Abstract
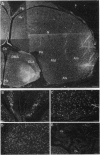
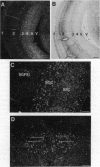
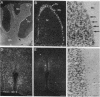
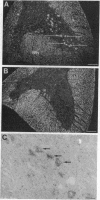
The infectious agent (prion) responsible for transmissible spongiform encephalopathies in humans and animals is composed primarily of a 33- to 35-kDa glycoprotein called PrPSc (scrapie isoform of prion protein), which is a posttranslationally modified form of the normal cell-surface protein PrPC. Little is known about the function of PrPC. Interestingly, chPrP, the chicken homologue of PrPC, copurifies with a factor from brain that stimulates synthesis of acetylcholine receptors on skeletal muscle cells. Using in situ hybridization, we report here that chPrP mRNA is widely distributed in cholinergic and noncholinergic neurons throughout the adult central nervous system, including those in the telencephalic striata, thalamus and hypothalamus, optic tectum, medulla, cerebellum, and spinal cord. The mRNA is present in the brain and spinal cord as early as embryonic day 6 and is also found in dorsal root ganglia, retina, intestine, and heart. Our data suggest that if chPrP serves to regulate acetylcholine receptor number on postsynaptic targets, this is not its only function. It is likely that the protein plays a more widespread role in the central nervous system and perhaps elsewhere, possibly one related to intercellular communication, adhesion, or recognition. The chicken embryo represents an attractive experimental system in which to investigate the normal developmental function of PrPC.
Full text
PDF
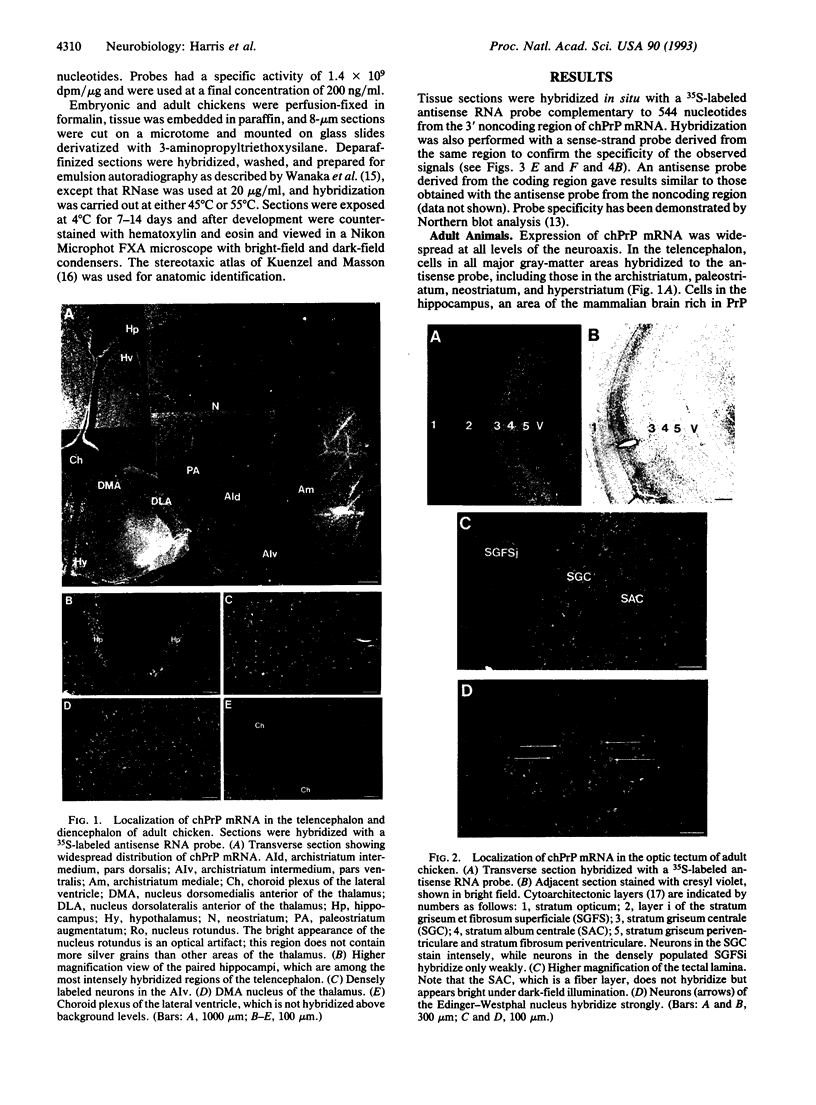
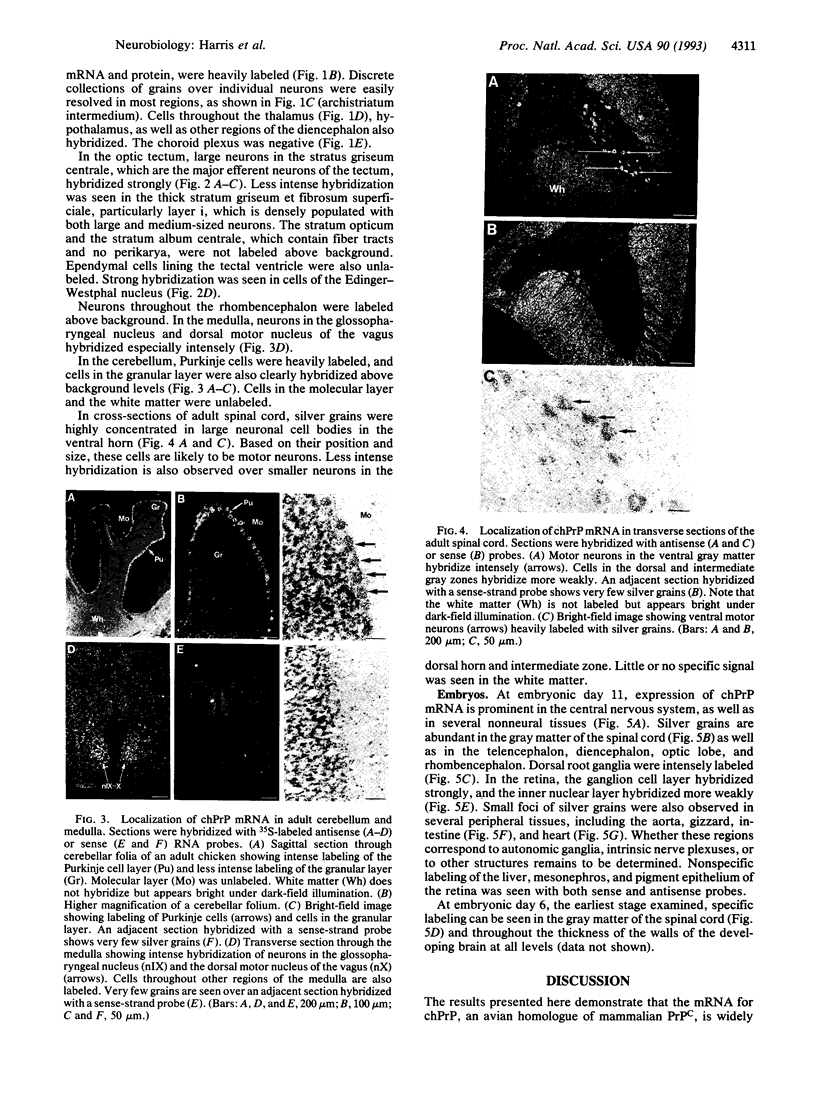
