) by appropriate reaction to the Conditioned Stimulus (CS) preceding the noxious stimulus. Active avoidance learning acquisition and its retention was tested by the method of Spignoli et al.[12] The apparatus used, was the conventional shuttle avoidance box (Techno, India), which consisted of two grid-floor compartments (29 × cm 29 × 25 cm each) separated by a plexiglass transparent partition with a single opening (14 cm × 17 cm) and buzzer.
The rats were placed individually on the right compartment of a shuttle box and allowed to adapt for 15 s. Thereafter, the rats were exposed to a 15 s acoustic buzzer stimulus (CS) followed by both the acoustic stimulus and electric shock (UCS; 1.5 mA, 50 Hz) through the grid floor of the right for 30 s. Jumping to the un-electrified adjacent (safe) left compartment during CS was designated as conditioned response (CR1) while jumping to the safe left chamber during the initial 15 s adaptation period was designated as anticipatory CR2. The number of trials required by the animal to reach the criterion of two consecutive correct responses represents the learning rate. A 60-min inter-trial interval period was maintained. For statistical analysis, rats not reaching criterion within eight trials was arbitrarily assigned a score of 9. All the rats were subjected to this training schedule and were retested 24 h later and at day 9 (after a gap of 1 week) for retention of the learned task. Besides CR1, CR2 and trial scores, total time taken and the total number of shocks received to reach criterion was also recorded.
Statistical analysis
The data, expressed as mean ± standard deviation, were subjected to Kruskal-Wallis one-way analysis of variance (ANOVA). Inter group comparisons were made by Mann-Whitney U-test (two-tailed) for only those responses, which yielded significant treatment effects in the ANOVA test. P < 0.05 was considered to be statistically significant.
Results
Transfer latency on elevated plus maze
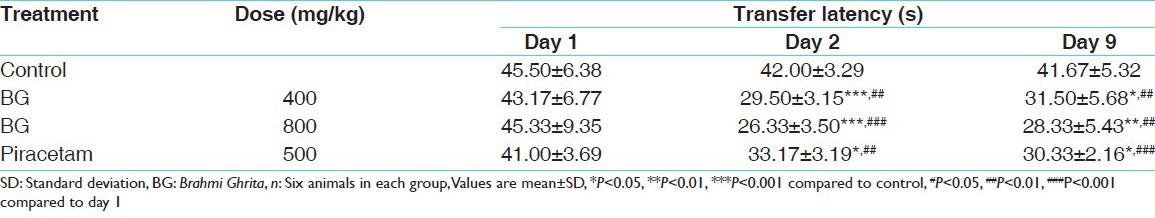
Transfer latency of the control group animal was 45, 42 and 41 s. respectively on day 1, 2 and 9, on elevated plus maze. It was decreased in animals treated with BG 400, BG 800 and piracetam 500 by 30%, 38% and 21% after 24 h and 24%, 31%, 26% respectively at day 9, compared to day 9 of the control group. BG 400 treated rats showed decreased transfer latency 31%, 27% on day 2 and day 9 respectively compared with day 1 of the same group. BG 800 treated rats showed decreased transfer latency 44%, 37% on day 2 and day 9 respectively compared with day 1 of the same group. Piracetam treated rats showed decreased transfer latency 19%, 26% on day 2 and day 9 respectively compared with day 1 of the same group [Table 1].
Table 1.
Effect of BG on transfer latency in elevated plus maze test
Passive avoidance test
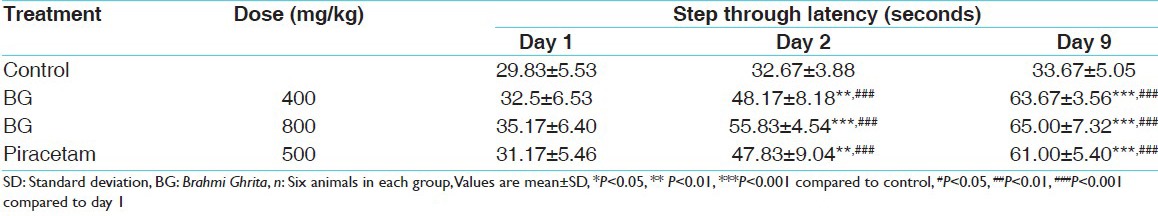
Step through latency on passive avoidance test is increased in animal treated with BG 400, BG 800 and piracetam 500 as 50%, 71%, 46% after 24 h and 90%, 94%, 84% respectively at day 9. BG 400 treated rats showed increased step through latency 53%, 97% on day 2 and day 9 respectively compared with day 1 of the same group. BG 800 treated rats showed increased step through latency 59%, 85% on day 2 and day 9 respectively compared with day 1 of the same group. Piracetam treated rats showed increased step through latency 19%, 26% on day 2 and day 9 respectively compared with day 1 of the same group [Table 2].
