Abstract
Background
Subclinical infections, manifest as biofilms, are considered an important cause of capsular contracture. Acellular dermal matrices (ADMs) are frequently used in revision surgery to prevent recurrent capsular contractures.
Objective
We sought to identify an association between capsular contracture and biofilm formation on breast prostheses, capsules, and ADMs in a tissue expander/implant (TE/I) exchange clinical paradigm.
Methods
Biopsies of the prosthesis, capsule, and ADM from patients (N = 26) undergoing TE/I exchange for permanent breast implant were evaluated for subclinical infection. Capsular contracture was quantified with Baker Grade and intramammary pressure. Biofilm formation was evaluated with specialized cultures, rtPCR, bacterial taxonomy, live:dead staining, and scanning electron microscopy (SEM). Collagen distribution, capsular histology, and ADM remodeling were quantified following fluorescent and light microscopy.
Results
Prosthetic devices were implanted from 91 to 1115 days. Intramammary pressure increased with Baker Grade. Of 26 patients evaluated, one patient had a positive culture and one patient demonstrated convincing evidence of biofilm morphology on SEM. Following PCR amplification 5 samples randomly selected for 16S rRNA gene sequencing demonstrated an abundance of suborder Micrococcineae, consistent with contamination.
Conclusions
Our data suggest that bacterial biofilms likely contribute to a proportion, but not all diagnosed capsular contractures. Biofilm formation does not appear to differ significantly between ADMs or capsules. While capsular contracture remains an incompletely understood but common problem in breast implant surgery, advances in imaging, diagnostic, and molecular techniques can now provide more sophisticated insights into the pathophysiology of capsular contracture.
Level of Evidence
Capsular contracture (CC) is the most common cause of implant failure in cosmetic and reconstructive breast surgery.1 Proposed mechanisms for CC continue to evolve, usually implicating an upstream inflammatory event leading to abnormal downstream collagen or myofibroblast deposition.2-10 While several CC studies investigated signaling pathways mediated by transforming growth factor-ß (TGF-ß),3 tumor necrosis factor-stimulated gene-6 (TSG-6),5 or leukotriene antagonist-mediated immunomodulation,8,11,12 others focused on the impact of subclinical infection or biofilms.13-21 An association between bacteria and CC is supported by studies implementing increasingly sophisticated culture techniques,19 recently accompanied by electron and confocal microcopy, and molecular biology.13,14,16,18 Techniques mitigating periprosthetic bacterial contamination that reduce the rate of CC support this association.14,22,23
Retrospective reviews and technique papers have suggested that acellular dermal matrices (ADMs) reduce CC in revision breast augmentation.24-26 In primates, ADMs contain less smooth muscle actin compared to capsule, suggesting that ADMs may provide a barrier to the host inflammatory response.27 In humans, ADMs are characterized by less granulation tissue, vascular proliferation, fibrosis, chronic inflammation, and fewer giant cells.28 The primary purpose of this prospective cross-sectional study was to identify biofilms on the surface of textured tissue expanders (TEs), ADMs, and the submuscular capsule at the time of implant exchange during prosthetic breast reconstruction. We hypothesized that patients with Baker Grade III or greater CC would be more likely to have a biofilm present than patients with lower Baker Grades. The secondary purpose of this study was to describe the cellular populations and collagen type in patients with various levels of CC. We hypothesized that patients with CC will demonstrate an increased cellularity, and an altered ratio of type I to type III collagen in both capsular tissue and incorporated ADM.2,29
METHODS
Patient Selection
Of 147 women undergoing exchange of a TE for a breast implant (TE/I) with authors (TMM, MMT) at the Washington University School of Medicine from Jan 2013 to June 2014, 63 were randomly selected for participation.30 Randomization was performed with software available online through www.randomizer.org30 (Wesleyan University, Middletown, Connecticut, USA).
Twenty-six patients who received radiation, chemotherapy, endocrine therapy, or who had a previous flap or soft tissue envelope so thin that a biopsy would cause contour deformity, were excluded. Twelve patients meeting inclusion criteria declined to participate. Twenty-five women meeting inclusion criteria who received their last expander fill 4 to 8 weeks prior to exchange were enrolled in this cross-sectional study. A patient with a frank Grade IV CC following breast augmentation with a textured implant was included as a positive control (n = 1). After randomization and exclusions,30 power analysis demonstrated that our sample size was sufficient based on the low risk of biofilm formation (1 in 26 patients), or effect size, for the cohort of patients evaluated. At the time of enrollment, clinical history, duration of implantation, device size, style, fill volume, and manufacturer were prospectively recorded. This study meets ethical guidelines for human research conduct and is approved by the Human Research Protection Office at the Washington University in St. Louis School of Medicine (Institutional Review Board # 201101959) and is also registered with clinicaltrials.gov (identification # NCT01060046).
Evaluation of Capsular Contracture
We chose to evaluate CC with both Baker Grade and applanation tonometry as clinically, Baker Grade is widely used to measure capsular contracture while applanation tonometry is used less frequently but may be a more sensitive and reproducible measure.31,32 On enrollment, these metrics were prospectively recorded for every patient.
Specimen Collection
Biopsies (∼2.5 cm2) were obtained from the submuscular capsule, TE shell, and ADM. A ∼1.5 × 0.5 cm segment was collected immediately in an anaerobic transport pack for culture and a 1.0 × 0.5 cm piece was placed in RNAlater (Sigma Co., St. Louis, MO) to evaluate the transcriptome. Remaining portions of the biopsy were aseptically maintained for live:dead stain, or stored per protocol for H&E staining, quantitative assessment of Type I and III collagen, scanning electron microscopy (SEM), and immunohistochemistry (IHC).33,34
EVALUATION FOR BIOFILM
Specimen Culture
Samples of the implant were coated in saline and vortexed for 30 s, while ADM and autologous capsules were minced. The resulting materials were plated on sheep's blood agar, chocolate agar, and pre-reduced Brucella blood agar. The blood and chocolate agar plates were incubated at 35°C in 5% carbon dioxide for 3 days and the Brucella plates incubated for 5 days at 35°C in anaerobic conditions.
PCR Amplification and Sequencing of Bacterial 16S rRNA Genes
Specimens from capsule, breast prosthesis, and ADM stored in RNAlater were evaluated with real-time quantitative polymerase chain reactions (qPCR) in all patients (n = 26). Further classification was limited to five randomly selected patients as a result of scant findings with qPCR.30 Fourteen PCR amplicons, representing all nine 16S variable regions, were constructed using the Fluidigm Access Array System (San Francisco, CA). Five ng/µl of cDNA were input into each reaction. The sample inlets consisted of 1× High Fidelity FastStart Reaction Buffer without MgCl2, 4.5 nM MgCl2, 5% DMSO (Roche), 200 µM PCR Grade Nucleotide Mix (Roche), 0.05 U/μL FastStart High Fidelity Enzyme Blend (Roche Diagnostics Corporation, Indianapolis, IN) 1× Access Array Loading Reagent (Fluidigm), 1 µL DNA, and water. The primers were added to the assay inlets at 200 nM forward and reverse primers with 1× Access Array Loading Reagent. Following PCR amplification, the samples were harvested on the BioMark HD system (Fluidigm). Each sample was harvested and indexed using unique 10 base pair sequences with 14 rounds of PCR to incorporate each index sequence. All samples were pooled into 48 sample libraries and cleaned using bead purification. The samples were loaded on a MiSeq instrument for sequencing (Illumina, San Diego, CA).
Sequencing Data Analysis
Of the 14 PCR amplicons sequenced, only reads from one amplicon that covers the 16S V1 and V2 variable regions were used for downstream analyses. Analysis of the V1-V2 region reads was performed using the QIIME pipeline.35 Open-reference operational taxonomic units (OTUs) were classified using a custom-built reference database created from 16S sequences contained in the NCBI set of full microbial genomes.36 Reads were clustered into OTUs by the open source software package Quantitative Insights into Microbial Ecology (QIIME) using the UCLUST clustering algorithm at a threshold of 97% similarity.37 Representative sequences for each OTU were classified taxonomically using the Ribosomal Database Project (RDP) classifier,38 using minimum confidence of 80% against the NCBI taxonomy for the custom database. Taxonomy was called to the suborder level. If a given order did not have an annotated suborder then the suborder was set to the order.