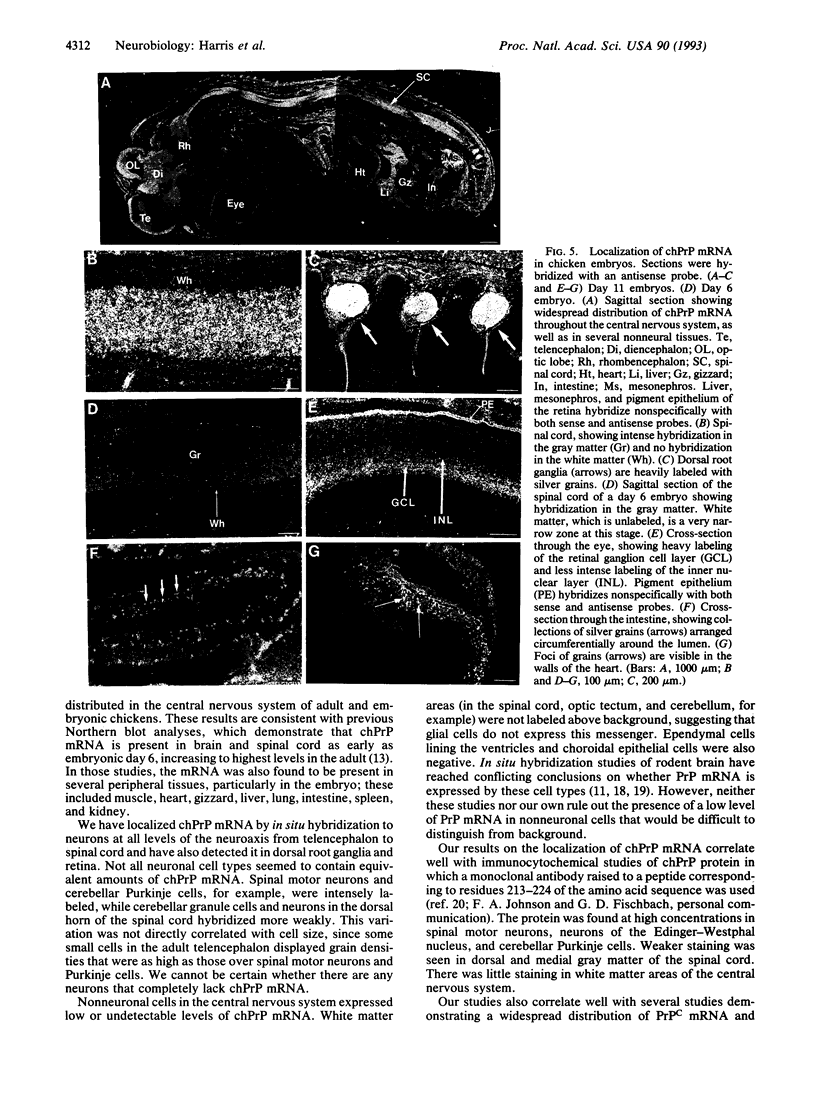

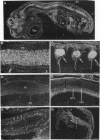
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bendheim P. E., Brown H. R., Rudelli R. D., Scala L. J., Goller N. L., Wen G. Y., Kascsak R. J., Cashman N. R., Bolton D. C. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology. 1992 Jan;42(1):149–156. doi: 10.1212/wnl.42.1.149. [DOI] [PubMed] [Google Scholar]
- Borchelt D. R., Scott M., Taraboulos A., Stahl N., Prusiner S. B. Scrapie and cellular prion proteins differ in their kinetics of synthesis and topology in cultured cells. J Cell Biol. 1990 Mar;110(3):743–752. doi: 10.1083/jcb.110.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown H. R., Goller N. L., Rudelli R. D., Merz G. S., Wolfe G. C., Wisniewski H. M., Robakis N. K. The mRNA encoding the scrapie agent protein is present in a variety of non-neuronal cells. Acta Neuropathol. 1990;80(1):1–6. doi: 10.1007/BF00294214. [DOI] [PubMed] [Google Scholar]
- Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H. P., DeArmond S. J., Prusiner S. B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992 Apr 16;356(6370):577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- Cashman N. R., Loertscher R., Nalbantoglu J., Shaw I., Kascsak R. J., Bolton D. C., Bendheim P. E. Cellular isoform of the scrapie agent protein participates in lymphocyte activation. Cell. 1990 Apr 6;61(1):185–192. doi: 10.1016/0092-8674(90)90225-4. [DOI] [PubMed] [Google Scholar]
- DeArmond S. J., Mobley W. C., DeMott D. L., Barry R. A., Beckstead J. H., Prusiner S. B. Changes in the localization of brain prion proteins during scrapie infection. Neurology. 1987 Aug;37(8):1271–1280. doi: 10.1212/wnl.37.8.1271. [DOI] [PubMed] [Google Scholar]