Table 2.
Effect of BG on step through latency in passive avoidance test
Active avoidance test
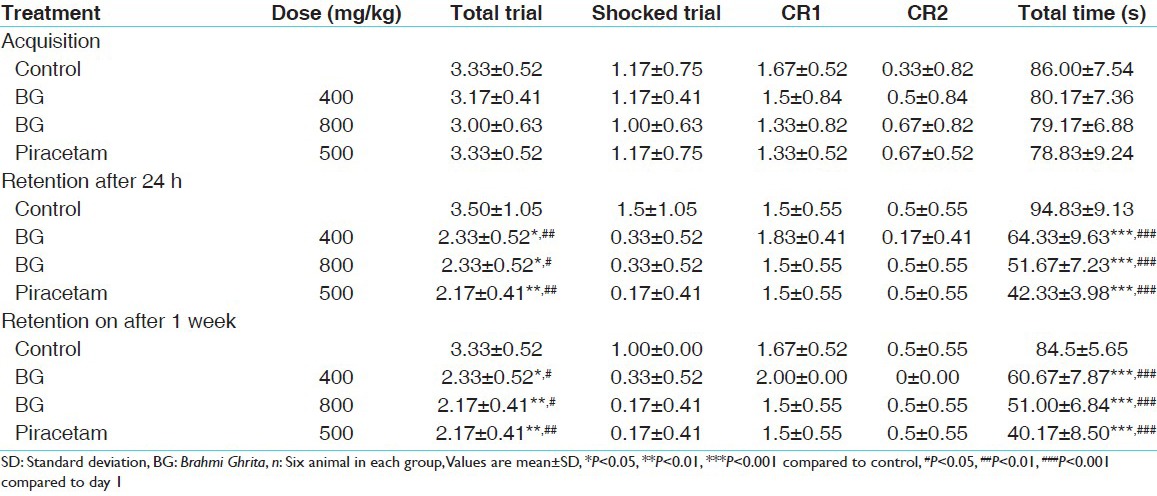
In active avoidance model, rats treated with BG took significant less number of total trials, shock trial and total time for jumping into safe compartment in 2nd and 9th day when compared with control. Total time taken by BG 400, BG 800 and piracetam was decreased as 31%, 45%, 55% after 24 h and 28%, 39%, 52% respectively at day 9 compared with the control. BG 400 treated rats showed decreased total time 19%, 24% on day 2 and day 9 respectively compared with day 1 of the same group. BG 800 treated rats showed decreased total time 34%, 35% on day 2 and day 9 respectively compared with day 1 of the same group. Piracetam treated rats showed decreased total time 46%, 49% on day 2 and day 9 respectively compared with day 1 of the same group [Table 3].
Table 3.