CHARACTERIZATION OF CELLULARITY AND COLLAGEN DISTRIBUTION
Several semi-quantitative methods of assessing capsular contracture histologically on the basis of cellularity,4,9,23,29,39 vascularity,39,40 fibrosis,39 or collagen distribution or orientation are reported.4,9,23,29 However, in the absence of a widely-recognized histologic grading system, we chose to study capsule cellularity and collagen distribution, which have previously been linked to capsular contracture, and about which we have recently reported.41
Histopathology: Remodeling Characteristics
Histological preparations from ADM specimens were evaluated based on six remodeling characteristics: cellular infiltration, cell type, host extracellular matrix (ECM) deposition, scaffold degradation, fibrous encapsulation, and neovascularization. The evaluating pathologist, independent of this study, was blinded as to the source and nature of the specimen. A single slide of each specimen was evaluated under light microscopy at 100× magnification using a previously-reported semi-quantitative scoring system.42-44 Scores ranged from 0 to 3, with higher scores representing more robust remodeling characteristics. A mean composite remodeling score was calculated from the six component remodeling scores for ADM remodeling. Specimens from the submuscular breast capsule of each patient were evaluated for cellularity, cell type, and vascularity using the same scoring system.
Histopathology: Immunohistochemistry and Fluorescent Imaging
Prior to dehydration, prosthetic ADM and TE specimens from five randomly selected patients were placed in phosphate buffered solution (PBS) and immediately evaluated for viable bacteria using a live:dead stain (Abcam, Cambridge, MA).30 Samples (0.5 cm2) were rinsed in 4% PBS, and stained with cell permeable (Ex/Em 488/515 nm) and nonpermeable dyes (Ex/Em 488/615 nm) per manufacturer protocol. When ready for IHC analysis, specimens stored at −80°C were sectioned (∼5-10 µm), blocked with 5% normal goat serum and diluted with 0.3% Triton-X in PBS for 1 h. Nuclei were labeled with DAPI and anti-human smooth muscle actin monoclonal antibody using a TRITC-conjugated goat anti-rabbit IgG as the secondary antibody (Life Technologies, Grand Island, NY). Sections were evaluated with an Olympus BX-51 fluorescent microscope and an Olympus FV1000 spectral scanning confocal microscope with a multi-line 458, 488, and 515 nm laser as well as 405 nm, 561 nm, and 633 nm lasers. (Olympus Corporation, Melville, NY).
Histopathology: Collagen Distribution
Sirius Red/Fast Green (SR/FG) stained specimens were prepared and evaluated as we have previously reported to differentiate Type I (red) from Type III (green) collagen.34,43-45 Slides were photographed under cross-polarized light using an Axioskop 40® microscope and Axiocam® camera (Carl Zeiss®, Thornwood, NY) at 400× magnification (n = 10 photographs per specimen). Axiovision 4.7® (Zeiss®) software was used to determine the areas (µm2) occupied by Type I and III collagen and calculate the collagen I:III ratio.
Scanning Electron Microscopy (SEM)
Specimens oriented with methylene blue and a microsuture at the time of biopsy were received in 4% paraformaldehyde and 0.01% glutaraldehyde in Sorensen's buffer. Specimen processing consisted of a rinse in buffer, DDI water, followed by post-fixing in 1% osmium tetroxide for 1 h and dehydration in graded concentrations of ethanol. Specimens were processed in a Tousimis Samdri-780a critical point dryer, and sputter coated with gold-palladium alloy using a Tousimis Samsputter 2a (Rockville, MD). SEM was performed with a Hitachi S2600 (Schaumburg, IL) with 15-20 kV accelerating voltage. Specimens were initially scanned for irregularities at low magnification and then at least five separate locations (all four corners and center) of each specimen were systematically imaged. Areas with heterogeneous morphology were also analyzed.
Statistical Analysis
Mean and standard deviation or median and interquartile range (IQR) were calculated when appropriate based on nature and normality. A two-tailed Wilcoxon signed-rank test or Mann-Whitney U test compared ordinal or non-normally distributed variables between groups. The Chi-square test analyzed differences of race and a two-tailed independent samples t-test was used to analyze age differences between the two types of ADM. Alpha was set a-priori at 0.05. Statistics were performed using IBM SPSS Statistics version 22 software (Armonk, NY, USA) and were reviewed by an independent biostatistician.
RESULTS
Patient Characteristics
Patients ranging in age from 40 to 64 (49.6 ± 5.5) underwent mastectomy for either cancer prophylaxis or to treat ductal carcinoma in situ or a stage Ia invasive ductal carcinoma. Their demographics are summarized in Table 1. All breast prostheses were textured, and the ADM used was Alloderm - Ready to Use (RTU), or Regenerative Tissue Matrix (RTM; LifeCell Corporation, Branchburg, New Jersey). In one case, Seri Scaffold (Allergan; Irvine, CA) biological mesh was used instead of an ADM. Duration of implantation ranged from 91 to 1115 days (Figure 1, Table 2) thus providing sufficient time for biofilm formation.16,46,47 Baker Grade, applanation tonometry, and duration of implantation by type of ADM are reported in Table 2. Median alloimplant duration was significantly greater for Alloderm RTM than RTU (P < 0.001), attributable to the more longstanding availability of the RTM product. Age, race, applanation tonometry score, and Baker Grade did not differ significantly based on alloimplant type.
Table 1.
Summary of Patient Characteristics*
| Age in years | 49.6 ± 5.5 (40-64) |
| BMI (kg/m2) | 27.0 ± 3.0 (21.9-33.8) |
| Race | |
| White | n = 18 (69%) |
| Black | n = 6 (23%) |
| Asian | n = 2 (8%) |
| Duration implanted (days) | 172 [141.75, 574.5] |
| Tissue expander type | |
| Allergan 133MV | n = 20 (80%) |
| Allergan 133MX | n = 5 (20%) |
| Culture results | n = 1 (4%) Staphylococcus lugdunensis |
| n = 25 (96%) No growth | |
*Data are reported as Median ± SD (range), Median [Interquartile Range], or n (%) as appropriate.
Figure 1.
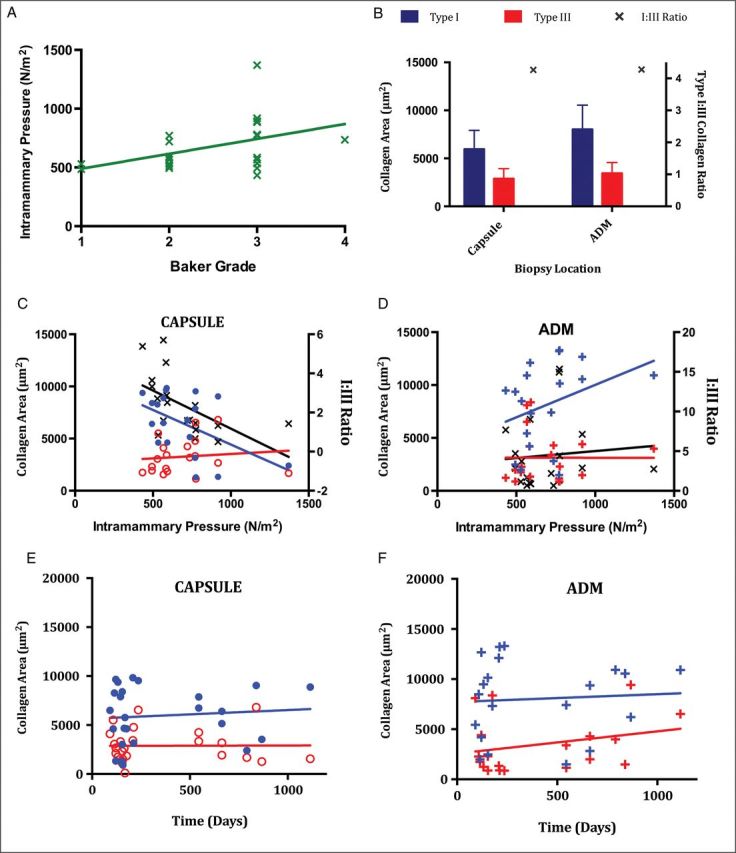
(A) Baker Grade (1 to 4) was assessed utilizing the modification published by Spear for breast reconstruction,31 and was correlated with applanation tonometry used to calculate intramammary pressure (N/m2).32 Intramammary pressure (green “x”) is plotted against clinical Baker Grade. (B) Pooled mean Type I and III collagen values and I:III collagen ratio. Type I collagen is blue, Type III collagen is red, and “x” represents the I:III collagen ratio. (C) Capsular Type I and III collagen as a function of intramammary pressure. (D) ADM Type I and III collagen as a function of intramammary pressure. (E) Capsular Type I and III collagen area versus duration of implantation. (F) ADM Type I and III collagen area versus duration of implantation. Each data point is derived from a mean of values from 10 sample fields taken from the same biopsy specimen. Type I collagen in capsule (filled blue circle), Type I collagen in ADM (blue “+”), Type III collagen in capsule (empty red circle), Type III collagen in ADM (red “+”), I:III collagen ratio (black “x”). Straight lines represent best-fit linear regression plots for variables including Type I collagen (blue line), Type III collagen (red line), and ratio (black line).