- Falls D. L., Rosen K. M., Corfas G., Lane W. S., Fischbach G. D. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the neu ligand family. Cell. 1993 Mar 12;72(5):801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Gabriel J. M., Oesch B., Kretzschmar H., Scott M., Prusiner S. B. Molecular cloning of a candidate chicken prion protein. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9097–9101. doi: 10.1073/pnas.89.19.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A., Falls D. L., Johnson F. A., Fischbach G. D. A prion-like protein from chicken brain copurifies with an acetylcholine receptor-inducing activity. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7664–7668. doi: 10.1073/pnas.88.17.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris D. A., Huber M. T., van Dijken P., Shyng S. L., Chait B. T., Wang R. Processing of a cellular prion protein: identification of N- and C-terminal cleavage sites. Biochemistry. 1993 Feb 2;32(4):1009–1016. doi: 10.1021/bi00055a003. [DOI] [PubMed] [Google Scholar]
- Kretzschmar H. A., Prusiner S. B., Stowring L. E., DeArmond S. J. Scrapie prion proteins are synthesized in neurons. Am J Pathol. 1986 Jan;122(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. I. Normal morphology and cytoarchitectonic development. Brain Res. 1971 May 21;28(3):391–419. doi: 10.1016/0006-8993(71)90053-9. [DOI] [PubMed] [Google Scholar]
- Lazarini F., Deslys J. P., Dormont D. Regulation of the glial fibrillary acidic protein, beta actin and prion protein mRNAs during brain development in mouse. Brain Res Mol Brain Res. 1991 Jul;10(4):343–346. doi: 10.1016/0169-328x(91)90093-d. [DOI] [PubMed] [Google Scholar]
