Abstract
Two types of RNA:DNA associations can lead to genome instability: the formation of R-loops during transcription and the incorporation of ribonucleotide monophosphates (rNMPs) into DNA during replication. Both ribonuclease (RNase) H1 and RNase H2 degrade the RNA component of R-loops, whereas only RNase H2 can remove one or a few rNMPs from DNA. We performed high-resolution mapping of mitotic recombination events throughout the yeast genome in diploid strains of Saccharomyces cerevisiae lacking RNase H1 (rnh1Δ), RNase H2 (rnh201Δ), or both RNase H1 and RNase H2 (rnh1Δ rnh201Δ). We found little effect on recombination in the rnh1Δ strain, but elevated recombination in both the rnh201Δ and the double-mutant strains; levels of recombination in the double mutant were ∼50% higher than in the rnh201 single-mutant strain. An rnh201Δ mutant that additionally contained a mutation that reduces rNMP incorporation by DNA polymerase ε (pol2-M644L) had a level of instability similar to that observed in the presence of wild-type Pol ε. This result suggests that the elevated recombination observed in the absence of only RNase H2 is primarily a consequence of R-loops rather than misincorporated rNMPs.
Keywords: RNase H1, RNase H2, loss of heterozygosity, mitotic recombination, microarrays
RNA transcripts can stably associate with the DNA template during transcription, giving rise to R-loop structures containing an RNA:DNA hybrid and an unpaired DNA strand. Such R-loops can stall DNA replication forks and are a major source of recombination events that are stimulated by high levels of transcription (reviewed by Aguilera and Garcia-Muse 2012). Transcription-associated recombination (TAR) is generally strongest when the transcription machinery and a replication fork approach each other head on (Prado and Aguilera 2005), but there is also an orientation-independent component to such conflicts (Azvolinsky et al. 2009). Importantly, TAR is highly elevated when RNA processing is perturbed and R-loops accumulate, or when R-loop removal mechanisms are disabled. The RNA component of R-loops can be removed by the Sen1 RNA–DNA helicase (Mischo et al. 2011) or degraded by ribonuclease (RNase) H1 or RNase H2 (reviewed in Cerritelli and Crouch 2009), which are generally considered to be functionally redundant.
R-loops in yeast have been quantified genome-wide using immunoprecipitation with an antibody specific to DNA:RNA hybrids, followed by hybridization to a microarray or by DNA sequencing. This type of analysis has been done in wild type (Chan et al. 2014; El Hage et al. 2014), mRNA processing defective (Chan et al. 2014), RNase H-defective (Chan et al. 2014; El Hage et al. 2014), and RNA–DNA helicase (Sen1)-defective strains (Chan et al. 2014). In wild-type strains, Chan et al. (2014) observed RNA–DNA hybrid accumulation at Ty retrotransposons, near telomeres, within the ribosomal RNA (rRNA) gene cluster, and at highly transcribed GC-rich genes. El Hage et al. (2014) found hybrid accumulation in wild-type strains at highly transcribed genes, within the rRNA gene cluster, near tRNA genes, and near Ty retrotransposons. They also reported, however, that RNA:DNA accumulation in Ty retrotransposons was primarily a consequence of reverse transcription rather than R-loop formation. In strains lacking both RNases H1 and H2, both groups found increased R-loop accumulation, with the positions of the R-loops being similar to those observed in wild-type strains.
In addition to stable RNA:DNA association via R-loops, ribonucleotides (rNMPs) can be embedded into DNA during replication, either as remnants of Okazaki fragments or by direct incorporation (reviewed by Williams and Kunkel 2014). Despite the efficiency with which replicative DNA polymerases discriminate between rNTP and dNTP precursors, there are ∼15,000 rNMPs inserted into yeast DNA during each round of replication, which translates into ∼1 rNMP per 1000 nucleotides (Nick McElhinny et al. 2010b). rNMPs in budding yeast genomic DNA have recently been mapped to single-nucleotide resolution by several groups (Clausen et al. 2015; Koh et al. 2015; Reijns et al. 2015). In contrast to R-loops, which are removed by RNase H1 (encoded by RNH1) or RNase H2 (catalytic subunit encoded by RNH201), only RNase H2 is capable of removing of single rNMPs embedded in DNA (Cerritelli and Crouch 2009). In the absence of error-free removal by RNase H2, error-prone removal of single rNMPs can be initiated by Topoisomerase 1 (Top1), resulting in short deletions (Kim et al. 2011; Williams et al. 2013). Although genetic instability in the absence of RNase H1 and H2 is generally assumed to reflect persistent R-loops, pathways that promote DNA-damage bypass during replication are essential in the complete absence of RNase H activity (Lazzaro et al. 2012). The requirement for bypass activity suggests that contiguous tracts of rNMPs in genomic DNA may also contribute to genetic instability.
In addition to the specific case of recombination associated with highly elevated transcription, yeast strains with reduced RNase H activity generally exhibit elevated genomic instability. Haploid strains lacking RNase H2, for example, have increased recombination between direct repeats (Ii et al. 2011; Potenski et al. 2014). Top1 is required for this increase in recombination, suggesting that the initiating lesion is a Top1-mediated nick at an rNMP (Potenski et al. 2014). Contributions of RNase H activity to genome integrity have additionally been assessed by measuring artificial chromosome stability or loss of heterozygosity (LOH) on an endogenous yeast chromosome (Wahba et al. 2011). Mutations eliminating either RNase H1 or RNase H2 increased the rate of loss of an artificial chromosome, and lack of both enzymes had a synergistic effect. With regard to LOH, however, an increase was observed only in an rnh1Δ rnh201Δ double-mutant background.
It is clear that yeast strains lacking both RNase H1 and RNase H2 activity have elevated levels of recombination, but the relative contributions of R-loops and rNMPs have not been explored. In addition, most previous studies have used assays that are limited either to a single locus or a single chromosome arm without subsequent mapping of repair events. In the current study, we examine the location and distribution of LOH events that occur throughout the yeast genome or on the 1-Mb right arm of chromosome IV. Analyses were done in single-mutant rnh1Δ and rnh201Δ diploids, as well as in double-mutant rnh1Δ rnh201Δ diploids. We found that the rnh1Δ strain had levels of recombination indistinguishable from those in wild type, while LOH was elevated in an rnh201Δ strain. Experiments done under conditions of reduced rNMP incorporation into genomic DNA indicated that the elevated recombination in the rnh201Δ single-mutant background reflected primarily the accumulation of R-loops. In all LOH assays, instability was further increased in the rnh1Δ rnh201Δ double-mutant relative to the rnh201Δ single-mutant strain. These findings have relevance to human disease, where hypomorphic mutations in RNase H2 lead to the neurodegenerative disorder Aicardi-Goutiéres syndrome (Crow et al. 2006) and have been associated with systemic lupus erythematosus (Günther et al. 2015).
Materials and Methods
Strain construction
Haploid strains with various gene deletions were constructed by one-step transplacement in isogenic derivatives of W303-1A (genotype of derivative: MATa ade2-1 can1-100 ura3-1 ho::hisG his3-11,15 leu2-3,112 trp1-1) and YJM789 (genotype of derivative: MATα ade2-1 ho::hisG gal2ura3) genetic backgrounds using polymerase chain reaction (PCR)-generated DNA fragments containing a selectable drug-resistance marker. Deletions were confirmed using PCR. To introduce the pol2-M644L mutant allele, two-step transplacement was done using the AgeI-digested plasmid p173-pol2-M644L (Nick McElhinny et al. 2010a).
LOH on chromosome IV was examined using hybrid diploid strains formed by mating W303-1A derivatives with YJM789 derivatives. Diploids had a URA3 gene from Kluyveromyces lactis (URA3-Kl) inserted near the right telomere of chromosome IV (Saccharomyces Genome Database, SGD coordinate 14954320) on the W303-1A-derived homolog, and an ADE2 gene inserted at the allelic position on the YJM789-derived homolog. For all experiments, at least two isolates of each diploid genotype were analyzed. In addition, for each diploid, at least 10 tetrads were dissected to confirm the correct genotypes.
A complete list of strains and the details of their construction are provided in Supporting Information, Table S1 and File S1. Plasmids and primers used in the strain construction are listed in Table S2 and Table S3, respectively. Table S4 summarizes which strains were used in different assays of LOH.
Accumulation of recombination events in subcultured cells
Mutant and wild-type strains were serially passaged on rich YPD medium (yeast, peptone, dextrose: 1% yeast extract, 2% bacto-peptone, 2% dextrose; 2% agar for plates) to allow accumulation of recombination events genome-wide. In each passage, a strain was grown from a single cell into a colony. This procedure was repeated 10 or 20 times, corresponding to ∼250 and ∼500 cell divisions, respectively. Genomic DNA from subcultured strains was examined using whole-genome single-nucleotide polymorphism (SNP) arrays.