Table 2.
Patient Characteristics by Alloimplant Typea
| Alloderm (RTU) | Alloderm (RTM) | Seri Surgical Scaffold | P-value | |
|---|---|---|---|---|
| Number of patients | 16 (64%) | 8 (32%) | 1 (4%) | _ |
| Age | 49.13 ± 5.22 | 51.88 ± 6.27 | 46 | 0.23 |
| Race | 0.441 | |||
| White | 10 (63%) | 6 (75%) | 1 (100%) | |
| Black | 4 (25%) | 2 (25%) | ||
| Other | 2 (12%) | |||
| Implant duration (days) | 154 [121, 208] | 663 [545, 791] | 153 | <0.001 |
| Applanation tonometry (N/m2) | 567 [510, 780] | 733.5 [721, 774] | 775 | 0.130 |
| Baker grade | 3 [2, 3] | 3 [2, 3] | 3 | 0.336 |
aData are reported as Median [Interquartile Range], or n (%) as appropriate. P < 0.05 statistically significant.
EVALUATION FOR BIOFILM
Molecular and Microbiology
While 77 of 78 cultures from minced and vortexed implant shell, ADM, and capsule biopsies demonstrated no growth, a single tissue expander shell rendered a positive culture for Staphylococcus luadunensis.48 Using bacterial 16S sequencing, the predominant taxa identified was suborder Micrococcineae of class Actinobacteria (Figure 2). Micrococcineae strains are aerobic gram-positive bacteria generally found in soil, sediment, and water environments. The low complexity of the samples and the fact that Micrococcineae is naturally found on the human skin suggests that the MiSeq reads were from contaminates.
Figure 2.
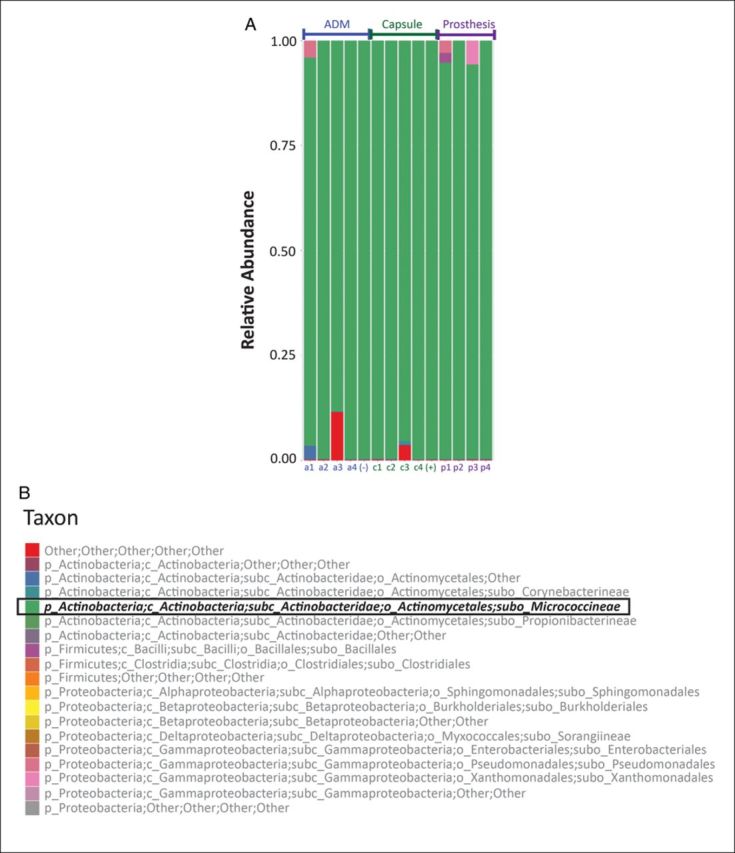
The relative abundance of each bacterial taxa in all sequenced samples color-coded by cluster (A). In all samples the majority taxa was suborder Micrococcineae of class Actinobacteria colored green (B). Samples from five patients are shown. Patients 1 through 4 contributed samples of ADM (a1-a4), capsule (c1-c4), and prosthesis (p1-p4). A frank CC specimen contains prosthesis and capsule together and serves as positive control (+). A fifth piece of nonimplanted Alloderm RTU served as a negative control piece of ADM (−).
Scanning Electron Microscopy
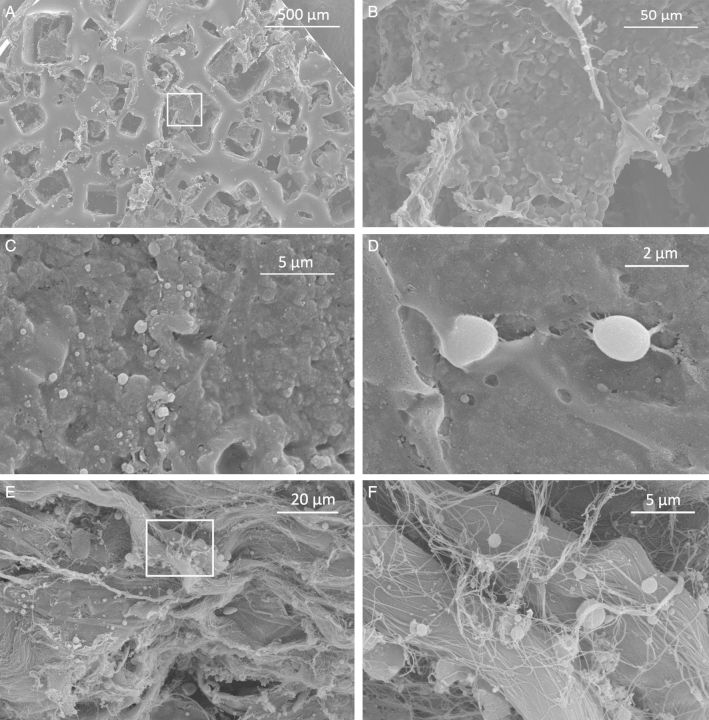
No evidence of biofilm formation was noted in 25 of 26 patients evaluated with SEM (Figure 3). Textured TEs demonstrate morphology consistent with Biocell® technology (Figure 3A). While regions of the textured surface were covered with an amorphous, lobulated substance, their size was not consistent with bacteria (Figure 3B). Submuscular biopsies were devoid of biofilms (Figure 3C), but it is feasible that some of these spheres are consistent with sporadic cocci (Figure 3D). ADMs were characterized by strands of collagen with variable thickness and orientation (Figure 3E), and infrequently, with sparse spheres that may represent persistent bacteria (Figure 3F).
Figure 3.
Representative scanning electron micrographs. (A) Textured surface (Biocell®) of an Allergan 133MV tissue expander (50× magnification). Panel B magnifies the contents of the white box. (B) Magnified view of amorphous material over surface of prosthesis. There is no evidence of structures whose size or morphology resemble bacteria (500× magnification). (C) Submuscular capsule devoid of biofilms (5000x magnification). (D) A ∼2 µm sphere appears to be adherent to submuscular capsule. This structure is smaller than a blood cell but larger than a typical bacteria (10,10,000× magnification). (E) ADM (Alloderm RTM) with numerous bundles of collagen. Panel F magnifies the contents of the white box (1000× mag). (F) with a few spheres <1 µm, but no discrete biofilm (5000× magnification).
Morphologic evidence suggestive of biofilm formation was noted in specimens derived from a single patient with Baker Grade III capsule, an intramammary pressure of 777 N/m2, and negative cultures (Figure 4). Spheres whose size and morphology were consistent with cocci appeared to congregate on both the capsule (Figure 4C and D) and ADM (Figure 4E and F) of this specimen. We did not identify any structures consistent with bacteria on the surface of this TE. A patient with a severe, Baker Grade 4 CC, 13 years following primary aesthetic breast augmentation, presented with an intramammary pressure of 945 N/m2 and negative cultures. SEM demonstrated collagen fibers of variable orientation, thickness, and density as well as both lymphocytes and macrophages, but no obvious biofilm in this positive control (Figure 5).