Effect of BG in active avoidance test
Discussion
In BG, Brahmi has maximum percentage so that properties shown by BG were mainly dependent on Brahmi. Transfer latency on modified elevated plus maze might be shortening if animal has previous experience of entering the open arm and shortened transfer latency could be related to memory. In modified elevated plus maze test transfer latency of BG 400, BG 800 and piracetam 500 treated rats was significantly reduced when compared to control group on day 2 and 9, as well as to the same group on day 1. It may be due to increase in learning and memory because shortening of transfer latency is related to increased memory. In passive avoidance test, Rats of the control group took less time for jumping in to dark chamber (where electric shock was provided on day 1) as compared to rats treated with BG 400 and BG 800 and piracetam 500. Rats treated with both dose of BG and piracetam spent more time in illuminated chamber than the control group. This showed that rats of the control group have less memorizing capacity i.e. they forget electric shock, which was given in dark chamber just after jumping in to dark chamber and jumped in to dark chamber earlier than both dose of BG and piracetam 500 treated rats. This showed that step through latency of BG 400, BG 800 and piracetam 500 treated rats was significantly increased when compared with control group on day 2 and 9 as well as to the same group on day 1. Increase in step through latency may be indicative of increase in memory. Thus BG and piracetam increases learning and memory. BG and piracetam treated rats took significantly fewer trials, shocked trials and total time to re-learn the task as compared to control group. In this test, most of the rats treated with BG jumped into safe compartment before providing electric shock, but most of the rats of the control group jumped into safe compartment after exposure to electric shock. Thus total shock trial in BG and piracetam treated rat was less as compared to control group. In the same way total time and total trial required by BG and piracetam treated rats was less as compared to rats of the control group. In active avoidance model, we observed that of BG and piracetam treated rats required less trial and time to reach the criterion of conditioned avoidance learning, when tested after 24 h and 1 week. This indicates that the rats treated with BG and piracetam memorize the training process, which was provided on day 1. Thus, we can say that BG is helping in learning and memory. BG contained B. monneri, C. pluricaulis, A. calamus, S. lappa and Puran Ghrita. Out of these B. monneri, might have reversed the depletion of acetylcholine, it is reported to reduce the choline acetylase activity and decrease muscarinic, cholinergic receptor binding in the frontal cortex and hippocampus, induced by neurotoxins, like colchicine.[13] It has been suggested to alleviate cholinergic degeneration,[14] lower norepinephrine and increase 5-hydroxytryptamine levels in the hippocampus, hypothalamus and cerebral cortex and also reported to possess antioxidant activity. Antioxidants present in it decreased nitrite, nitrate and lipid per oxidation and significantly improved catalase activity.[15] In this way, Brahmi increases the acetyl choline concentration,[16] which may be related to memory. C. pluricaulis is recommended as a brain tonic to promote intellect and memory, eliminate nervous disorders and to treat hypertension.[17] Alcoholic extract of C. pluricaulis showed enhanced Neuro-Peptide Y (NPY) synthesis in brain and induced increased brain protein content and increased acquisition.[18] The exact mechanisms by which activity of NPY receptors is able to affect memory are unknown. However, there is strong evidence that excitatory amino acids, particularly glutamate, play a significant role in normal hippocampal functioning associated with memory and NPY has been established as a potent presynaptic receptor inhibitor of excitatory synaptic transmission in the rat hippo campus, probably by, inhibition of glutamate release.[19] Rhizomes of A. calamus are used as brain tonic in a weak memory psychoneurosis and epilepsy. Methanolic extracts of the A. calamus roots, contain essential oil β-asarone, which showed inhibitory effect on acetylcholinesterase.[20] Old clarified butter is especially good for healing the mind. It is also considered as auspicious and is given in mental disorders with no clear physical cause, along with Sanskrit mantras. The effectiveness of such clarified butter formulations in mental disorders shown to be effective.[21]
Piracetam is well-known nootropic agent. The mechanisms through which piracetam can improve cognitive performance are not clearly delineated. It affects both glutaminergic[22] and cholinergic[23] neurotransmission and actions on both of these systems have been proposed for piracetam's behavioral effects. BG and piracetam both improved learning and memory in all experimental model but there was some difference found in their effect, i.e. BG has less effect than piracetam on learning and memory, but these difference were not statistically significant in all experimental model. From the above, we can say that BG and piracetam influence learning and memory by the same mechanism. The effect of BG on learning and memory may also be due to synergetic action of B. monneri, C. pluricaulis, A. calamus.