- Lieberburg I. Developmental expression and regional distribution of the scrapie-associated protein mRNA in the rat central nervous system. Brain Res. 1987 Aug 11;417(2):363–366. doi: 10.1016/0006-8993(87)90465-3. [DOI] [PubMed] [Google Scholar]
- Manson J., West J. D., Thomson V., McBride P., Kaufman M. H., Hope J. The prion protein gene: a role in mouse embryogenesis? Development. 1992 May;115(1):117–122. doi: 10.1242/dev.115.1.117. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Hay B., Lingappa V. R., Lieberburg I., Prusiner S. B. Developmental expression of prion protein gene in brain. Dev Biol. 1987 May;121(1):105–110. doi: 10.1016/0012-1606(87)90143-6. [DOI] [PubMed] [Google Scholar]
- Mobley W. C., Neve R. L., Prusiner S. B., McKinley M. P. Nerve growth factor increases mRNA levels for the prion protein and the beta-amyloid protein precursor in developing hamster brain. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9811–9815. doi: 10.1073/pnas.85.24.9811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Scrapie prions. Annu Rev Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- Sorenson E. M., Parkinson D., Dahl J. L., Chiappinelli V. A. Immunohistochemical localization of choline acetyltransferase in the chicken mesencephalon. J Comp Neurol. 1989 Mar 22;281(4):641–657. doi: 10.1002/cne.902810412. [DOI] [PubMed] [Google Scholar]
- Wanaka A., Johnson E. M., Jr, Milbrandt J. Localization of FGF receptor mRNA in the adult rat central nervous system by in situ hybridization. Neuron. 1990 Sep;5(3):267–281. doi: 10.1016/0896-6273(90)90164-b. [DOI] [PubMed] [Google Scholar]