Abstract
Background: A minority of women with urinary incontinence (UI) and even fewer with fecal incontinence (FI) report having discussed it with a health care provider in the past year. Thus our aim was to evaluate whether the use of an electronic pelvic floor assessment questionnaire (ePAQ-PF) improves communication about incontinence in primary care.
Methods: Women 40 years and older who were scheduled for an annual wellness physical at an internal medicine clinic between August 2007 and August 2008 were randomized to complete the ePAQ-PF prior to (n = 145) or after (n = 139) their visit. Clinicians of women in the intervention group received the ePAQ-PF report prior to the visit. Outcome measures from clinic note abstraction included mention of UI (primary) and FI. Participant-reported outcome measures included discussion of UI and FI and initiator of discussion.
Results: Discussions of UI was more common in the intervention group than the control group: (27% vs. 19%; odds ratio [OR], 1.6 95% confidence interval [95%CI] 0.9–2.8, particularly for women over 60 (33% vs. 12%; OR 3.8, 95%CI 1.2–11.8) and for women with UI (42% vs. 25%; OR 2.2, 95%CI 1.1–4.1). The intervention primarily led to an increase in clinician-initiated UI discussions which were more common in the intervention group (18% vs. 4%, OR 4.8, 95%CI 1.9–12.0) Participants in the intervention group more frequently reported discussion of FI (14% vs. 6%; OR 2.5, 95%CI 1.1–6.0) which was clinician initiated in over half the cases (9% vs. 3%; OR 3.5, 95%CI 1.1–11.0).
Conclusions: Use of the ePAQ-PF prior to clinic visits increases discussion of UI and FI, particularly clinician-initiated discussion. These findings suggest that such instruments may increase the detection and treatment of this often “silent” affliction.
Introduction
Urinary and fecal incontinence are important health problems that can strongly impact quality of life and lead to decreased work productivity and employment as well as increased social isolation.1 In population-based studies that examined UI occurring at least monthly, prevalence ranges from 25% to 45% in women over age 40 years.2–4 In active clinic populations, prevalence rates of UI are usually higher than population-based ones, with two studies reporting past month UI prevalence rates of over 50%5,6 and one study reporting a past week UI prevalence of 44%.7 Recent studies of monthly fecal incontinence (FI) range from 5.4% for women over 20 years to 10%–15% for women over 60 years.8–10 UI and FI are highly co-morbid, with 20% of women with UI also suffering from FI and 50% of women with FI also reporting UI.11
Patients often do not report or discuss their incontinence symptoms with a healthcare provider.12 Only 12%–53%13–18 of female patients with UI report having ever discussed UI with a healthcare provider. For patients with FI, the problem of underreporting is more severe. Only 10% of women report having spoken to a physician in the previous year,8 and 20% of patients report having ever spoken to a physician about FI.19 Even when patients with FI see a gastroenterologist, only 50% mention the symptom without being asked.20 In addition, primary care doctors screen for UI only 16% of the time,21 and 85%–90% of conversations about UI are initiated by the patient.15 Since there are effective treatment options for both UI and FI, this lack of discussion results in many patients suffering unnecessarily from incontinence. Even when treatments do not cure incontinence, they can improve quality of life by decreasing the frequency or volume of incontinence and by providing patients more effective methods of coping with the problem.
Because misconceptions about incontinence and its treatments are an important barrier to help seeking, some have proposed greater education for patients and clinicians to encourage screening. Educational interventions for patients appear to have limited impact on treatment seeking behavior, at least with short term follow-up.22 A 3-hour seminar on the Agency for Health Care Policy and Research Guidelines to Physicians also did not greatly impact screening rates.23 Focusing on educating patients and healthcare providers about incontinence does not address communication barriers or the systemic issues of time pressures, discomfort about discussing the problem, and mismatch of expectations between doctors and patients.