Figure 4.
Scanning electron microscopy strongly suggested biofilm formation in capsular, ADM, and prosthetic biopsies obtained from one patient. This patient had a Baker Grade III capsule, intramammary pressure of 777 N/m2, and negative cultures. (A) Submuscular capsule specimen demonstrates several macrophages (M) and crenated erythrocytes (cRBC) (1000× magnification). Panel B magnifies the contents of the white inset box. (B) Magnified inset shows lymphocytes (L) (5000× magnification). (C) Submuscular capsule with macrophages (M) and crenated erythrocytes (cRBC). Panel D magnifies the contents of the white inset box (1000× magnification). (D) At increased magnification, several spheres whose sizes (<1 µm) are consistent with cocci are apparent as well as a larger lymphocyte (L). These are interconnected by strands of material whose appearance may represent extracellular biofilm matrix (15,000× magnification). (E) ADM (Alloderm RTU) demonstrates a more uniform morphology disrupted by focal areas of heterogeneity including a macrophage (M). Panel F magnifies the contents of the white inset box (1000× magnification). (F) Magnified inset demonstrates focal areas where cocci appear to congregate. Early biofilm formation is feasible in this region. No bacteria where identified on the surface of the prosthesis in this subject. PCR demonstrated scant Micrococcineae in this specimen (5000× magnification).
Figure 5.
Scanning electron microscopy of a patient with clinically frank (Baker Grade IV) CC 13 years following breast augmentation. (A) Randomly distributed collagen bundles of variable thickness are identified (1000× magnification). Panel B magnifies the contents of the white inset box. (B) No biofilm, bacterial remnant, or individual bacteria noted at 5000× magnification. (C) Thickened capsular specimen devoid of obvious bacteria, notable for structures whose morphology and size are consistent with macrophages (M) (1000× magnification) and (D) with magnification, lymphocytes (L) (5000× magnification).
CHARACTERIZATION OF CELLULARITY AND COLLAGEN DISTRIBUTION
Light, Fluorescent, and Confocal Microscopy
Cell type, cellularity, and vascularity scores of capsular and ADM samples are reported in Table 3. Cellular infiltration scores were significantly greater in capsules (P < 0.05), but there were no differences in cell type or vascularity. Intramammary pressure increased significantly (P = 0.026) with Baker Grade (Figure 1A).
Table 3.
Histologic Grading of Submuscular Capsule and ADM Remodeling
| Histologic Criteria | Submuscular Capsule Median [IQR] | ADM Remodeling Median [IQR] | P-value |
|---|---|---|---|
| Cell type | 2.00 [2.00, 3.00] | 2.25 [2.00, 3.00] | 0.314 |
| Cellular infiltration | 3.00 [3.00, 3.00] | 3.00 [2.00, 3.00] | <0.02 |
| ECM deposition | 2.92 [2.00, 3.00] | ||
| Scaffold degradation | 2.19 [1.00, 3.00] | ||
| Fibrous encapsulation | 2.88 ± [2.00, 3.00] | ||
| Neovascularization | 3.00 [3.00, 3.00] | 3.00 [2.88, 3.00] | 0.286 |
| Mean composite | 2.64 ± [2.00, 3.00] |
P < 0.05 statistically significant.
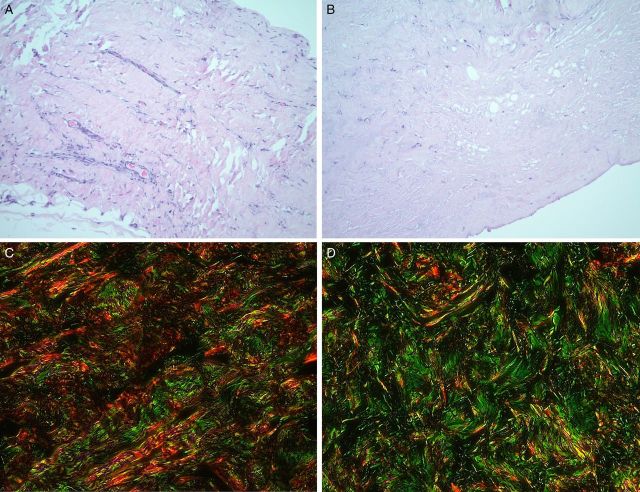
Type I collagen and the I:III ratio decreased with an increase in intramammary pressure in capsular tissue (Figure 1C) but not within the ADM (Figure 1D). Type I and III collagen content remained relatively stable in specimens biopsied at both early and late time points (Figures 1E and F, 3C and D), and the mean ratio of type I:III collagen remained the same (4.3:1) in biopsies harvested from the submuscular capsule and the ADM (Figures 1B, and 6C and D). Mean collagen content was higher overall in biopsied ADM than capsular specimens.
Figure 6.
Representative samples stained with H&E biopsied from (A). Submuscular capsule and (B) ADM. To assess collagen, samples from the (C) submuscular capsule and (D) ADM were labeled with Sirius Red (Type I collagen) and Fast Green (Type III collagen).
Live:dead stain failed to identify any viable cells in the processed prosthetic specimens (n = 5). Immunohistochemistry confirmed the presence of myofibroblasts within all evaluated capsules (n = 10). Qualitatively, DAPI-stained nuclei were densely distributed in capsular tissue, and less densely distributed in ADMs, with no staining on the breast implant surface. Semi-quantitative evaluation of H&E-stained biopsies from the capsule (Figure 6A) and ADM (Figure 6B) revealed collagen distributed in an organized fashion (Figure 6C and D) with 100 to 150 cells and 6 to 10 blood vessels per high-powered field (Table 3).
DISCUSSION
Our results show no correlation between biofilm formation and CC. This discrepancy may relate to how CC is defined, which in most studies, involves Baker Grade, applanation tonometry, or the breast augmentation classification.49-53 These metrics, however, are influenced not only by CC, but also the unique density and volume of the prosthesis, and the elasticity and surface area of the surrounding soft tissue envelope. For example, in two patients with histologically identical capsules, the one with pre-existing glandular ptosis who receives only a breast augmentation with a moderate profile saline implant will have a lower intramammary pressure compared to a patient with dense breasts who receives a high projecting cohesive gel implant with concomitant mastopexy. In this case, with the same capsule histologically, one may be a Baker 1 and the other a Baker 3. Baker Grade and applanation tonometry are still extremely useful, particularly when repeat measures of intramammary pressure are serially applied to the same breast in a longitudinal study design. However, in a cross-sectional study design when different breasts are compared to each other, additional variables are introduced that impact breast “firmness” that may or may not be the result of changes to the capsule itself. While Baker Grade in particular was not originally intended to evaluate the periprosthetic capsule around tissue expanders, we utilized this scale along with applanation tonometry in the absence of a more specific clinical grading system and also supplemented this recognized deficiency with a robust histologic analysis. The unintended widespread use of Baker Grade as a ubiquitous, subjective method to analyze capsular contracture is recognized, and its adaptation for classifying prosthetic breast reconstruction has been reported.31 More robust techniques for evaluating capsular contracture including clinical assessment with ultrasound elastography,54 computed tomography,55 or histologically have been described,2,9,29,39,56-58 but have yet to be widely adopted.
In our study, the presence of isolated cocci on SEM and the identification of the bacterial subclass Micrococcineae on bacterial taxonomy (Figure 2) suggest either sample contamination or the presence of persister cells. A dense population of bacteria in a biofilm will use quorum sensing to regulate gene expression and modulate virulence and antibiotic expression.59,60 Persister cells existing in or out of a biofilm are nonreplicating, possess intracellular mechanisms immune to antibiotic corruption,61 and require complex strategies to eradicate.62-64 The concentration and distribution of both persister cells and biofilms is dynamic.65 It is possible that biofilms escaped detection in our study because at the time of biopsy, causative organisms existed as antibiotic-tolerant persister cells with the capacity to form latent biofilms. Persister cells may also provide a mechanism by which biofilms can ultimately migrate to involve more widespread areas of the prosthesis surface and its adjacent capsule.