Rates of LOH on chromosome IV
Single colonies grown on rich medium were diluted and plated onto solid synthetic dextrose medium supplemented with all amino acids and bases except arginine. The concentration of adenine was reduced to 10 μg/ml to allow the Ade− sectors to develop their red color. Each plate contained ∼1000 colonies. The plates were screened for red/white sectored colonies using a dissecting microscope. Single colonies derived from the red and white sides of each sectored colony were isolated for subsequent phenotypic and physical analysis. To form a red/white sectored colony, the crossover must occur in the first division after the cells are plated. Thus, the frequency of red/white colonies divided by the total number of colonies is also the rate of sectored colony formation per cell division.
To produce a reciprocal crossover (RCO) using the sectoring assay, both daughter cells containing the recombination products must be viable. For each strain, we examined the daughter-cell viability by following the division of 88 unbudded cells on solid medium. After ∼60 min of growth at 30°, the mother and daughter cells were separated through micromanipulation. After 2 days at 30°, the growth of individual cells into colonies was assessed. For all strains, at least 75% of the unbudded cells produced two viable daughter cells.
In separate experiments, we measured the rate of loss of the URA3-Kl marker by monitoring the rate of colonies resistant to 5-fluoro-orotate (5-FOA) as described in detail in File S1. To measure rates, we determine the frequencies of 5-FOAR derivatives in 10–20 individual cultures. These frequencies are converted to rates of 5-FOA resistance (the number of 5-FOAR isolates generated per cell division) using the method of the median (Lea and Coulson 1949). At least two independently derived diploid isolates were used for each estimate. Confidence intervals were obtained as described in Altman (1991).
Microarray hybridization and analysis
Diploids used to monitor LOH were heterozygous for ∼55,000 SNPs. Chromosome IV-specific microarrays monitored ∼1000 SNPs on the right arm of chromosome IV, and whole-genome microarrays monitored ∼13,000 SNPs distributed throughout the genome (St Charles et al. 2012, 2013). On our custom Agilent microarrays, each SNP was represented by at least four 25-base oligonucleotides: probes identical to the Watson and Crick strands of the W303-1A-derived SNP and probes identical to the Watson and Crick strands of the YJM789-derived SNP. The sequences used for the oligonucleotides for these arrays are described in St Charles et al. (2012, 2013).
DNA samples derived from the red or white sides of sectored colonies were labeled with a Cy5-tagged fluorescent nucleotide (St Charles et al. 2012). DNA from a wild-type heterozygous control sample was labeled with a Cyanine3 (Cy3)-tagged fluorescent nucleotide. The labeled control and experimental DNAs were then mixed and hybridized to the microarrays. The hybridization levels for each Cy3- and Cy5-labeled sample were determined using a GenePix scanner. The ratio of Cy5 to Cy3 fluorescence (i.e., the ratio of medians) was normalized for each array (St Charles et al. 2012). If the normalized ratio of medians of a given sample for both W303-1A- and YJM789- derived oligonucleotides was 1, the locus is heterozygous. LOH events result in a normalized ratio of medians >1.5 for oligonucleotides representing one homolog and a signal <0.5 for oligonucleotides representing the other homolog. We also required that at least two adjacent SNPs reflect LOH to be included in the dataset. In addition to detecting LOH events, the SNP microarrays revealed deletion and duplication events (reduced or elevated hybridization levels for SNPs from one homolog with no alteration in the level of hybridization of SNPs on the other homolog) and ploidy changes (trisomy).
For each SNP on the microarray, our analysis allows us to conclude whether the analyzed strain is homozygous for the W303-1A-derived SNP, the YJM789-derived SNP, or retains heterozygosity. The SGD coordinates for LOH events, deletions/duplications, and ploidy changes in the subcultured strains are shown in Table S5, Table S6, and Table S7, respectively. SGD coordinates for LOH events in sectored colonies are in Table S8. The patterns of LOH, deletions/duplications, and aneuploidy for subcultured strains are shown schematically in Figure S1, Figure S2, and Figure S3; the location of LOH events on the chromosomes in subcultured strains is summarized in Figure S4, Figure S5, and Figure S6. The patterns of LOH for sectored colonies are shown schematically in Figure S7.
Association of genome features with recombination events
The transition between heterozygous and homozygous SNPs should contain the site of the recombination-initiating lesion (St Charles and Petes 2013; Yin and Petes 2013). Breakpoint regions in subcultured mutant strains were examined to determine if specific chromosome elements (for example, autonomously replicating sequence, ARS elements) were overrepresented at the breakpoints. For interstitial LOH events (gene conversions), we used association windows that extended between the two heterozygous flanking SNPs that were closest to the homozygous SNPs at the termini of the conversion tracts. The association windows for terminal LOH events were defined by including the sequences located 10 kb centromere-proximal and 10 kb centromere-distal to the first homozygous SNP. For RCO events associated with sectored colonies, we used association windows that extended from the centromere-proximal heterozygous SNP of the event to the most centromere-distal homozygous SNP of the event. Other details of the association analysis are described in File S1.
The references for the locations of the chromosome elements in the genome are in Table S9, and the number of elements per genomic microarray are in Table S10. After defining the association windows for LOH events (as described above), we tallied the number of specific genomic elements within the association windows for each genotype for each type of experiment (whole-genome or chromosome IV-specific analyses). The total numbers of genomic elements within and outside of the association windows were compared to the expected numbers by χ2 analysis. The expected numbers were based on the total numbers of elements in the genome, and the amount of DNA inside and outside of the association windows for all strains analyzed (Song et al. 2014). Because multiple genome features were tested for association with recombination windows, we applied a correction of the P-value (Hochberg and Benjamini 1990). The association analyses for the subcultured strains and the sectored colonies are in Table S11 and Table S12, respectively.
Calculations of gene conversion tract lengths
Gene conversion tract lengths were calculated for RCO events in the sectoring assay and for interstitial LOH events in subcultured strains. Tract lengths were measured by calculating the distances between the midpoints of heterozygous to homozygous transitions on the farthest edges of both sides of the tracts.
Data availability
Strains are available upon request. The contents of File S1, Table S1, Table S2, Table S3, Table S4, Table S5, Table S6, Table S7, Table S8, Table S9, Table S10, Table S11, and Table S12, and Figure S1, Figure S2, Figure S3, Figure S4, Figure S5, Figure S6, and Figure S7 are described in the text. Microarray data are available at GEO with the accession number GSE73334.
Results
To assess the contributions of incorporated ribonucleotides (rNMPs) and R-loops to genome instability and recombination in RNase H-defective strains, we compared genome instability in the following six diploid strains: wild type (KO198), rnh1Δ (KO73/KO187), rnh201Δ (KO75/KO188/KO135), pol2-M644L (KO234), rnh201Δ pol2-M644L (KO244), and rnh1Δ rnh201Δ (KO5/KO132/KO189); the complete genotypes of all strains are given in Table S1. The rnh1Δ and rnh201Δ mutants lack RNase H1 and the catalytic subunit of RNase H2, respectively (reviewed in Cerritelli and Crouch 2009). The pol2-M644L allele encodes a mutant form of the catalytic subunit of DNA polymerase ε (Pol ε) that results in the insertion of 70% fewer rNMPs than the wild-type enzyme (Nick McElhinny et al. 2010a). Genome instability in mutant and wild-type diploid strains was monitored using two approaches. The first was to examine LOH throughout the genome in cells that were serially passaged for ∼500 divisions (20 cycles of growth from a single cell to a colony). The second approach involved identification of cells that had undergone a crossover on the right arm of chromosome IV. The locations of LOH events were determined by microarray analysis. Details of these systems are described further below.
Genome-wide mapping of LOH events in subcultured RNase H-defective strains
Experiments utilized diploid strains that were derived by mating two sequence-diverged haploids (W303-1A and YJM789) and were heterozygous for ∼55,000 single-nucleotide SNPs (St Charles et al. 2012). We developed oligonucleotide-based microarrays that detect LOH for ∼13,000 SNPs distributed throughout the genome, allowing mapping of events to ∼1-kb resolution. Each heterozygous SNP on the microarray was represented by at least four 25-base oligonucleotides: two identical to the W303-1A form of the SNP and two identical to the YJM789 form. DNA from the serially passaged strains was labeled with Cy5-fluorescent dNTPs and mixed with DNA from a wild-type heterozygous control strain, which was labeled with Cy3-fluorescent dNTPs. The combined sample was then hybridized to a whole-genome microarray and the hybridization signal of DNA from the passaged strain was normalized to that from the heterozygous control strain (details in Materials and Methods). A normalized ratio of about one for both the YJM789 and W303-1A SNP indicated that the subcultured sample had maintained heterozygosity at that position. LOH, as a consequence of a crossover, resulted in an elevation in the hybridization signal for strain-specific SNPs derived from one homolog and a reduction in the signal of SNPs derived from the other homolog. Heterozygous deletions or duplications resulted in a loss or an increase, respectively, in the hybridization of SNPs specific for one strain with no alteration in the hybridization level of the other strain-specific SNPs. Changes in ploidy were also readily detected.