Recently there has been renewed interest in implementing and adopting health information technology to improve the quality of care. While most of the focus has been on electronic medical records, computerized interviewing is another way that health information technology can improve a patient's experience of care. Our goal was to evaluate whether an electronic pelvic floor questionnaire could increase communication about UI and FI in primary care. There is evidence that patients are more comfortable reporting incontinence on a self-report questionnaire than a face-to-face interview,24,25 and computerized interviewing has been tested in other stigmatizing conditions and found to be acceptable to patients26 who also may be more candid on computerized questionnaires.27
In designing an intervention to improve communication about UI and FI in primary care, we relied on the model of treatment decision making developed by Charles and Gafni28 and later adapted to agenda setting in primary care.29 While they conceive of an ideal model of a shared approach in which setting the visit agenda is negotiated between patient and clinician, they also recognize other models such as the paternalistic model in which the doctor is the primary person who decides on the visit agenda. We recognize that there are a range of practice styles of physicians (from more paternalistic to more patient centered), as well as a range of patient communication styles from passive (lets the clinician set the agenda) to active. Therefore, we wanted our intervention to improve communication regardless of the styles of the participants and clinicians. Thus we chose a questionnaire that provides an easy-to-read summary of a woman's incontinence symptoms. Giving the results to the clinician enables her to identify the problem when deciding on the agenda for the visit. By giving the results to the patient, we hoped to facilitate the patient placing incontinence on the visit agenda if it is important to her. Having the questionnaire results in hand may also help counter barriers such as patient embarrassment by giving them an alternative way to report symptoms.
Materials and Methods
Trial design
This was a stratified (by clinician) randomized (1:1 allocation ratio), parallel group study conducted in a single outpatient clinic.
Participants
Participants were women aged 40 years and older who were scheduled for a routine physical at an academically affiliated women's health internal medicine clinic in Wisconsin between August 9, 2007, and August 8, 2008. Main exclusion criteria were inability to speak English and inability to complete the questionnaire. The clinic sent letters (n = 1990) to women over 40 scheduled for an annual visit during this time frame informing them of the study. Only those who returned a response form prior to their appointment expressing interest in the study were assessed for eligibility.
Intervention
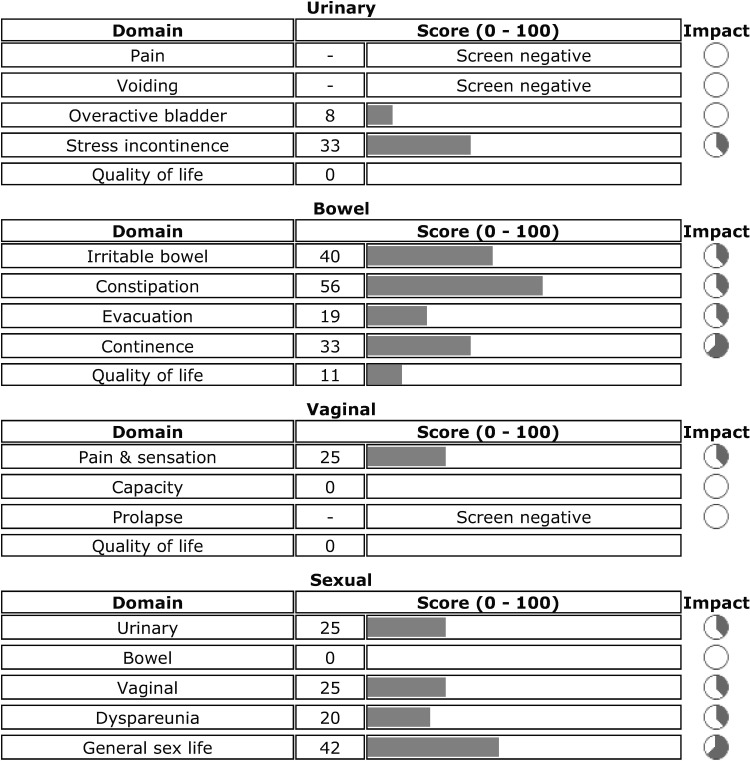
The electronic pelvic floor questionnaire (ePAQ-PF) was developed and validated in the United Kingdom using female subjects in primary and secondary care (urogynecology)30,31 to improve the assessment of pelvic floor disorders and to decrease the burden posed by paper based questionnaires.30 It was developed from existing validated paper-based instruments and refined through consultation with specialists in urology, gynecology, colorectal surgery, and sexual medicine.30 The ePAQ-PF assesses urinary (35 items maximum), bowel (33 items), vaginal (20 items), and sexual function (27 items) and uses an adaptive testing technique that will vary the number of items of the questionnaire depending on patient responses. The ePAQ-PF cover report lists severity scores for each symptom along with an indicator of how problematic the symptoms are for the patient and quality of life scores for each domain (Fig. 1). Thus, it is meant to enable the clinician to focus on the most bothersome symptoms of a patient and to save the clinician time.