We studied the TE/I exchange paradigm to obtain a large number of human specimens from the submuscular capsule, ADM, and prosthesis at multiple time points post-implantation. Based on previous work, we anticipated identifying more biofilms than we found,66 particularly noting our use of textured expanders.18,66 Low bacterial yield resulting from our chosen methodologies or sampling bias may explain missing biofilms by SEM since <0.1% of the surface was imaged in this study. Previous evaluation of craniofacial miniplates,67 or breast implants in swine,16 assessed surface areas that were several orders of magnitude smaller, significantly reducing the impact of sampling bias. If biofilms were present in these capsules and they were simply missed due to sampling bias, it would suggest that bacteria may only require a sporadic or heterogeneous interaction with the capsule either physically or temporally to elicit a contracture.16,67
Some evidence has suggested that bacterial biofilms are a component of “the principal pathogenic pathway to development of CC”.13-15,17-19,21,58,66,68-77 Indirect evidence demonstrated lower CC rates with periprosthetic delivery of antibiotics via irrigation20,22 or an impregnated mesh.14 Deliberate inoculation of the periprosthetic pocket with bacteria leads to CC in animal models.14,16,18 In this study, however, specimens from only one of 26 patients demonstrated evidence of a microbial biofilm.
Confirming the presence of a biofilm—“a structured community of bacterial cells enclosed in a self-produced polymeric matrix and adherent to an inert or living surface”78—is challenging and cost-prohibitive for routine clinical practice. A multimodality or “full circle” approach originally described by Amann et al79 represents a more complete approach that includes imaging with electron or confocal microscopy to demonstrate “growth in place”80 of a biofilm morphologically,67,80-84 and verification of the presence, viability, and species of bacteria with techniques like PCR, coupled PCR-mass spectrometric (PCR-MS) assay,83,85 or fluorescent-in situ hybridization (FISH).83,85,86 Bacterial genomic DNA amplification techniques are extremely sensitive, but not specific for a biofilm since individual and even nonviable bacteria render a positive result. Sophisticated imaging techniques to identify bacterial biofilms have been reported in other clinical implantable device paradigms,67,87,88 but are limited (Table 4) in the human CC literature.13,15,17 Moreover, studies reporting the use of PCR or a live:dead stain to identify bacterial biofilms on breast implants are currently limited to studies in animal models.13,14,18 Sonication has been effectively utilized by many investigators to dislodge bacteria from a biofilm to increase the sensitivity of bacterial culture.19,75 By its very mechanism of action, however, sonication disrupts and converts biofilm-derived bacteria to a planktonic form and precludes confirmation of their biofilm origin with subsequent direct imaging techniques. Under optimized experimental conditions,89 sonication is an important adjunct to bacterial culture with widespread utility.19,75 However, PCR—as we have employed—is a more sensitive diagnostic modality,81,90,91 and reports that sonication can damage or alter bacterial growth remain.89,92 Since biofilms are not visually confirmed, the source of bacteria cultured following sonication, or evaluated with PCR, may include a biofilm or planktonic bacteria derived from the prosthesis or an environmental contaminate.81,92,93 In our study, it is feasible that relative to sonication, vortexing less effectively dislodged bacteria from the prosthesis, ADM, or capsule and reduced the sensitivity of our cultures. For this reason, we also used real-time PCR to increase the sensitivity of our detection, classify bacteria (Figure 2), and with the aid of live:dead stain, confirm viability. However, even if all of these other assays were positive, including implementation of sonication, in the absence of their direct visualization in a colony (Figures 3-5) the presence of biofilms still could not have been confirmed.
Table 4.
Focused Review of Methods Studies Have Used to Examine an Association Between Subclinical Infection and Capsular Contracture
| Study | Implant Host | Sample Size | Duration of Implantation | Clinical Method for Evaluating Capsule | Histologic Method for Evaluating Capsule | Culture Method | IHCa/Light Microscopy | SEMb | Molecular Biology |
|---|---|---|---|---|---|---|---|---|---|
| Pajkos et al15 | Human | 27 breasts, 19 Baker III/IV | 0.4 to 26 years (mean 9.2 years) | Baker Grade | No | Sonication, broth | No | Yes | No |
| Jacombs et al14 | Swine | 121 breasts | 19 wks | Baker Grade | No | Sonication, broth | Live:Dead stain | Yes | rtPCR, genomic DNA |
| Rieger et al19 | Human | 121 breasts | Augmentation: Mean 4.0 years (0.1 to
32); Reconstruction: Mean 3.0 months (1 to 6) |
Baker Grade | No | Sonication, broth | No | No | No |
| Tamboto et al16 | Swine | 51 breasts | 13 weeks | Baker Grade | No | Sonication, broth | No | Yes | No |
| Allan et al13 | Swine/Human | 6 swine implants; 1 human implant | 20 weeks (swine); rapidly developing Grade III (human) |
Baker Grade | No | Sonication, broth | No | Yes | Ica gene evaluation |
| Jacombs et al14 | Swine | 28 breasts | 16 weeks | Applanation Tonometry + Baker Grade | Contracted capsule had greater volume and mass and reduced surface area | Sonication, broth & PCR-based bacterial viability | No | Yes | Yes |
| Adams et al22 | Human | 330 breasts aesthetic augmentation; 44 breasts augmentation-mastopexy; 99 breasts reconstructions | 6 to 75 months | Baker Grade | No | Impact of antibiotic pocket irrigation study | No | No | No |
| Giordano et al20 | Human | 660 breasts (aesthetic augmentation through IMF incision) | 24 ± 13 months (no pocket irrigation); 22 ± 3 months (pocket irrigation) |
“Modified” Baker Grade | No | Impact of antibiotic pocket irrigation study | No | No | No |
| Marques et al29 | Rabbit | 31 rabbits | 4 weeks | Intracapsular Pressure of Implant + Baker Grade | Yes | Incubation in broth | No bacterial assessment. Capsule assessed. | No | No |
| Rieger et al75 | Human | 13 patients; 22 breasts | 10.4 years (0.25 to 30) | Baker Grade | No | Sonication, agar plates | No | No | No |
| Bergmann et al58 | Rat | 80 breasts | 60 days | No | Yes | Sonication, broth | No bacterial assessment. Capsule assessed. | No | No |
| Del Pozo77 | Human | 45 breasts | (+)Cap Con 16.4 (0.65-33.87) years (−)Cap Con 14.8 (0.46-24.49) years |
Baker Grade | No | Sonication, agar plates | No | No | No |
aIHC: immunohistochemistry. bSEM: scanning electron microscopy.
A causative relationship between biofilm formation and CC is difficult to prove.20 Bacteria can initiate an upstream inflammatory reaction by elaborating toxins or inciting an immune response, which may lead to fibrosis and CC.3 Whether biofilms impact the capsule locally or can influence remote capsular tissue with which they are not in contact is unknown. The species, concentration, and environmental triggers that cause a bacterial biofilm to trigger a CC rather than an unrelated immune response have not been described.1 Bacteria may form biofilms as a survival mechanism to ensure viability when exposed to hostile proinflammatory environments. It is therefore possible that stressful stimuli independently lead to both a biofilm and inflammation, rather than the biofilm causing the inflammation. Strong evidence shows that antimicrobial therapy induces biofilm formation in some clinical paradigms to confer resistance.94-96 Contrary to evidence favoring antibiotic pocket irrigation to attenuate CC rates,20,22 recent cohort studies comparing triple antibiotic to saline irrigation demonstrate no difference in CC rates.94,97,98 Elucidating a relationship between biofilm formation and CC merits further study.
ADMs may be more prone to biofilm formation as they are associated with more bacterial adhesion than proline or vicryl mesh.99 Still, ADMs remain a reported strategy to reduce the contractile properties of the periprosthetic pocket,26 and our study identified too few biofilms to reliably identify a relationship between biofilms, ADMs, and CC.
CONCLUSION
Our study does not refute an association between biofilms and CC. Rather, it shows that in a clinical setting, identifying a biofilm with multimodality methodology that includes direct imaging is challenging where sampling of a relatively large surface area may be prohibitive. Identification of a molecular mechanism by which potentially proinflammatory etiologies such as bacteria actually trigger contracture histopathologically is also required. Finally, it underscores the need for more specific clinical and histologic methodologies to differentiate capsular pathology from characteristics of the prosthesis or noncapsular soft tissue envelope that may also be influencing breast compliance. Modern advances in molecular biology and imaging continue to provide better tools to develop a more sophisticated understanding and characterization of CC.