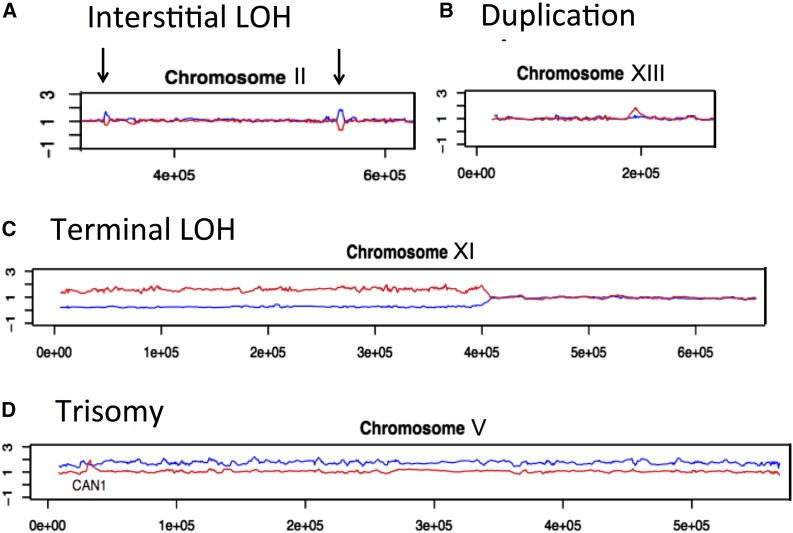
The four common classes of events observed by this analysis are shown in Figure 1. In this depiction, red and blue lines represent levels of hybridization to W303-1A- and YJM789-derived SNPs, respectively. An interstitial LOH event is shown in Figure 1A; from previous studies (St Charles et al. 2012; St Charles and Petes 2013; Yin and Petes 2013), we know that these events reflect gene conversion, the nonreciprocal transfer of sequences from one homolog to the other. In this example, sequences of the YJM789 copy of chromosome II were copied into the W303-1A copy of chromosome II at two different locations. Figure 1B shows a duplication of sequences derived from the W303-1A copy of chromosome XIII; such events are often generated by unequal recombination between two nonallelic Ty elements or other repeated genes (Song et al. 2014). Figure 1C shows a terminal LOH event that includes most of the left arm of chromosome XI. This pattern of LOH can result from a crossover or break-induced replication (BIR) event between homologs. Lastly, Figure 1D shows the pattern of hybridization expected for chromosome V trisomy with a duplication of the YJM789-derived homolog. No chromosome loss events were observed in our experiments. Schematic depictions of all of the LOH patterns observed in these strains are shown in Figure S1 (terminal and interstitial LOH), Figure S2 (deletions/duplications), and Figure S3 (aneuploidy).
Figure 1.
Classes of events found in subcultured strains. In each panel, the x-axis represents the chromosome coordinate, and the y-axis represents the ratio of hybridization medians. The red and blue lines reflect the hybridization signal of the W303-1A-specific- and YJM789-specific oligonucleotides, respectively. The events in this figure are from the genome of a single passaged isolate of the rnh1Δ rnh201Δ double mutant.
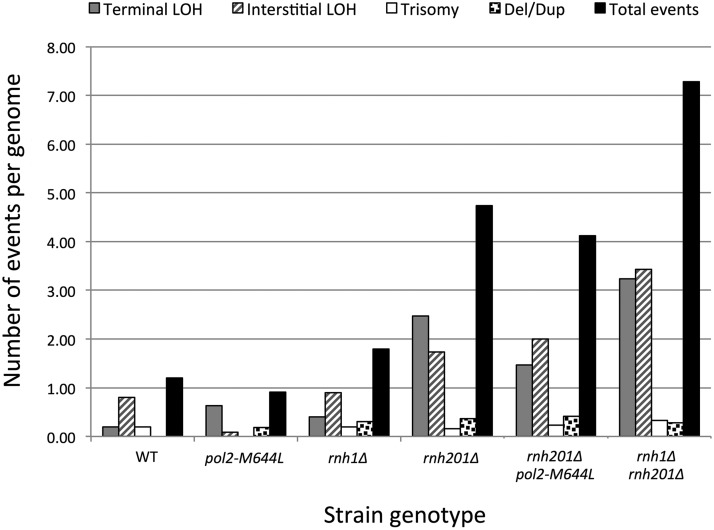
We determined the numbers of events of various classes described above after 10–21 colonies of each genotype were subcultured 20 times, which corresponds to ∼500 generations. These data are summarized in Figure 2. The average number of events (sum of all four classes) was about one per strain in the wild-type, rnh1Δ, and pol2-M644L strains. There was no difference in the number of events for these three genotypes (Wilcoxon rank sum test, P > 0.05). In contrast, the average number of events was elevated three- to fourfold in the rnh201Δ single- and rnh201Δ pol2-M644L double-mutant strains relative to wild type, and about sevenfold in the rnh1Δ rnh201Δ double mutant. Differences in the average number of total events per strain primarily reflected differences in the number of terminal and interstitial LOH events. By the Wilcoxon rank sum test, all three of these strains had significantly (P < 0.001) more genome alterations than in wild type, and the rnh1Δ rnh201Δ strain had significantly (P < 0.001) more rearrangements than either the rnh201Δ or the rnh201Δ pol2-M644L strain.
Figure 2.
Genome instability of subcultured wild type, pol2-M644L, rnh1Δ, rnh201Δ, rnh201Δ pol2-M644L, and rnh1Δ rnh201Δ strains. The total number of events per genome is indicated for each genotype as a black bar. For wild-type, pol2-M644L, rnh1Δ, rnh201Δ, rnh201Δ pol2-M644L, and rnh1Δ rnh201Δ strains, 10, 12, 10, 19, 17, and 22 genomes were analyzed by whole-genome microarrays, respectively. Since the wild-type strain was subcultured 10 times, and the mutant strains were subcultured 20 times (∼500 cell divisions), we doubled the number of events observed in the wild-type strain to make the comparisons among the strains valid.
The lack of an increase in recombination in the rnh1Δ strain, the greater instability in the rnh201Δ mutant, and the even greater instability in the rnh1Δ rnh201Δ double mutant could be explained in two ways. First, it is possible that the recombinogenic lesions primarily reflect persistent R-loops and RNase H2 is the primary enzyme involved in their removal, with RNase H1 playing a back-up role. The alternative possibility is that the primary recombinogenic lesions are misincorporated rNMPs, with RNase H2 acting as the most important enzyme in their removal and RNase H1 having a relatively minor effect. Data obtained with the rnh201Δ pol2-M644L double-mutant strain argue against the second possibility. As described previously, the pol2-M644L allele encodes a mutant Pol ε that incorporates only one-third as many rNMPs as the wild-type enzyme (Nick McElhinny et al. 2010a). If rNMPs are the major recombinogenic lesion in the rnh201Δ single mutant, then the level of instability in the rnh201Δ pol2-M644L double mutant should decrease to one-third of the level observed in rnh201Δ single mutant. No significant decrease in instability was observed (P = 0.37), however, indicating that rNMPs embedded into DNA by Pol ε are not the major source of instability in the rnh201Δ single-mutant strain. The most likely interpretation of the data in Figure 2 is that the hyperrecombination (hyperrec) phenotype is driven primarily by persistent R-loops, which are present at inconsequential levels in the rnh1Δ single mutant, high levels in the rnh201Δ single mutant, and even higher levels in the rnh1Δ rnh201Δ mutant.
In addition to determining the number of LOH events and other genomic alterations, we used the microarray data to map the location of the transitions between heterozygous and homozygous SNPs. The coordinates of the SNPs adjacent to the transitions of LOH events are presented in Table S5, Table S6, and Table S7. These coordinates are based on the June 2008 (SGD/sacCer2) version of the SGD available on the University of California Santa Cruz Genome Browser (http://genome.ucsc.edu/).
Several types of analyses were performed with these data. First, simple interstitial LOH events (class b1-2 from Figure S1) were used to estimate the length of the gene conversion tract associated with each event. The conversion events that produce interstitial LOH are both conversion events that are unassociated with crossovers and conversion events in which the recombinant chromosomes cosegregate into the same cell. Tract size was estimated by averaging the distance between the closest heterozygous sites flanking the transition (the maximum tract size) and the sites of homozygous SNPs closest to the transitions (the minimum tract size). A summary of conversion tract sizes is in Table S5. The median conversion tract lengths (in parentheses) for each strain were: rnh201Δ (11.8 kb), rnh1Δ rnh201Δ (11.8 kb), and rnh201Δ pol2-M644L (14.2 kb). The median length of conversion events in the wild-type strain (based on only four events) was 12.7 kb, similar to the lengths observed in the mutant strains.