FIG. 1.
Sample electronic pelvic floor questionnaire (e-PAQ-PF) output. Source: e-PAQ-PF output reproduced with permission of Dr. Stephen Radley.
Participants were randomized to complete the ePAQ-PF prior to or after their appointment. Participants in the intervention (pre-visit) group were asked to arrive 30 minutes prior to their appointment in order to complete the ePAQ-PF. After registering for their appointment and consenting to the study, they completed the ePAQ-PF. Their results were printed and copies were distributed to the clinician and participant. After their visit they completed the post-visit questionnaire. The control group was asked to stay 30 minutes after their appointment. They reviewed and signed the consent form prior to their appointment. After their appointment, they completed the ePAQ-PF and the post-visit questionnaire. The ePAQ-PF results were printed and a copy was given to the participant. All participants received the standard clinic intake paper forms, which included a question about urinary incontinence, prior to their visit.
Objectives and hypotheses
Our primary objective was to determine whether administration of the ePAQ-PF and dissemination of the results to patient and clinician prior to a routine clinic visit increases rates of UI discussion. Our secondary objective was to assess the impact on rates of FI discussion. We hypothesized that participants in the pre-visit ePAQ group would have higher rates of discussion of UI and/or FI compared with participants in the post-visit ePAQ group.
Outcomes
The primary outcome was mention of UI in the clinic note. Secondary outcomes included patient report of UI discussion and clinician-initiated discussion of UI. We also evaluated the impact of the ePAQ-PF on the treatment of UI counting referrals as treatment along with actual treatments as determined by reporting in the clinic note. Finally, we noted the percent of participants with UI and UI clinic note mention who reported no prior incontinence discussion and the frequency of assigning UI diagnostic codes for the visit in this subgroup. Similar outcomes were assessed for FI. We planned to perform this analysis for the whole sample, the age subgroups 40–59 years and 60 years and older, and for the subgroups reporting urinary or fecal incontinence. We used abstraction forms to standardize collection of chart data.
Sample size
Sample size calculations were based on estimates from the literature. We used a conservative estimate of monthly UI prevalence of 25%.3 We assumed that in the control arm, 25% of women with UI would mention it during the visit and that in the intervention group, and clinicians would see the ePAQ results at least 95% of the time. We assumed that clinicians would only record UI discussion 60% of the time based on reported frequency of UI treatment.16 Thus, we expected a 3.75% rate of UI mention in the clinic note for the control group and a 14.25% rate in the intervention group. The proposed total sample size of 234, or 117 patients per arm, provided 80% power to detect this difference using Pearson's chi-square test with a two-sided 5% level.
Randomization
Participants were randomized within strata defined by clinicians. The randomization list was computer generated using a permuted block design (n = 8).32 Participants were assigned sequentially to their clinician block in the order that their responses arrived. Participants were randomized prior to consent so they could be told whether they needed to come early or stay late after their appointment. The primary researcher—who was not a clinician in the clinic and thus knew nothing about the participants other than their name, contact information, and clinician—generated the sequence and allocated potential participants.