Disclosures
Dr Myckatyn receives consulting, speaker, and research grant fees from LifeCell (Bridgewater, NJ) and Allergan (Irvine, CA), and consulting fees from Andrew Technologies (Wheeling, IL). Products from Allergan (breast prostheses) and LifeCell (acellular dermal matrices) were used in this study, but there was no industry funding or involvement with this study. Dr Tenenbaum receives consulting fees from Seintra (Santa Barbara, CA) and Andrew Technologies (Wheeling, IL). No products from these companies were used in this study. None of the other authors have anything to disclose.
Funding
This study was supported by a grant from the Aesthetic Surgery Education and Research Foundation to Dr Terence Myckatyn. These funds were used exclusively for specimen processing, laboratory costs, and assistance with statistical analysis.
Acknowledgements
We are very grateful to the Aesthetic Surgery Education Research Foundation, whose generous grant to Dr Myckatyn supported this study. The authors are also grateful to Dr Carey-Ann Burnham (Medical Director, Clinical Microbiology at the Washington University School of Medicine) for her expertise regarding cultures derived from breast implant and ADM prostheses. We are also grateful to Dr Andres Roma (Department of Anatomic Pathology, Cleveland Clinic, Cleveland, Ohio) for histologic grading of H&E stained biopsies of capsules and ADMs, and Ms. Piyaraj Newton (Division of Plastic and Reconstructive Surgery Research Laboratory, Washington University School of Medicine) for assistance with immunohistochemistry. We thank the Genome Technology Access Center in the Department of Genetics at Washington University School of Medicine for help with genomic analysis. The Center is partially supported by a NCI Cancer Center Support Grant (#P30 CA91842) to the Siteman Cancer Center and by an ICTS/CTSA Grant (# UL1TR000448) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Scanning electron microscopy was performed by Jaclynn Lett, Senior Research Technician in the Electron Microscopy Facility within the Research Center for Auditory and Vestibular Studies at Washington University School of Medicine. This facility is supported by the National Institutes of Health NIDCD Grant P30DC04665. This publication is solely the responsibility of the authors and does not necessarily represent the official view of NCRR or NIH.
REFERENCES
- 1.Adams WP., Jr. Capsular contracture: what is it? What causes it? How can it be prevented and managed? Clinics in Plastic Surgery. 2009;361:119-126, vii. [DOI] [PubMed] [Google Scholar]
- 2.Moyer KE, Ehrlich HP. Capsular contracture after breast reconstruction: collagen fiber orientation and organization. Plastic and Reconstructive Surgery. 2013;1314:680-685. [DOI] [PubMed] [Google Scholar]
- 3.Katzel EB, Koltz PF, Tierney R, et al. The impact of Smad3 loss of function on TGF-beta signaling and radiation-induced capsular contracture. Plastic and Reconstructive Surgery. 2011;1276:2263-2269. [DOI] [PubMed] [Google Scholar]
- 4.Marques M, Brown SA, Cordeiro ND, et al. Effects of fibrin, thrombin, and blood on breast capsule formation in a preclinical model. Aesthet Surg J. 2011;313:302-309. [DOI] [PubMed] [Google Scholar]
- 5.Tan KT, Baildam AD, Juma A, Milner CM, Day AJ, Bayat A. Hyaluronan, TSG-6, and inter-alpha-inhibitor in periprosthetic breast capsules: reduced levels of free hyaluronan and TSG-6 expression in contracted capsules. Aesthet Surg J. 2011;311:47-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.San-Martin A, Dotor J, Martinez F, Hontanilla B. Effect of the inhibitor peptide of the transforming growth factor beta (p144) in a new silicone pericapsular fibrotic model in pigs. Aesthetic Plastic Surgery. 2010;344:430-437. [DOI] [PubMed] [Google Scholar]
- 7.Zimman OA, Toblli J, Stella I, Ferder M, Ferder L, Inserra F. The effects of angiotensin-converting-enzyme inhibitors on the fibrous envelope around mammary implants. Plastic and Reconstructive Surgery. 2007;1207:2025-2033. [DOI] [PubMed] [Google Scholar]
- 8.D'Andrea F, Nicoletti GF, Grella E, et al. Modification of cysteinyl leukotriene receptor expression in capsular contracture: Preliminary results. Annals of Plastic Surgery. 2007;582:212-214. [DOI] [PubMed] [Google Scholar]
- 9.Wolfram D, Rainer C, Niederegger H, Piza H, Wick G. Cellular and molecular composition of fibrous capsules formed around silicone breast implants with special focus on local immune reactions. Journal of Autoimmunity. 2004;231:81-91. [DOI] [PubMed] [Google Scholar]
- 10.Siggelkow W, Faridi A, Spiritus K, Klinge U, Rath W, Klosterhalfen B. Histological analysis of silicone breast implant capsules and correlation with capsular contracture. Biomaterials. 2003;246:1101-1109. [DOI] [PubMed] [Google Scholar]
- 11.Bastos EM, Neto MS, Alves MT, et al. Histologic analysis of zafirlukast's effect on capsule formation around silicone implants. Aesthetic Plastic Surgery. 2007;315:559-565. [DOI] [PubMed] [Google Scholar]
- 12.Grella E, Grella R, Siniscalco D, et al. Modification of cysteinyl leukotriene receptors expression in capsular contracture: follow-up study and definitive results. Annals of Plastic Surgery. 2009;632:206-208. [DOI] [PubMed] [Google Scholar]
- 13.Allan JM, Jacombs AS, Hu H, Merten SL, Deva AK. Detection of bacterial biofilm in double capsule surrounding mammary implants: findings in human and porcine breast augmentation. Plastic and Reconstructive Surgery. 2012;1293:578e-580e. [DOI] [PubMed] [Google Scholar]
- 14.Jacombs A, Allan J, Hu H, et al. Prevention of biofilm-induced capsular contracture with antibiotic-impregnated mesh in a porcine model. Aesthet Surg J. 2012;327:886-891. [DOI] [PubMed] [Google Scholar]
- 15.Pajkos A, Deva AK, Vickery K, Cope C, Chang L, Cossart YE. Detection of subclinical infection in significant breast implant capsules. Plastic and Reconstructive Surgery. 2003;1115:1605-1611. [DOI] [PubMed] [Google Scholar]
- 16.Tamboto H, Vickery K, Deva AK. Subclinical (biofilm) infection causes capsular contracture in a porcine model following augmentation mammaplasty. Plastic and Reconstructive Surgery. 2010;1263:835-842. [DOI] [PubMed] [Google Scholar]
- 17.Deva A, Chang LC. Bacterial biofilms: a cause for accelerated capsular contracture? Aesthet Surg J. 1999;192:130-133. [Google Scholar]
- 18.Jacombs A, Tahir S, Hu H, et al. In vitro and in vivo investigation of the influence of implant surface on the formation of bacterial biofilm in mammary implants. Plastic and Reconstructive Surgery. 2014;1334:471e-480e. [DOI] [PubMed] [Google Scholar]
- 19.Rieger UM, Mesina J, Kalbermatten DF, et al. Bacterial biofilms and capsular contracture in patients with breast implants. The British Journal of Surgery. 2013;1006:768-774. [DOI] [PubMed] [Google Scholar]
- 20.Giordano S, Peltoniemi H, Lilius P, Salmi A. Povidone-iodine combined with antibiotic topical irrigation to reduce capsular contracture in cosmetic breast augmentation: a comparative study. Aesthet Surg J. 2013;335:675-680. [DOI] [PubMed] [Google Scholar]
- 21.van Heerden J, Turner M, Hoffmann D, Moolman J. Antimicrobial coating agents: can biofilm formation on a breast implant be prevented? Journal of Plastic, Reconstructive & Aesthetic Surgery: JPRAS. 2009;625:610-617. [DOI] [PubMed] [Google Scholar]
- 22.Adams WP, Jr., Rios JL, Smith SJ. Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. Plastic and Reconstructive Surgery. 2006;118:(7 Suppl):46S-52S. [DOI] [PubMed] [Google Scholar]
- 23.Marques M, Brown SA, Rodrigues-Pereira P, et al. Animal model of implant capsular contracture: effects of chitosan. Aesthet Surg J. 2011;315:540-550. [DOI] [PubMed] [Google Scholar]
- 24.Salzberg CA, Ashikari AY, Koch RM, Chabner-Thompson E. An 8-year experience of direct-to-implant immediate breast reconstruction using human acellular dermal matrix (AlloDerm). Plastic and Reconstructive Surgery. 2011;1272:514-524. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell GP, Gabriel A. Use of the acellular dermal matrix in revisionary aesthetic breast surgery. Aesthet Surg J. 2009;296:485-493. [DOI] [PubMed] [Google Scholar]
- 26.Namnoum JD, Moyer HR. The role of acellular dermal matrix in the treatment of capsular contracture. Clinics in plastic surgery. 2012;392:127-136. [DOI] [PubMed] [Google Scholar]
- 27.Stump A, Holton LH, 3rd, Connor J, Harper JR, Slezak S, Silverman RP. The use of acellular dermal matrix to prevent capsule formation around implants in a primate model. Plastic and Reconstructive Surgery. 2009;1241:82-91. [DOI] [PubMed] [Google Scholar]
- 28.Basu CB, Leong M, Hicks MJ. Acellular cadaveric dermis decreases the inflammatory response in capsule formation in reconstructive breast surgery. Plastic and Reconstructive Surgery. 2010;1266:1842-1847. [DOI] [PubMed] [Google Scholar]
- 29.Marques M, Brown SA, Cordeiro ND, et al. Effects of coagulase-negative staphylococci and fibrin on breast capsule formation in a rabbit model. Aesthet Surg J. 2011;314:420-428. [DOI] [PubMed] [Google Scholar]
- 30.Urbaniak GC, Plous S. Research Randomizer [computer program]. Version 4.02013.