In the second type of analysis, we mapped the location of interstitial and terminal LOH events in yeast genome to search for potential hotspots. As shown in Figure S4, Figure S5, and Figure S6, LOH events in the rnh201Δ, rnh1Δ rnh201Δ, and rnh201Δ pol2-M644L mutants were widely distributed throughout the genome with little evidence for strong hotspots of recombination. For strains with large numbers of LOH events (rnh201Δ, rnh201Δ pol2-M644L, and rnh1Δ rnh201Δ), we also examined regions near the recombination breakpoints for enrichment of various chromosome elements. The rationale for this analysis is that the breakpoints are likely to be located at or near the site of the recombination-initiating DNA lesion. For each simple terminal and interstitial LOH event, we calculated an association window likely to contain the relevant lesion. For interstitial events, the association windows were the DNA sequences between the heterozygous SNPs that most closely flanked the LOH region (details are provided in Materials and Methods and File S1). For terminal LOH events, we used a 20-kb window, extending 10 kb centromere-proximal and centromere-distal from the homozygous SNP located at the transition point. After calculating the association window for each event, we determined the total number of each chromosome element/motif located within these windows. Based on the total number of an element in the genome and the fraction of genome located within and outside of the windows, we calculated an expected number of elements within and outside of the windows. We then compared the observed numbers of events to the expected numbers by χ2 analysis, correcting the P-values for multiple comparisons (Hochberg and Benjamini 1990). The list of the elements used in our analysis and the expected numbers of such elements are given in Table S9 and Table S10, respectively. The results of this analysis for the subcultured strains are in Table S11.
In our previous studies, breakpoints of spontaneous recombination events in wild-type strains were enriched for replication fork-stalling motifs (St Charles and Petes 2013); a similar enrichment was observed for events associated with low levels of DNA Pol α (Song et al. 2014). In the current analysis, we first looked for overrepresented or underrepresented genomic elements in the subcultured rnh201Δ, rnh201Δ pol2-M644L, and rnh1Δ rnh201Δ strains. Based on results of several other groups, we expected overrepresentation of regions that accumulate R-loops and/or rNMPs among the recombination breakpoints. R-loop formation is promoted by high GC content, the ability to form G4 quadruplex structures on the nontranscribed strand, high levels of transcription, and unusually long genes (reviewed by Aguilera and Garcia-Muse 2012; Hamperl and Cimprich 2014). We found no significant enrichment for these factors (Table S11). Additionally, genomic sites of R-loop accumulation have been measured directly in wild-type and rnh1Δ rnh201Δ strains (Chan et al. 2014; El Hage et al. 2014). Surprisingly, these sites also were not enriched at the LOH breakpoints in our study. An exception may be the ribosomal RNA gene tandem array, where two independent rnh1Δ rnh201Δ diploids had LOH events before subculturing began, indicating a high level of instability. We also found that one of 19 rnh201Δ and three of 17 rnh201Δ pol2-M644L subcultured colonies had LOH events in the rDNA; there were none among the 10 wild-type colonies analyzed. This difference, however, is not significant by the Fisher exact test (P = 0.56).
We also looked for overrepresentation of sites of RNA polymerase II subunit Rbp3 accumulation during S phase (Fachinetti et al. 2010) and binding sites for the Rrm3 helicase (Azvolinsky et al. 2009) at LOH breakpoints. No significant associations were observed for any of three mutant strains (Table S11). Lastly, we calculated whether LOH events located within 25 kb of the telomere were overrepresented in the datasets. We found an overrepresentation of LOH events near the telomere in the rnh201Δ single mutant (χ2 analysis; P-value of 0.002), but not in the rnh201Δ pol2-M644L or rnh1Δ rnh201Δ double mutant.
Frequency of recombination events on the right arm of chromosome IV
In addition to examining patterns of unselected LOH in subcultured mutant strains, we also employed a system that identifies cells that have undergone a RCO or BIR event on the ∼1 Mb right arm of chromosome IV. As will be discussed below, this system allows inference of whether the recombination-initiating lesion occurred in G1 or S/G2 of the cell cycle. In the same hybrid genetic background used for the whole-genome analyses, we constructed diploids in which YJM789-derived copy of chromosome IV had an insertion of ADE2 near the telomere of the right arm. The W303-1A-derived homolog had an insertion of URA3 at the same position (Figure 3). The diploid was also homozygous for the ade2-1 allele at the ADE2 locus on chromosome XV. Strains without a wild-type ADE2 gene on chromosome IV form red colonies.
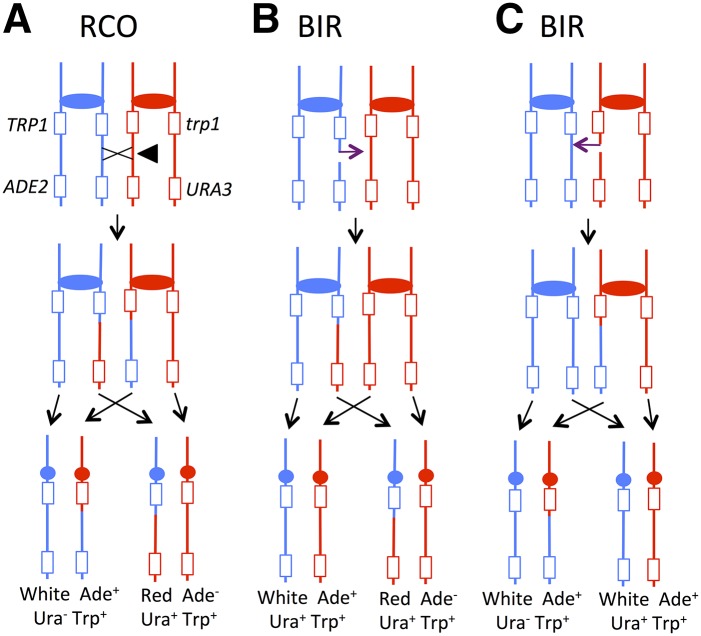
Figure 3.
Mechanisms of repair leading to terminal LOH events in the red/white sectoring system and in 5-FOA-resistant colonies. Blue and red lines represent the YJM789-derived and W303-derived chromosomes, respectively. Rectangles and circles represent genes and centromeres, respectively. In two of the three types of repair in this figure, a red/white sectored colony is formed. (A) A double-strand break (DSB) leads to a reciprocal crossover (RCO) event. After the first mitotic division following the crossover, the sectored colony has reciprocal LOH products in the white, Ura− and the red Ura+ sides of the sector. (B) A DSB on the YJM789-derived homolog repairs through break-induced replication (BIR) of the W303-1A-derived homolog, leading to a nonreciprocal terminal LOH event. Both sides of the red/white sector are Ura+. (C) A DSB on the W303-1A-derived homolog repairs by BIR, using the YJM789-derived homolog as a template. Although no red/white sectored colony is formed, one half of the colony is Ura− and the other half is Ura+.
An RCO event between the ADE2/URA3 insertions and CEN4 can produce a red/white sectored colony in which the red side of the sector is Ura+ and the white side is Ura− (Figure 3A). In contrast, a BIR event initiated by a break on the YJM789-derived homolog produces a red/white sectored colony in which both sides are Ura+ (Figure 3B). It should be noted that a red/white sectored colony in which both sectors are Ura+ also can result from loss of the YJM789-derived chromosome, producing the red side of the sector. In this case, however, the red side of the sector would be Trp−. Of 173 red/white Ura+/Ura+ sectors examined, the red sector was Trp+ in all but one, indicating that chromosome loss does not significantly contribute to sector formation. It should be noted that if a BIR-initiating break occurs on the W303-1A-derived homolog, a red/white sectored colony is not formed (Figure 3C).
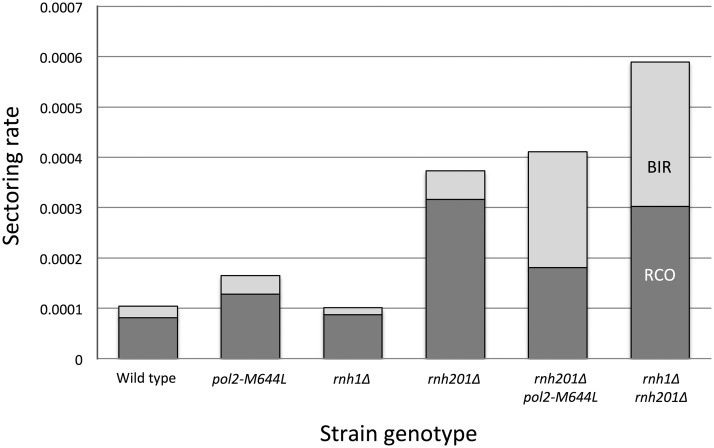
Using this system, we quantitated RCO and BIR events on the right arm of chromosome IV in two different ways. First, we counted red/white sectored colonies relative to total colonies in the following diploid strains: wild type, rnh1Δ, pol2-M644L, rnh201Δ, rnh201Δ pol2-M644L, and rnh1Δ rnh201Δ. Purified colonies from the red and white sectors were checked to determine whether they were Ura+ or Ura− to distinguish between BIR and RCO events. A summary of this analysis is shown in Figure 4. When normalized to the rate of sectored colonies observed in wild type, the rate of sectored colonies was 1.0 in rnh1Δ, 1.6 in pol2-M644L, 3.6 in rnh201Δ, 4.0 in rnh201Δ pol2-M644L, and 5.7 in rnh1Δ rnh201Δ. The sectoring results were in broad agreement with genome-wide analysis done using subcultured colonies: rnh1Δ and pol2-M644L were indistinguishable from wild type (P = 0.92 and P = 0.16, respectively; contingency χ2), sectoring was elevated in rnh201Δ relative to wild type (P < 0.001), the presence of the pol2-M644L allele did not reduce sectoring in the rnh201Δ background (P = 0.60), and sectoring was elevated in the rnh1Δ rnh201Δ double mutant relative to the rnh201Δ single mutant (P = 0.002). Relative to wild type, there were changes in the distribution between BIR and RCO events in the rnh201Δ pol2-M644L and rnh1Δ rnh201Δ strains, where the BIR events were a larger fraction of LOH events. This difference was statistically significant only for the rnh201Δ pol2-M644L strain (P = 0.04 by Fisher exact test). In our analysis of subcultured colonies, it was not possible to distinguish between RCO and BIR events.