Conclusion
BG has beneficial effect on learning and memory in the dose of 400 and 800 mg/kg body weight of normal rats in modified elevated plus maze, passive avoidance and active avoidance test. There was an apparent difference in learning and memory capacity between Brahmi Ghrita and piracetam, but these differences was not statistically significant. Hence, Brahmi Ghrita can be used as memory enhancer.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Vaidya Jadavaji Trikamji Acharya., editor. Varanasi: Chaukhambha Orientalia; 2011. Agnivesha, Charaka, Dridhbala. Charaka Samhita, Chikitsa Sthana, Apasmara Chikitsa Adhyay, 10/25; p. 475. Reprint ed. [Google Scholar]
- 2.Singh HK, Dhawan BN. Neuropsycopharmacological effects of the Ayurvedic nootropic Bacopa monneri Linn.(Brahmi) Indian J Pharmacol. 1997;29:359–65. [Google Scholar]
- 3.Kumar V, Singh PN, Muruganandam AV, Bhattacharya SK. Effect of Indian Hypericum perforatum Linn on animal models of cognitive dysfunction. J Ethnopharmacol. 2000;72:119–28. doi: 10.1016/s0378-8741(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 4.Cho JY, Baik KU, Jung JH, Park MH. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398:399–407. doi: 10.1016/s0014-2999(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 5.Fulzele SV, Joshi SB, Dorle AK. Thesis. Nagpur: Nagpur University; 2002. Studies on formulation rational and quality assessment of some indigenous medicinal preparations. Ph. D. [Google Scholar]
- 6.Yadav KD, Reddy KR. Standardization of Brahmi Ghrita with special reference to its pharmaceutical study. Int J Ayurvedic Med. 2012;3:16–21. [Google Scholar]
- 7.Itoh J, Nabeshima T, Kameyama T. Utility of an elevated plus-maze for the evaluation of memory in mice: Effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology (Berl) 1990;101:27–33. doi: 10.1007/BF02253713. [DOI] [PubMed] [Google Scholar]
- 8.Hock FJ. Involvement of nitric oxide-formation in the action of losartan (DuP 753): Effects in an inhibitory avoidance model. Behav Brain Res. 1994;61:163–7. doi: 10.1016/0166-4328(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 9.Kings RA, Glasser RL. Duration of electroconvulsive shock-induced retrograde amnesia in rats. Physiol Behav. 1970;5:335–9. doi: 10.1016/0031-9384(70)90107-1. [DOI] [PubMed] [Google Scholar]
- 10.Vogel GH, Vogel WH. Vol. 413. Berlin: Springer Verlog; 1997. Drug Discovery and Evolution: Pharmacological Assays; pp. 292–16. [Google Scholar]
- 11.D Amato MR. New York: McGraw Hill; 1970. Experimental Psychology, Methodology, Psychophysics and Learning; pp. 381–416. [Google Scholar]
- 12.Spignoli G, Pepeu G. Oxiracetam prevents electroshock-induced decrease in brain acetylcholine and amnesia. Eur J Pharmacol. 1986;126:253–7. doi: 10.1016/0014-2999(86)90055-5. [DOI] [PubMed] [Google Scholar]
- 13.Russo A, Borrelli F. Bacopa monniera, a reputed nootropic plant: An overview. Phytomedicine. 2005;12:305–17. doi: 10.1016/j.phymed.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Sara SJ. Noradrenergic-cholinergic interaction: Its possible role in memory dysfunction associated with senile dementia. Arch Gerontol Geriatr Suppl. 1989;1:99–108. [PubMed] [Google Scholar]
- 15.Saraf MK, Prabhakar S, Anand A. Neuroprotective effect of Bacopa monniera on ischemia induced brain injury. Pharmacol Biochem Behav. 2010;97:192–7. doi: 10.1016/j.pbb.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 16.Pathak SR. Thesis. Varanasi: Banaras Hindu University; 1990. A neurohumoral study on brain function in stress anti-stress drug with special reference to the effect of some plant product. Ph. D. [Google Scholar]
- 17.Manyam BV. Dementia in Ayurveda. J Altern Complement Med. 1999;5:81–8. doi: 10.1089/acm.1999.5.81. [DOI] [PubMed] [Google Scholar]
- 18.Khare CP. Heidelberg: Springer-Verlag; 2007. Indian Medicinal Plants an Illustrated Dictionary. [Google Scholar]
- 19.Colmers WF. Modulation of synaptic transmission in hippocampus by neuropeptide Y: Presynaptic actions. Ann N Y Acad Sci. 1990;611:206–18. doi: 10.1111/j.1749-6632.1990.tb48932.x. [DOI] [PubMed] [Google Scholar]
- 20.Oh MH, Houghton PJ, Whang WK, Cho JH. Screening of Korean herbal medicines used to improve cognitive function for anti-cholinesterase activity. Phytomedicine. 2004;11:544–8. doi: 10.1016/j.phymed.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 21.Chandre R, Narasimha Murthy VSSS, Singh RH. Evaluation of the efficacy of kushmandaghrta in the management of depressive illness. Aryavaidyan. 2004;18:87–90. [Google Scholar]
- 22.Isaacson JS, Nicoll RA. Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus. Proc Natl Acad Sci U S A. 1991;88:10936–40. doi: 10.1073/pnas.88.23.10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wurtman RJ, Magil SG, Reinstein DK. Piracetam diminishes hippocampal acetylcholine levels in rats. Life Sci. 1981;28:1091–3. doi: 10.1016/0024-3205(81)90685-8. [DOI] [PubMed] [Google Scholar]