- 31.Spear SL, Baker JL., Jr. Classification of capsular contracture after prosthetic breast reconstruction. Plastic and Reconstructive Surgery. 1995;965:1119-1123. discussion 1124. [PubMed] [Google Scholar]
- 32.Moore JR. Applanation tonometry of breasts. Plastic and Reconstructive Surgery. 1979;631:9-12. [DOI] [PubMed] [Google Scholar]
- 33.Hunter DA, Moradzadeh A, Whitlock EL, et al. Binary imaging analysis for comprehensive quantitative histomorphometry of peripheral nerve. Journal of Neuroscience Methods. 2007;1661:116-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cavallo JA, Gangopadhyay N, Dudas J, et al. Remodeling Characteristics and collagen distribution of biologic scaffold materials biopsied from postmastectomy breast reconstruction sites. Ann Plast Surg. 2015;751:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;75:335-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.NCBI. Full Microbial Genomes 2013. ftp://ftp.ncbi.nlm.nih.gov/genomes/Bacteria/ Accessed 2 Sept. 2013.
- 37.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;2619:2460-2461. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology. 2007;7316:5261-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plastic and Reconstructive Surgery. 2007;1201:275-284. [DOI] [PubMed] [Google Scholar]
- 40.Guijarro-Martinez R, Miragall Alba L, Marques Mateo M, Puche Torres M, Pascual Gil JV. Autologous fat transfer to the cranio-maxillofacial region: updates and controversies. Journal of cranio-maxillo-facial Surgery: Official Publication of the European Association for Cranio-Maxillo-Facial Surgery. 2011;395:359-363. [DOI] [PubMed] [Google Scholar]
- 41.Myckatyn TM, Cavallo JA, Sharma K, et al. The impact of chemotherapy and radiation therapy on the remodeling of acellular dermal matrices in staged, prosthetic breast reconstruction. Plastic and Reconstructive Surgery. 2015;1351:43e-57e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;8812:2673-2686. [DOI] [PubMed] [Google Scholar]
- 43.Cavallo JA, Greco SC, Liu J, Frisella MM, Deeken CR, Matthews BD. Remodeling characteristics and biomechanical properties of a crosslinked versus a non-crosslinked porcine dermis scaffolds in a porcine model of ventral hernia repair. Hernia. 2013. PubMed PMID: 23483265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cavallo JA, Roma AA, Jasielec MS, et al. Remodeling characteristics and collagen distribution in biologic scaffold materials explanted from human subjects after abdominal soft tissue reconstruction: an analysis of scaffold remodeling characteristics by patient risk factors and surgical site classifications. Surg Endosc. 2014;286:1852-1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown SR, Melman L, Jenkins E, et al. Collagen type I:III ratio of the gastroesophageal junction in patients with paraesophageal hernias. Surgical Endoscopy. 2011;255:1390-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cho H, Jonsson H, Campbell K, et al. Self-organization in high-density bacterial colonies: efficient crowd control. PLoS Biol. 2007;511:e302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bester E, Kroukamp O, Wolfaardt GM, Boonzaaier L, Liss SN. Metabolic differentiation in biofilms as indicated by carbon dioxide production rates. Applied and Environmental Microbiology. 2010;764:1189-1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Onyango LA, Hugh Dunstan R, Roberts TK, Macdonald MM, Gottfries J. Phenotypic variants of staphylococci and their underlying population distributions following exposure to stress. PloS one. 2013;810:e77614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bengtson BP, Van Natta BW, Murphy DK, Slicton A, Maxwell GP. Style 410 highly cohesive silicone breast implant core study results at 3 years. Plastic and Reconstructive Surgery. 2007;120(7 Suppl 1):40S-48S. [DOI] [PubMed] [Google Scholar]
- 50.Cunningham B, McCue J. Safety and effectiveness of Mentor's MemoryGel implants at 6 years. Aesthetic Plastic Surgery. 2009;333:440-444. [DOI] [PubMed] [Google Scholar]
- 51.Spear SL, Murphy DK, Allergan Silicone Breast Implant USCCSG. Natrelle round silicone breast implants: core study results at 10 years. Plastic and Reconstructive Surgery. 2014;1336:1354-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Niechajev I, Jurell G, Lohjelm L. Prospective study comparing two brands of cohesive gel breast implants with anatomic shape: 5-year follow-up evaluation. Aesthetic Plastic Surgery. 2007;316:697-710. [DOI] [PubMed] [Google Scholar]
- 53.Stevens WG, Nahabedian MY, Calobrace MB, et al. Risk factor analysis for capsular contracture: a 5-year Sientra study analysis using round, smooth, and textured implants for breast augmentation. Plastic and Reconstructive Surgery. 2013;1325:1115-1123. [DOI] [PubMed] [Google Scholar]
- 54.Prantl L, Englbrecht MA, Schoeneich M, Kuehlmann B, Jung EM, Kubale R. Semiquantitative measurements of capsular contracture with elastography - First results in correlation to Baker Score. Clin Hemorheol Microcirc. 2014;584:521-528. [DOI] [PubMed] [Google Scholar]
- 55.Katzel EB, Koltz PF, Tierney R, et al. A novel animal model for studying silicone gel-related capsular contracture. Plastic and Reconstructive Surgery. 2010;1265:1483-1491. [DOI] [PubMed] [Google Scholar]
- 56.Wilflingseder P, Hoinkes G, Mikuz G. Tissue reactions from silicone implant in augmentation mammaplasties. Minerva Chir. 1983;3812:877-880. [PubMed] [Google Scholar]
- 57.Bergmann PA, Liodaki ME, Mauss KL, et al. [Histological and immunohistochemical study of capsular contracture in an animal model—a comparison of two implants according to a modification of Wilflingseder's classification]. Handchirurgie, Mikrochirurgie, plastische Chirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft fur Handchirurgie : Organ der Deutschsprachigen Arbeitsgemeinschaft fur Mikrochirurgie der Peripheren Nerven und Gefasse 2012;444:220-226. [DOI] [PubMed] [Google Scholar]
- 58.Bergmann PA, Tamouridis G, Lohmeyer JA, et al. The effect of a bacterial contamination on the formation of capsular contracture with polyurethane breast implants in comparison with textured silicone implants: an animal study. Journal of Plastic, Reconstructive and Aesthetic Surgery: JPRAS. 2014;6710:1364-1370. [DOI] [PubMed] [Google Scholar]
- 59.Siehnel R, Traxler B, An DD, Parsek MR, Schaefer AL, Singh PK. A unique regulator controls the activation threshold of quorum-regulated genes in Pseudomonas aeruginosa. Proceedings of the National Academy of Sciences of the United States of America. 2010;10717:7916-7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh PK, Schaefer AL, Parsek MR, Moninger TO, Welsh MJ, Greenberg EP. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;4076805:762-764. [DOI] [PubMed] [Google Scholar]
- 61.Lewis K. Persister cells, dormancy and infectious disease. Nature Reviews. Microbiology. 2007;51:48-56. [DOI] [PubMed] [Google Scholar]
- 62.Wood TK, Knabel SJ, Kwan BW. Bacterial persister cell formation and dormancy. Applied and Environmental Microbiology. 2013;7923:7116-7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kint CI, Verstraeten N, Fauvart M, Michiels J. New-found fundamentals of bacterial persistence. Trends in Microbiology. 2012;2012:577-585. [DOI] [PubMed] [Google Scholar]