Figure 4.
Rate of red/white sector formation in RNase H-defective strains. The numbers of sectors among total colonies screened for wild type, pol2-M644L, rnh1Δ, rnh201Δ, rnh201Δ pol2-M644L, and rnh1Δ rnh201Δ strains were 14/134864, 44/266267, 22/218008, 126/337864, 59/143669, and 78/132302, respectively. Dark and light gray bars correspond to RCO and BIR events, respectively, among sectored colonies.
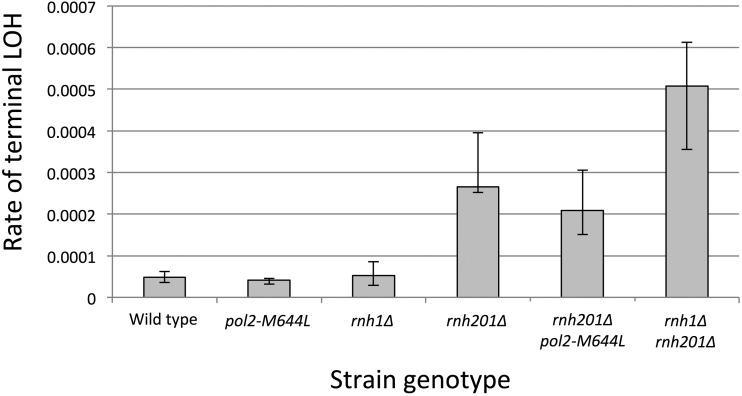
Our estimates of the rates of sectored colonies were based on a nonselective screening procedure and involved a relatively small number of sectored colonies, from 14 for the wild type to 126 for the rnh201Δ strain. Since both BIR events that initiate on the W303-1A homolog and RCO events can result in derivatives that are Ura− and, therefore, selectable on medium containing 5-FOA (Figure 3, A and C), we also determined the rates of 5-FOAR colonies in the same strains used for the red/white sector analysis. These results (Figure 5) are also in good agreement with the LOH data from whole-genome analysis of the subcultured mutants (Figure 2). The wild-type, rnh1Δ, and pol2-M644L strains had similar rates of LOH on the right arm of chromosome IV (∼5 × 10−5/cell division). The rnh201Δ and rnh201Δ pol2-M644L strains had rates that were elevated ∼5-fold above wild type, and the rnh1Δ rnh201Δ double mutant had a rate ∼10-fold higher than wild type. Though the rates of LOH were similar for the rnh201Δ and rnh201Δ pol2-M644L strains, it should be noted that there was a significant, ∼20% decrease in the double mutant (P = 0.016 by Mann–Whitney test).
Figure 5.
Rate of terminal LOH on chromosome IV in RNase H mutant diploids. LOH was assessed by measuring the rate of 5-FOA resistance, which corresponds to loss of the URA3 marker near the end of chromosome IV. For wild-type, pol2-M644L, rnh1Δ, rnh201Δ, rnh201Δ pol2M644L, and rnh1Δ rnh201Δ diploids, 17, 23, 16, 17, 25, and 19 independent cultures were used to derive the rates of instability, respectively.
Mapping RCO events on the right arm of chromosome IV
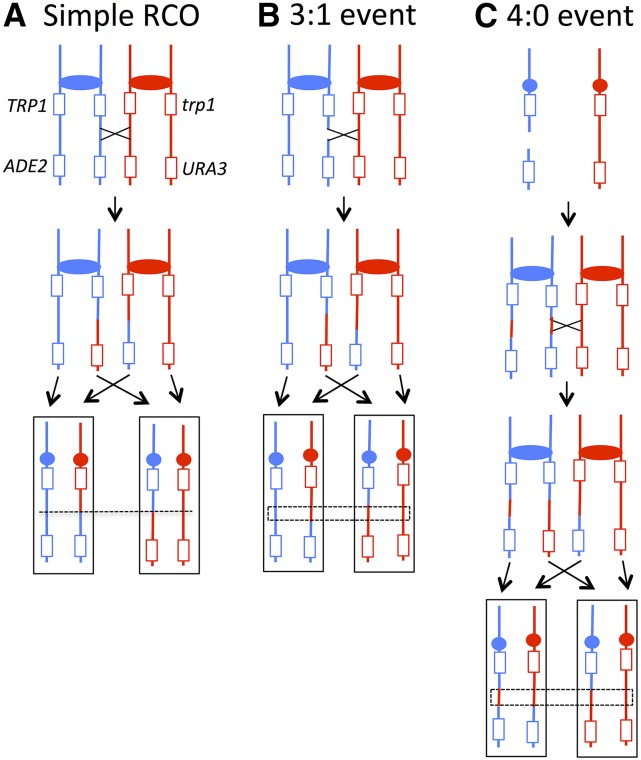
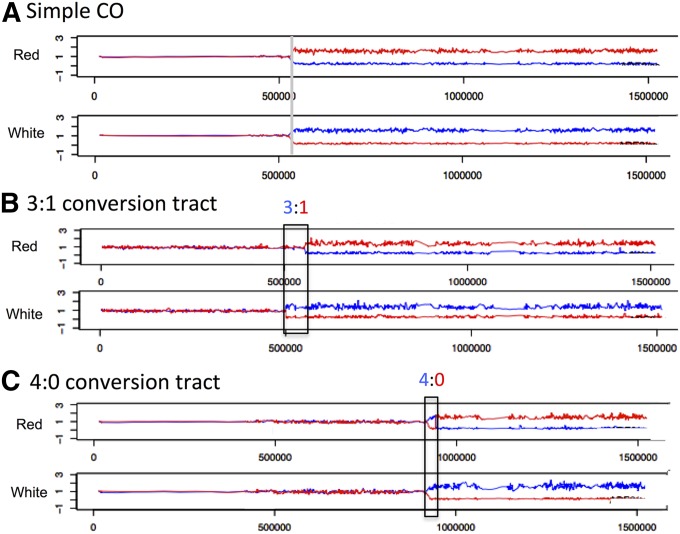
The red and white sides of sectored colonies were analyzed using chromosome IV-specific microarrays, which allows the position of crossovers and their associated gene conversion tracts to be mapped (St. Charles et al. 2012; St. Charles and Petes 2013). For RCO events unassociated with gene conversion, the transition between heterozygous and homozygous SNPs occurs at exactly the same position in both sectors, indicated by the dotted line in Figure 6A. Gene conversion events in which one sister chromatid is broken and subsequently repaired using a nonsister chromatid result in an LOH pattern in which the breakpoints between heterozygous and homozygous SNPs are at different positions in the two sectors (Figure 6B). In the region boxed in Figure 6B, three of the chromatids have SNPs specific to W303-1A and one has SNPs specific to YJM789. This type of conversion is, therefore, defined as a 3:1 conversion. We infer that the DNA lesion that initiated the crossover likely occurred in S or G2 of the cell cycle because only one sister chromatid received information from the homologous donor chromosome. Another common LOH pattern observed for spontaneous crossovers on chromosome IV is a 4:0 conversion (Figure 6C). This class of event reflects the repair of two sister chromatids broken at approximately the same position. The 4:0 pattern of conversion likely reflects double-strand break (DSB) formation in G1 that is subsequently replicated to give two broken chromatids (Lee and Petes 2010). Microarray analyses of the red and white sectors with these patterns are in Figure 7. In the boxed region in Figure 7B, in the red sector, the hybridization signal is about one, indicating one copy of W303-1A-derived SNPs and one copy of YJM789-derived SNPs; in the white sector, the ratio of hybridization indicates that there are two copies of YJM789-derived SNPs and no copies of W303-1A-derived SNPs within the boxed region. Thus, this region represents a 3:1 conversion tract. In Figure 7C, in the boxed regions of both the red and white sectors, the hybridization signals indicate that there are two copies of YJM789-derived SNPs and no copies of W303-1A-derived SNPs, as expected for a 4:0 conversion event. Among spontaneous crossovers, an additional pattern of conversion associated with crossovers is a hybrid 4:0/3:1 tract. This pattern is consistent with a G1-associated DSB in which the repair of the two broken sister chromatids results in gene conversion tracts of different lengths (St Charles and Petes 2013).
Figure 6.