Blinding (masking)
Given the nature of the study, participants were not blinded, and clinicians were not blinded to participants in the intervention group. Clinicians were blinded to the identities of control participants. Although the primary chart abstractor had access to participant assignment, chart abstraction was performed without reference to the assignment. For quality control purposes, two additional chart abstractors who were blinded to randomization assignment abstracted a sample of the charts. With respect to the primary outcome, UI mention in the clinic note, agreement rates with the primary chart abstractor were 95% (39/41) and 98% (176/180) respectively.
Statistical methods
Baseline characteristics for both groups were summarized using mean and standard deviation for continuous variables and percentages for categorical variables. Rates of dichotomous outcome variables (e.g. UI mention in the clinic note) were compared between groups using Pearson's chi-square test. Unadjusted odds ratios were used as a relative measure of effect and were calculated using logistic regression. All analyses were performed on the intention to treat principle, including all subjects based on assigned group regardless of ePAQ completion status at the time of visit. A nominal two-sided p-value of 0.05 was regarded as statistically significant. All analyses were performed in SAS version 9.3 (SAS Institute Inc, Cary NC). To evaluate for the potential of clinician effect, we used the Mantel-Haenszel test statistic. We also performed a logistic regression analysis to evaluate whether results changed after controlling for covariates (age, severity score, quality of life score, impact score, number of visits to a healthcare provider in the past year, and length of time seeing clinician.)
The study was approved by the Health Sciences Institutional Review Board at the University of Wisconsin–Madison.
Results
Participant flow, recruitment, and participation rates
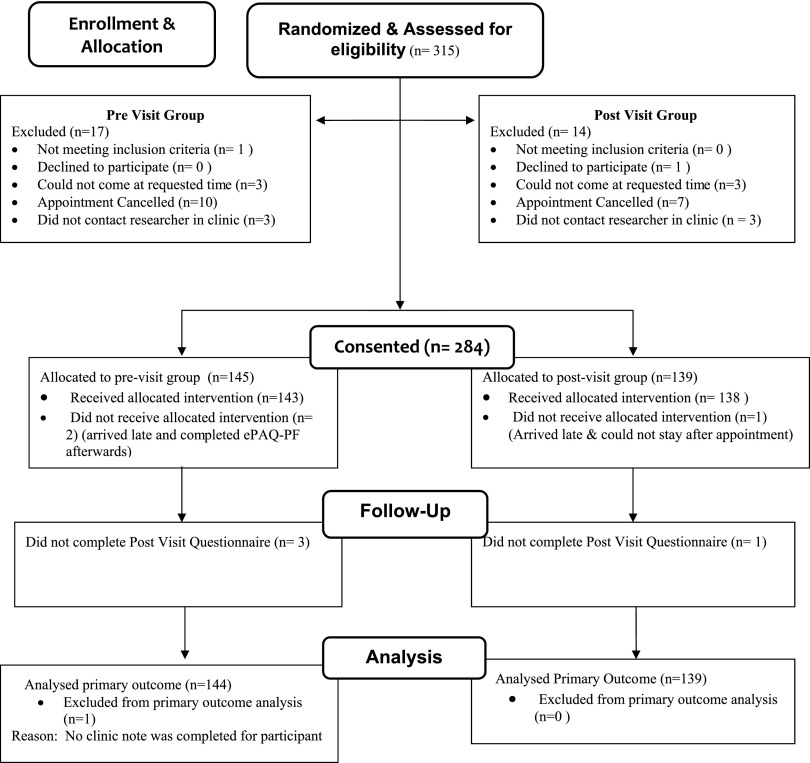
Recruitment letters were initially distributed in July 2007. Study visits occurred between August 9, 2007, and August 8, 2008; the last post visit questionnaire was received by August 15, 2008. Those who expressed interest prior to their scheduled appointment were randomized (n = 334). Participant flow is illustrated in Figure 2. (An additional 14 responses were received after the respondent's scheduled appointment and thus were not assessed for eligibility or randomized.) Potential participants were then contacted by telephone to describe the study and to confirm their participation. Nineteen women were never reached. Thus, 315 women were contacted and assessed for eligibility and of these, 97.5% (307) agreed to participate. Reasons for ineligibility included inability to speak/read English (1), inability to come at requested time (6), or refused (1). Of those agreeing to participate, 284 (92.5%) entered the study. Reasons for not participating after being confirmed were appointment cancelled (17) or failed contact between the researcher and subject at the clinic (6). The overall participation rate of those contacted was 90%.
