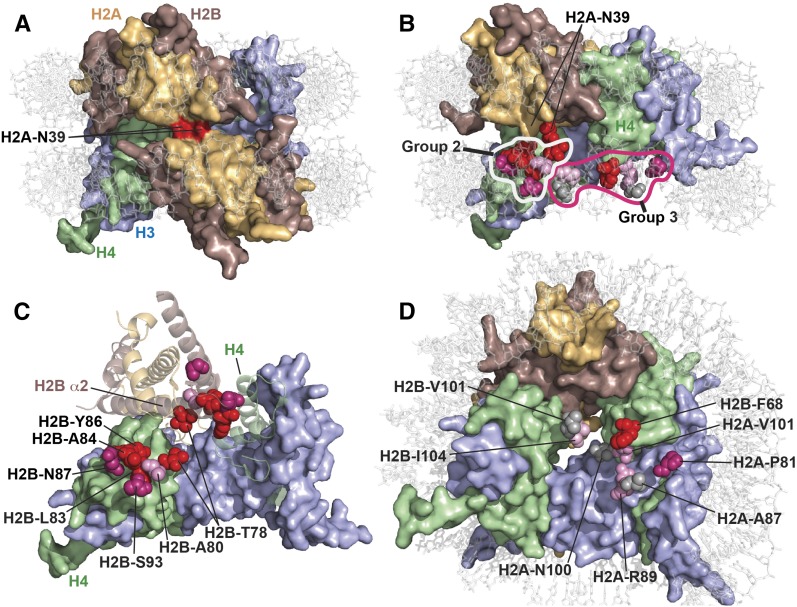
Figure 2.
Physical environments of the suppressor mutations reveal spatial domains of nucleosomes important for Spt6 function. Residues mutated in spt6-F249K suppressors were mapped to the yeast nucleosome structure (PDB 1ID3) (White et al. 2001) using PyMol (Schrodinger). (A) Top view of a nucleosome with both H2A-N39 residues in surface mode colored red. (B) As in A, except rotated to the left, with one H2A-H2B dimer removed and the H2A-N39 residue in the remaining dimer colored the same as the rest of the protein. The residues affected by suppressor mutations are shown as spheres for the missing copy of the dimer only, to allow them to be seen because they are otherwise buried within the structure. The first group of suppressors is comprised entirely of H2A-N39K, and the second and third groups are described in the text and here. Suppressors were scored for their effects on growth rate, temperature sensitivity, HUs, and Spt− phenotype in a spt6-F249K strain (see Figure 1), and the affected residues are color-coded here according to the aggregate score with red > purple > pink > gray. (C) A top view of the nucleosome is shown with the DNA removed to allow better visibility and the core proteins rotated slightly back into the plane of view. Both H3 molecules are shown in surface representation, one H4 is also a surface, while the other H4 molecule and one H2A-H2B dimer are rendered as ribbons, and the other H2A-H2B dimer is removed. The residues in Group 2 are identified for both H2A-H2B dimers and coded as in B. The residues in the front dimer are labeled and are shown nestling into the H4 surface. The residues in the back dimer are shown on the ribbon representation to highlight their clustering along and at the end of H2B helix α2. H2A-N39 is removed for clarity; it lies above and between the H2B-T78 residues. (D) A nucleosomal face is shown with Group 3 residues identified and colored as earlier. This group represents several environments and contains mostly weaker suppressors. The exception, H2B-F68, is strong and makes direct contact with the H4 surface.