Classes of red/white sectors resulting from RCO. As in Figure 3, blue and red lines indicate YJM789- and W303-1A-derived chromatids, respectively. Three common types of crossovers that result in a red Ura+ and a white Ura− sector can be distinguished by microarray analysis. (A) If a RCO is not associated with a gene conversion, the transition between heterozygous and homozygous SNPs occurs at the same position in the two sectors. Such crossovers provide no information about the likely timing of the recombinogenic DNA lesion. (B) A DSB formed in S or G2 on the YJM789-derived chromatid is associated with the nonreciprocal transfer of information from the W303-1A-derived chromatid, producing a 3:1 gene conversion event. In the dotted box, there are three chromosomes with W303-1A-derived sequences and only one chromosome with YJM789-derived sequences. By microarray analysis, the red sector loses heterozygosity at a more centromere-proximal location than the white sector. (C) A DSB occurs on the YJM789-derived homolog in G1, and the broken molecule is replicated to produce two sister chromatids that are broken at the same position. Repair of the two broken DNA molecules produces a 4:0 conversion tract as indicated by the dotted lines. Repair of one these breaks is associated with a crossover, producing the red/white sectored colony.
Figure 7.
Examples of microarrays showing simple crossovers, crossovers with 3:1 conversion tracts, and crossovers with 4:0 conversion tracts. The red and white sectors of sectored colonies were examined by chromosome IV-specific microarrays. The hybridization ratio of DNA derived from the sectors relative to heterozygous control DNA is shown on the y-axis; the red and blue lines represent hybridization to W303-1A-specific or YJM789-specific oliognucleotides, respectively. The x-axis shows the SGD coordinates on the right arm of chromosome IV. (A) In this sectored colony (KO135_5_2R and KO135_5_2W in Table S8), the red and white sectors have the same point of transition between heterozygous and homozygous markers (near SGD coordinate 530 kb), indicative of a simple crossover. (B) In this sectored colony (KO132_31_17R and KO132_31_17W in Table S8), the red sector has a transition point at about coordinate 500 kb, and the white sector has a transition near coordinate 555 kb. Thus, this sectored colony has a large (∼55 kb) 3:1 conversion tract associated with the crossover. In the boxed region, the red sector has one copy each of W303-1A- and YJM789-derived SNPs. In this region, the white sector has two copies of YJM789-derived SNPs and no copies of W303-1A-derived SNPs. (C) In this sectored colony (KO135_5_5R and KO135_5_5W in Table S8), there is a region of ∼20 kb that is homozygous for the YJM789-derived SNPs in both red and white sectors, consistent with a 4:0 conversion.
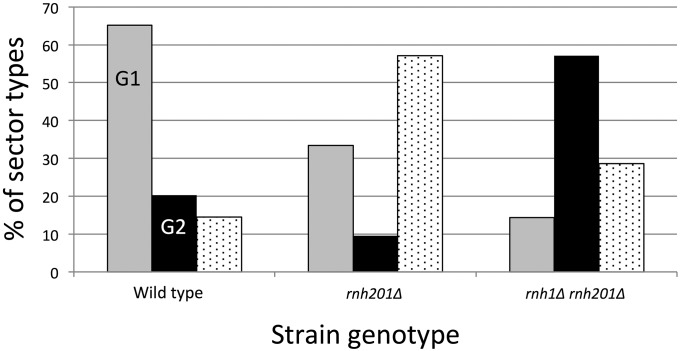
In our previous analyses of spontaneous RCOs in a wild-type strain, most events were consistent with DSB formation in G1. The ratio of the numbers of 4:0 (or 4:0/3:1 hybrid) tracts to 3:1 tracts to no detectable tracts was 90:28:20 (St Charles and Petes 2013). The corresponding ratios for the rnh201Δ and rnh1Δ rnh201Δ strains in the current analysis were 7:2:12 and 2:8:4, respectively. These data are summarized in Figure 8. Though the numbers of sectored colonies analyzed in the current study were relatively small, the distribution of events in the rnh201Δ or rnh1Δ rnh201Δ diploid was significantly different from that in the wild-type strain (P < 0.001, Fisher exact test). For the rnh201Δ single mutant, the difference was driven by an increase in events with no detectable conversion tracts; when just 3:1 and 4:0 events were considered, there was no significant difference from wild type (P = 1). For the rnh1Δ rnh201Δ double mutant, however, the difference reflected a strong shift from predominantly G1 events in wild type to predominantly S/G2 events in the double mutant. The distribution of events in the rnh201Δ single mutant also was significantly different from that in the rnh1Δ rnh201Δ double mutant (P = 0.016, Fisher exact test).
Figure 8.
Distribution of sector types by cell cycle stage in which the initiating lesion arose. Conversion tracts with a 4:0 region are indicative of a G1-associated DSB (gray bars), and 3:1 tracts indicate a G2-/S-phase DSB (black bars). The stippled bars show simple CO events in which the timing of the recombinogenic lesion cannot be inferred.
In the wild-type strain, only 20 of 138 (14%) RCOs had no detectable gene-conversion tract, whereas in the rnh201Δ strain 12 of 21 crossovers (57%) had no detectable tract (P < 0.001 by Fisher exact test). The simplest explanation of this difference is that the conversion tracts in the rnh201Δ strain are shorter than in the wild-type strain and, therefore, less likely to include a heterozygous SNP. Indeed, direct measurements of the conversion tract lengths confirmed this expectation, with tracts being significantly shorter in the rnh201Δ mutant than in wild type (P = 0.03, Wilcoxon rank sum test); conversion tract lengths in the rnh1Δ rnh201Δ strain were not significantly different from wild type (P = 0.63). The median tract lengths in the wild-type, rnh201Δ, and rnh1Δ rnh201Δ (95% confidence limits in parentheses) strains were 10.6 kb (8.2–13.6 kb; (St Charles and Petes 2013), 4.8 kb (1.7–17.1 kb), and 9.3 kb (2.2–19 kb), respectively. It should be emphasized that these conversion tracts are all associated with a crossover. The conversion tract lengths from the subcultured strains in Table S5 represent a mixture of conversion events that are associated and unassociated with crossovers.
Depictions of each class of sector identified in the current analysis are shown in Figure S7 and the coordinates of the breakpoints in each event are listed in Table S8. We analyzed events on chromosome IV in rnh201Δ and rnh1Δ rnh201Δ mutants for enrichment of various genetic elements using the same procedure as used for the subcultured strains. None of the genomic elements listed in Table S10 were significantly over- or underrepresented at recombination breakpoints on the right arm of chromosome IV (Table S12).
Discussion
Previous work has shown that strains lacking RNase H1 and/or RNase H2 have elevated levels of mitotic recombination and chromosome loss. RNase H2-defective haploids, for example, have elevated gene conversion between closely linked repeats (Potenski et al. 2014) and increased loss of markers flanked by direct repeats (Ii et al. 2011). Although loss of either RNase H1 or RNase H2 promoted loss of an artificial chromosome, loss of both enzymes was required to elevate LOH on chromosome III in a diploid background (Wahba et al. 2011). Our work extends analyses of instability to a genome-wide scale in diploid yeast strains that are partially (rnh1Δ and rnh201Δ single mutants) or completely (rnh1Δ rnh201Δ double mutant) defective in RNase H activity and uses microarrays to provide a high-resolution map of recombination events resulting in LOH. Significantly, in each of three assays used, LOH was elevated in the rnh201Δ, but not the rnh1Δ single mutant, and was further elevated in the rnh1Δ rnh201Δ double mutant. We additionally used a mutant DNA polymerase that lowers the direct incorporation of rNMPs into genomic DNA, allowing us to assess the relative contributions of R-loops vs. rNMPs to LOH in the rnh201Δ background. The discussion below focuses on three related issues: (1) the nature of the recombination-initiating lesion in strains lacking RNase H activity, (2) the timing of formation of the recombinogenic lesion, and (3) factors that regulate the distribution of recombination events associated with loss of RNase H activity.
Nature of the recombinogenic lesions in strains lacking RNH1 and/or RNH201
The DNA alterations that lead to elevated recombination in strains lacking RNH1 or RNH201 are likely rNMPs embedded in DNA (expected to accumulate in rnh201Δ strains) and/or R-loops (expected to accumulate in rnh1Δ and rnh201Δ strains). In the case of loss of RNase H2, the elevated level of intrachromosomal recombination between repeats is dependent on Topoisomerase I (Top1) (Potenski et al. 2014). Further genetic and biochemical studies suggest that Top1-mediated cleavage at rNMPs is followed by the sequential action of Srs2 and Exo1, which produces a single-strand gap (Potenski et al. 2014). Subsequent replication of a gap-containing chromosome would be expected to produce a broken, presumably recombinogenic, chromatid. That R-loop accumulation results in a hyperrec phenotype has been shown using mutants defective in transcript processing and/or in the removal of R-loops (Aguilera and Garcia-Muse 2012; Hamperl and Cimprich 2014). The corresponding recombinogenic DNA lesion could reflect either nicking of the unpaired DNA strand within an R-loop or conflicts between the replication fork and an R-loop. Either of these mechanisms would likely result in single broken sister chromatid in S phase. It is widely assumed that most, if not all, R-loops are redundantly processed by RNase H1 and RNase H2.