- 64.Vega NM, Allison KR, Khalil AS, Collins JJ. Signaling-mediated bacterial persister formation. Nature Chemical Biology. 2012;85:431-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature Reviews. Microbiology. 2004;22:95-108. [DOI] [PubMed] [Google Scholar]
- 66.Karau MJ, Greenwood-Quaintance KE, Schmidt SM, et al. Microbial biofilms and breast tissue expanders. BioMed Research International. 2013;2013:254940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jhass AK, Johnston DA, Gulati A, Anand R, Stoodley P, Sharma S. A scanning electron microscope characterisation of biofilm on failed craniofacial osteosynthesis miniplates. J Craniomaxillofac Surg. 2014;427:e372-e378. [DOI] [PubMed] [Google Scholar]
- 68.Kossovsky N, Heggers JP, Parsons RW, Robson MC. Acceleration of capsule formation around silicone implants by infection in a guinea pig model. Plastic and Reconstructive Surgery. 1984;731:91-98. [DOI] [PubMed] [Google Scholar]
- 69.Burkhardt BR, Fried M, Schnur PL, Tofield JJ. Capsules, infection, and intraluminal antibiotics. Plastic and Reconstructive Surgery. 1981;681:43-49. [DOI] [PubMed] [Google Scholar]
- 70.Shah Z, Lehman JA, Jr., Tan J. Does infection play a role in breast capsular contracture? Plastic and Reconstructive Surgery. 1981;681:34-42. [DOI] [PubMed] [Google Scholar]
- 71.Hurwitz PJ. Chronic infection as a possible cause of capsular contracture. Plastic and Reconstructive Surgery. 1987;793:504-505. [DOI] [PubMed] [Google Scholar]
- 72.Virden CP, Dobke MK, Stein P, Parsons CL, Frank DH. Subclinical infection of the silicone breast implant surface as a possible cause of capsular contracture. Aesthetic Plastic Surgery. 1992;162:173-179. [DOI] [PubMed] [Google Scholar]
- 73.Netscher DT, Weizer G, Wigoda P, Walker LE, Thornby J, Bowen D. Clinical relevance of positive breast periprosthetic cultures without overt infection. Plastic and Reconstructive Surgery. 1995;965:1125-1129. [DOI] [PubMed] [Google Scholar]
- 74.Aad G, Abbott B, Abdallah J, et al. Search for diphoton events with large missing transverse energy in 7 TeV proton-proton collisions with the ATLAS detector. Physical Review Letters. 2011;10612:121803. [DOI] [PubMed] [Google Scholar]
- 75.Rieger UM, Pierer G, Luscher NJ, Trampuz A. Sonication of removed breast implants for improved detection of subclinical infection. Aesthetic Plastic Surgery. 2009;333:404-408. [DOI] [PubMed] [Google Scholar]
- 76.Schreml S, Heine N, Eisenmann-Klein M, Prantl L. Bacterial colonization is of major relevance for high-grade capsular contracture after augmentation mammaplasty. Annals of Plastic Surgery. 2007;592:126-130. [DOI] [PubMed] [Google Scholar]
- 77.Del Pozo JL, Tran NV, Petty PM, et al. Pilot study of association of bacteria on breast implants with capsular contracture. J Clin Microbiol. 2009;475:1333-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;2845418:1318-1322. [DOI] [PubMed] [Google Scholar]
- 79.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiological Reviews. 1995;591:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nistico L, Hall-Stoodley L, Stoodley P. Imaging bacteria and biofilms on hardware and periprosthetic tissue in orthopedic infections. Methods Mol Biol. 2014;1147:105-126. [DOI] [PubMed] [Google Scholar]
- 81.Hall-Stoodley L, Stoodley P, Kathju S, et al. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunology and Medical Microbiology. 2012;652:127-145. [DOI] [PubMed] [Google Scholar]
- 82.Nistico L, Gieseke A, Stoodley P, Hall-Stoodley L, Kerschner JE, Ehrlich GD. Fluorescence “in situ” hybridization for the detection of biofilm in the middle ear and upper respiratory tract mucosa. Methods Mol Biol. 2009;493:191-213. [DOI] [PubMed] [Google Scholar]
- 83.Stoodley P, Conti SF, DeMeo PJ, et al. Characterization of a mixed MRSA/MRSE biofilm in an explanted total ankle arthroplasty. FEMS Immunology and Medical Microbiology. 2011;621:66-74. [DOI] [PubMed] [Google Scholar]
- 84.Stoodley P, Kathju S, Hu FZ, et al. Molecular and imaging techniques for bacterial biofilms in joint arthroplasty infections. Clinical Orthopaedics and Related Research. 2005437:31-40. [DOI] [PubMed] [Google Scholar]
- 85.Constantine RS, Constantine FC, Rohrich RJ. The ever-changing role of biofilms in plastic surgery. Plastic and Reconstructive Surgery. 2014;1336:865e-872e. [DOI] [PubMed] [Google Scholar]
- 86.Hoa M, Tomovic S, Nistico L, et al. Identification of adenoid biofilms with middle ear pathogens in otitis-prone children utilizing SEM and FISH. International Journal of Pediatric Otorhinolaryngology. 2009;739:1242-1248. [DOI] [PubMed] [Google Scholar]
- 87.McConoughey SJ, Howlin R, Granger JF, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiology. 2014;9:987-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kathju S, Nistico L, Tower I, Lasko LA, Stoodley P. Bacterial biofilms on implanted suture material are a cause of surgical site infection. Surgical Infections. 2014;155:592-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Monsen T, Lovgren E, Widerstrom M, Wallinder L. In vitro effect of ultrasound on bacteria and suggested protocol for sonication and diagnosis of prosthetic infections. J Clin Microbiol. 2009;478:2496-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Post JC, Preston RA, Aul JJ, et al. Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA. 1995;27320:1598-1604. [PubMed] [Google Scholar]
- 91.Hall-Stoodley L, Hu FZ, Gieseke A, et al. Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA. 2006;2962:202-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trampuz A, Piper KE, Jacobson MJ, et al. Sonication of removed hip and knee prostheses for diagnosis of infection. The New England journal of medicine. 2007;3577:654-663. [DOI] [PubMed] [Google Scholar]
- 93.Nistco L, Hall-Stoodley L, Stoodley P. Imaging bacteria and biofilms on hardware and periprosthetic tissue in orthopedic infections. New York: Humana Press; 2014. [DOI] [PubMed] [Google Scholar]
- 94.Hoffman LR, D'Argenio DA, MacCoss MJ, Zhang Z, Jones RA, Miller SI. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. 2005;4367054:1171-1175. [DOI] [PubMed] [Google Scholar]
- 95.Kaplan JB. Antibiotic-induced biofilm formation. Int J Artif Organs. 2011;349:737-751. [DOI] [PubMed] [Google Scholar]
- 96.Ghigo JM. Natural conjugative plasmids induce bacterial biofilm development. Nature. 2001;4126845:442-445. [DOI] [PubMed] [Google Scholar]
- 97.Drinane JJ, Bergman RS, Folkers BL, Kortes MJ. Revisiting Triple Antibiotic Irrigation of Breast Implant Pockets: A Placebo-controlled Single Practice Cohort Study. Plastic and Reconstructive Surgery. Global Open. 2013;17:e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drinane JJ, Kortes MJ, Bergman RS, Folkers BL. Evaluation of Antibiotic Irrigation Versus Saline Irrigation in Reducing the Long-Term Incidence and Severity of Capsular Contraction After Primary Augmentation Mammoplasty. Annals of Plastic Surgery. 2014. Epub ahead of print. PMID: 25144414. [DOI] [PubMed] [Google Scholar]
- 99.Liao EC, Nyame T, Lemon KP, Kolter R. High Throughput Assay for Bacterial Biofilm Formation on Biomaterials. Plastic and Reconstructive Surgery. 2009;124(4S):107. [DOI] [PMC free article] [PubMed] [Google Scholar]