In our experiments, we did not detect a hyperrec phenotype in rnh1Δ single mutants, but consistently observed a three- to fivefold increase in LOH in rnh201Δ strains. In all three assays, instability in the rnh1Δ rnh201Δ strains was elevated relative to that in the rnh201Δ single-mutant strains. One interpretation of the lack of a hyperrec phenotype in the rnh1Δ mutant and the substantial hyperrec phenotype in the rnh201Δ mutant is that stimulation of recombination upon loss of RNase H2 is solely a consequence of misincorporated rNMPs. Two arguments suggest that this extreme hypothesis is not correct. First, since the hyperrec phenotype is stronger in the rnh1rnh201 double mutant than in the rnh1 mutant, and RNase H1 has no activity on single ribonucleotides, the hyperrec phenotype of the rnh201 strain must reflect, at least in part, some other type of lesion than single ribonucleotides. Second, if all of the recombination events in the rnh201Δ mutant reflect persistent rNMPs in DNA, then the hyperrec phenotype should be substantially reduced in an rnh201Δ pol2-M644L strain. Prior studies have demonstrated that the presence of the pol2-M644L allele reduces the level of rNMPs in genomic DNA ∼70% relative to a strain with wild-type Pol 2 activity (Nick McElhinny et al. 2010a). Although we did observe a small reduction in LOH in the rnh201Δ pol2-M644L strain relative to the rnh201Δ strain in one of our LOH assays, the reduction was only ∼20%. In a similar assay, the rate of LOH in an rnh201Δ strain was reduced less than twofold by the pol2-M644L mutation (Conover et al. 2015).
Based on subtle effect of the pol2-M644L mutation, we suggest that most of the LOH in the rnh201Δ mutant reflects persistent R-loops rather than persistent rNMPs. Our data indicate that RNase H2 can remove most, if not all, of the R-loops that accumulate in the absence of RNase H1, but that RNase H1 can remove only a relatively small fraction of the R-loops that accumulate in the rnh201Δ mutant. Why this particular division of labor might not have been evident in prior studies could reflect the monitoring of instability in much smaller genetic intervals and/or the examination of RNase H activity only under conditions of pathological R-loop accumulation. The ability of RNase H2 to fully compensate for loss of RNase H1 activity may also be related to the induction of RNase H2 in strains that lack RNase H1 (Arudchandran et al. 2000).
Is there any role of misincorporated rNMPs in stimulating LOH in diploids? Several types of data suggest that there is. First, as discussed above, Potenski et al. (2014) defined a pathway in which Top1 acts on rNMPs to produce recombinogenic lesions; a similar pathway produces mutagenic DNA lesions (Kim et al. 2011). Second, we observed a small reduction in LOH in the rnh201Δ pol2-M644L strain relative to the rnh201Δ strain; this reduction was statistically significant, however, only for the assay measuring the rate of LOH on chromosome IV (Figure 5). Altogether, our data support the conclusion that recombination events in strains lacking RNase H1 and H2 are primarily a consequence of R-loop formation, with misincorporated rNMPs playing only a minor role. This conclusion, however, is based on the assumption that the reduced incorporation of ribonucleotides resulting from the pol2-M644L-encoded DNA polymerase is not affected by the rnh201 mutation or other aspects of the genetic background in the rnh201pol2-M644L diploid. In addition, we assume that the rnh201pol2-M644L strain does not have an alteration (for example, a significantly slower S phase) that affects the likelihood that the repair template is the sister chromatid rather than the homolog.
It should also be noted that triple mutant combination of rnh1Δ rnh201Δ pol2-M644G is synthetically lethal, whereas the double mutant rnh201Δ pol2-M644G is viable (Lazzaro et al. 2012). Since the pol2-M644G allele encodes a form of Pol ε that incorporates increased levels of ribonucleotides (Nick McElhinny et al. 2010a), this result was interpreted as indicating a possible role of RNase H1 in the removal of ribonucleotides (Lazzaro et al. 2012), although other interpretations of the synthetic lethality are possible.
Cell-cycle timing of recombinogenic lesions in strains lacking RNase H1 and/or H2
Most of the proposed models for the recombinogenic effects of R-loops or misincorporated rNMPs predict a DNA lesion that leads to one broken chromatid in an S- or G2-phase cell. Such a lesion could be repaired by sister-chromatid exchange (no observable LOH) or by an interaction with the homolog that is expected to result in an associated 3:1 conversion event. In a wild-type strain, only ∼30% of sectored colonies have the 3:1 pattern, indicating that most LOH is initiated in G1 (St Charles and Petes 2013). Most of the events in the rnh1Δ rnh201Δ double-mutant strain had the S/G2 pattern, however, with 8 of the 10 sectored colonies having 3:1 conversion tracts. This result is consistent with the recombinogenic lesion resulting from an interaction of the replication fork with an R-loop. In contrast, in the rnh201Δ strain, seven of the nine events had 4:0 or 3:1/4:0 conversion tracts indicative of a G1-initiated event, and thus were similar to wild type. One interpretation of this result is that RNase H2 might be specialized to remove R-loops that give rise to recombinogenic lesions outside of S phase. Indeed, immunological data suggest that ∼30% of pathological R-loops exert their recombinogenic effect outside of S phase (Wahba et al. 2011). The transcription of RNH201 is elevated prior to S phase and continues at a high level in S (Pramila et al. 2006); the transcription of RNH1 is not periodic (Pramila et al. 2006). Alternatively, this result could reflect the production of a double-stranded DNA break reflecting the processing of rNMPs that are close (<10 bp) together on opposite strands of duplex DNA.
Distribution of recombination events associated with loss of RNase H activity
With the possible exception of the ribosomal RNA genes, no strong hotspots for LOH were observed in the subcultured rnh201Δ, rnh201Δ pol2-M644L, or rnh1Δ rnh201Δ strains (Figure S4, Figure S5, and Figure S6). In particular, we found no correlation of LOH breakpoints with a number of chromosome elements expected to be associated with R-loop formation such as highly transcribed genes, intron-containing genes, or G4-forming motifs. We also observed no strong correlation between recombination breakpoints in subcultured mutant strains and regions of R-loop accumulation. Finally, we found no association between regions of Rpb3p (a subunit of RNA polymerase II) accumulation and recombination breakpoints in the rnh201Δ, rnh201Δ pol2-M644L, or rnh1Δ rnh201Δ strains. These observations suggest that R-loop associated recombinogenic lesions are widely distributed throughout the yeast genome and/or that a number of different factors each contribute to the hyperrec phenotype of strains lacking RNase H activity.
Although several studies reported that Ty elements accumulated RNA:DNA hybrids in strains lacking RNase H activity (Chan et al. 2014; El Hage et al. 2014), we found no enrichment of Ty elements among LOH events. We did, however, find that deletion and duplication events frequently resulted from homologous recombination between nonallelic Ty elements (Table S6). It is difficult to assess the significance of this observation since Ty elements are the primary type of large dispersed repeats in the yeast genome. In addition, nonallelic recombination between Ty elements is a common source of chromosome rearrangements in mutant yeast strains that do not accumulate R-loops (McCulley and Petes 2010; Song et al. 2014). Several other relevant factors should be mentioned. First, our experiments were performed in diploid strains with both MATa and MATα information, and previous studies showed that Ty transcription is repressed in such diploids (Errede et al. 1980). Second, RNA:DNA hybrids associated with Ty elements primarily involve cDNA copies rather than the genomic elements (El Hage et al. 2014). Finally, it should be pointed out that our genetic assays are fundamentally different than the physical analysis of R-loop formation. To be detected by our LOH assays, the recombination event stimulated by R-loop formation must involve an interaction of the broken chromosome with the other homolog; breaks that are repaired by equal sister-chromatid recombination are genetically silent.
A significant enrichment of LOH events near the telomere was observed in the rnh201Δ strain. Subtelomeric regions encode a telomeric-repeat-containing RNA (TERRA) that accumulates in strains lacking RNase H2 (Yu et al. 2014). Strains with increased levels of telomeric RNA:DNA hybrids have elevated rates of telomere–telomere recombination (Yu et al. 2014). If elevated R-loops at the telomere cause a partial defect in telomere elongation by telomerase, there may be increased degradation of the ends of the chromosome, resulting in elevated levels of telomere-associated LOH. We note that Hackett and Greider (2003) previously showed an increase in terminal LOH in strains that had telomerase defects.
Summary
Our genome-wide analysis of instability associated with loss of RNase H in yeast shows that RNase H2 activity is much more important than RNase H1 activity in the maintenance of genome stability. However, strains that lack both RNase H1 and RNase H2 have qualitative and quantitative differences in genome stability relative to strains that lack only RNase H2. Our results suggest that R-loops contribute to most of the genetic instability of strains lacking RNase H activity, and that RNase H2 is uniquely able to process a subpopulation of R-loops. These results are relevant to human pathologies associated with defects in RNase H2 as well as the particular species of RNA:DNA hybrids that serve as the triggers for autoimmune disease. In the specific case of Aicardi-Goutiéres syndrome, recent work suggests that RNA:DNA hybrids are the likely immunogenic trigger of disease (Lim et al. 2015).
Supplementary Material
Acknowledgments
We thank J. L. Argueso and D. Koshland for communicating unpublished information and all members of the Petes and Jinks-Robertson labs for useful suggestions. The research was supported by National Institutes of Health grants GM24110 and GM52319 to T.D.P., and GM038464 and GM101690 to S.J.-R. K.O. was supported by a National Science Foundation graduate research fellowship (1106401).
Note added in proof: See Conover et al. 2015 (pp. 951–961) in this issue for a related work.
Footnotes
Communicating editor: N. Hollingsworth
Supporting information is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.115.182725/-/DC1.
Literature Cited
- Aguilera A., Garcia-Muse T., 2012. R loops: from transcription byproducts to threats to genome stability. Mol. Cell 46: 115–124. [DOI] [PubMed] [Google Scholar]
- Altman, D. G., 1991 Practical Statistics for Medical Research, Chapman and Hall/CRC Press, Boca Raton, FL. [Google Scholar]
- Arudchandran A., Cerritelli S., Narimatsu S., Itaya M., Shin D. Y., et al. , 2000. The absence of ribonuclease H1 or H2 alters the sensitivity of Saccharomyces cerevisiae to hydroxyurea, caffeine and ethyl methanesulphonate: implications for roles of RNases H in DNA replication and repair. Genes Cells 5: 789–802. [DOI] [PubMed] [Google Scholar]
- Azvolinsky A., Giresi P. G., Lieb J. D., Zakian V. A., 2009. Highly transcribed RNA polymerase II genes are impediments to replication fork progression in Saccharomyces cerevisiae. Mol. Cell 34: 722–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerritelli S. M., Crouch R. J., 2009. Ribonuclease H: the enzymes in eukaryotes. FEBS J. 276: 1494–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan Y. A., Aristizabal M. J., Lu P. Y., Luo Z., Hamza A., et al. , 2014. Genome-wide profiling of yeast DNA:RNA hybrid prone sites with DRIP-chip. PLoS Genet. 10: e1004288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen A. R., Lujan S. A., Burkholder A. B., Orebaugh C. D., Williams J. S., et al. , 2015. Tracking replication enzymology in vivo by genome-wide mapping of ribonucleotide incorporation. Nat. Struct. Mol. Biol. 22: 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover H., Lujan S., M. Chapman, D. Cornelio, R. Sharif et al. 2015 Stimulation of chromosomal rearrangements by ribonucleotides. Genetics 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow Y. J., Leitch A., Hayward B. E., Garner A., Parmar R., et al. , 2006. Mutations in genes encoding ribonuclease H2 subunits cause Aicardi-Goutiéres syndrome and mimic congenital viral brain infection. Nat. Genet. 38: 910–916. [DOI] [PubMed] [Google Scholar]
- El Hage A., Webb S., Kerr A., Tollervey D., 2014. Genome-wide distribution of RNA-DNA hybrids identifies RNase H targets in tRNA genes, retrotransposons and mitochondria. PLoS Genet. 10: e1004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errede B., Cardillo T. S., Sherman F., Dubois E., Deschamps J., et al. , 1980. Mating signals control expression of mutations resulting from insertion of a transposable repetitive element adjacent to diverse yeast genes. Cell 22: 427–436. [DOI] [PubMed] [Google Scholar]
- Fachinetti D., Bermejo R., Cocito A., Minardi S., Katou Y., et al. , 2010. Replication termination at eukaryotic chromosomes is mediated by Top2 and occurs at genomic loci containing pausing elements. Mol. Cell 39: 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günther C., Kind B., Reijns M. A., Berndt N., Martinez-Bueno M., et al. , 2015. Defective removal of ribonucleotides from DNA promotes systemic autoimmunity. J. Clin. Invest. 125: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett J. A., Greider C. W., 2003. End resection initiates genomic instability in the absence of telomerase. Mol. Cell. Biol. 23: 8450–8461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamperl S., Cimprich K. A., 2014. The contribution of co-transcriptional RNA:DNA hybrid structures to DNA damage and genome instability. DNA Repair (Amst.) 19: 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg Y., Benjamini Y., 1990. More powerful procedures for multiple significance testing. Stat. Med. 9: 811–818. [DOI] [PubMed] [Google Scholar]
- Ii M., Ii T., Mironova L. I., Brill S. J., 2011. Epistasis analysis between homologous recombination genes in Saccharomyces cerevisiae identifies multiple repair pathways for Sgs1, Mus81-Mms4 and RNase H2. Mutat. Res. 714: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N., Huang S. Y., Williams J. S., Li Y. C., Clark A. B., et al. , 2011. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332: 1561–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh K. D., Balachander S., Hesselberth J. R., Storici F., 2015. Ribose-seq: global mapping of ribonucleotides embedded in genomic DNA. Nat. Methods 12: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro F., Novarina D., Amara F., Watt D. L., Stone J. E., et al. , 2012. RNase H and postreplication repair protect cells from ribonucleotides incorporated in DNA. Mol. Cell 45: 99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea D. E., Coulson C. A., 1949. The distribution of the numbers of mutants in bacterial populations. J. Genet. 49: 264–285. [DOI] [PubMed] [Google Scholar]
- Lee P. S., Petes T. D., 2010. Mitotic gene conversion events induced in G1-synchronized yeast cells by gamma rays are similar to spontaneous conversion events. Proc. Natl. Acad. Sci. USA 107: 7383–7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y. W., Sanz L. A., Xu X., Hartono S. R., Chédin F., 2015. Genome-wide DNA hypomethylation and RNA:DNA hybrid accumulation in Aicardi-Goutières syndrome. eLife 4 10.7554/eLife.08007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulley J. L., Petes T. D., 2010. Chromosome rearrangements and aneuploidy in yeast strains lacking both Tel1p and Mec1p reflect deficiencies in two different mechanisms. Proc. Natl. Acad. Sci. USA 107: 11465–11470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischo H. E., Gomez-Gonzalez B., Grzechnik P., Rondon A. G., Wei W., et al. , 2011. Yeast Sen1 helicase protects the genome from transcription-associated instability. Mol. Cell 41: 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick McElhinny S. A., Kumar D., Clark A. B., Watt D. L., Watts B. E., et al. , 2010a Genome instability due to ribonucleotide incorporation into DNA. Nat. Chem. Biol. 6: 774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick McElhinny S. A., Watts B. E., Kumar D., Watt D. L., Lundstrom E. B., et al. , 2010b Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc. Natl. Acad. Sci. USA 107: 4949–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenski C. J., Niu H., Sung P., Klein H. L., 2014. Avoidance of ribonucleotide-induced mutations by RNase H2 and Srs2-Exo1 mechanisms. Nature 511: 251–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F., Aguilera A., 2005. Impairment of replication fork progression mediates RNA polII transcription-associated recombination. EMBO J. 24: 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pramila T., Wu W., Miles S., Noble W. S., Breeden L. L., 2006. The Forkhead transcription factor Hcm1 regulates chromosome segregation genes and fills the S-phase gap in the transcriptional circuitry of the cell cycle. Genes Dev. 20: 2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijns M. A., Kemp H., Ding J., De Proce S. M., Jackson A. P., et al. , 2015. Lagging-strand replication shapes the mutational landscape of the genome. Nature 518: 502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Dominska M., Greenwell P. W., Petes T. D., 2014. Genome-wide high-resolution mapping of chromosome fragile sites in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 111: E2210–E2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Charles J., Petes T. D., 2013. High-resolution mapping of spontaneous mitotic recombination hotspots on the 1.1 Mb arm of yeast chromosome IV. PLoS Genet. 9: e1003434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Charles J., Hazkani-Covo E., Yin Y., Andersen S. L., Dietrich F. S., et al. , 2012. High-resolution genome-wide analysis of irradiated (UV and gamma-rays) diploid yeast cells reveals a high frequency of genomic loss of heterozygosity (LOH) events. Genetics 190: 1267–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L., Amon J. D., Koshland D., Vuica-Ross M., 2011. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol. Cell 44: 978–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. S., Kunkel T. A., 2014. Ribonucleotides in DNA: origins, repair and consequences. DNA Repair (Amst.) 19: 27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. S., Smith D. J., Marjavaara L., Lujan S. A., Chabes A., et al. , 2013. Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA. Mol. Cell 49: 1010–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y., Petes T. D., 2013. Genome-wide high-resolution mapping of UV-induced mitotic recombination events in Saccharomyces cerevisiae. PLoS Genet. 9: e1003894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T. Y., Kao Y. W., Lin J. J., 2014. Telomeric transcripts stimulate telomere recombination to suppress senescence in cells lacking telomerase. Proc. Natl. Acad. Sci. USA 111: 3377–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. The contents of File S1, Table S1, Table S2, Table S3, Table S4, Table S5, Table S6, Table S7, Table S8, Table S9, Table S10, Table S11, and Table S12, and Figure S1, Figure S2, Figure S3, Figure S4, Figure S5, Figure S6, and Figure S7 are described in the text. Microarray data are available at GEO with the accession number GSE73334.