FIG. 2.
Participant flow diagram.
Implementation
To avoid disrupting clinic workflow, when an intervention participant was called back prior to completing the full ePAQ, we instructed them to stop filling out the ePAQ-PF and partial results were printed and given to the participant and clinician. Overall, 99.6% (283) participants completed at least part of the ePAQ-PF, all of whom completed the urinary section and 98% (278) completed the entire bowel section. Two participants in the intervention group completed the entire ePAQ-PF after their appointment and one control participant did not complete the ePAQ-PF at all. If possible, participants who were unable to complete the ePAQ-PF prior to their appointment were asked to finish after the appointment, however, non-completion rates for the entire ePAQ-PF were still higher in the pre-visit group [12 (8%)] compared with the post-visit group [4 (3%)]. There were no adverse events in the study.
Baseline data
The demographics and visit characteristics of the two groups are shown in Table 1. Mean age of participants was 56 years (range 40–87). Participants were well educated, with the majority having attained a bachelor's or higher degree. The participants' UI and FI prevalence rates and other characteristics are shown in Table 2. Overall, when asked about incontinence in the past month, 64% reported UI, 15% reported FI, and 11% reported both UI and FI. In participants with past month incontinence, 78% of those with UI, 93% of those with FI, and 100% of those with double incontinence reported it to be a problem.
Table 1.
Demographic and Patient Characteristics Comparison
| Characteristic | ePAQ-PF intervention group | Control group | ||
|---|---|---|---|---|
| Age, mean ± SD | 144 | 56 ± 8.2 | 56 ± 9.8 | 139 |
| 40–60 years | 102 | 70% | 65% | 90 |
| ≥60 years | 43 | 30% | 35% | 49 |
| Income, n (%) | ||||
| <40 K | 9 | 6% | 8% | 11 |
| 40–80K | 37 | 26% | 30% | 41 |
| >80K | 81 | 57% | 51% | 70 |
| Refused | 15 | 11% | 10% | 14 |
| Highest education level, n (%) | ||||
| Less than bachelor's degree | 38 | 27% | 25% | 34 |
| Bachelor's degree | 43 | 30% | 36% | 49 |
| Masters, PhD, or professional | 61 | 43% | 40% | 55 |
| Married, n (%) | 113 | 80% | 72% | 100 |
| Lives alone, n (%) | 25 | 18% | 24% | 32 |
| New patient visit, n (%) | 10 | 7% | 10% | 14 |
| Years with MD/NP, mean ± SD | 140 | 6.1 ± 4.7 | 6.7 ± 5.3 | 136 |
| No. visits (any MD/NP) past year, mean ± SD | 142 | 2.8 ± 3.7 | 2.6 ± 2.8 | 137 |
ePAQ-PF, electronic pelvic floor assessment questionnaire; SD, standard deviation; MD/NP, doctor of medicine/nurse practitioner.
Table 2.
Baseline Incontinence Characteristics: Group Comparison
| Intervention | Control | |||
|---|---|---|---|---|
| Urinary incontinence characteristics | ||||
| UI past month, n (%) | 94 | 65% | 63% | 87 |
| UI duration >2 years, n (%) | 47 | 50% | 50% | 38 |
| SUI severity score, mean ± SD | 145 | 14.3 ± 13.72 | 12.5 ± 13.3 | 138 |
| SUI impact, n (%) | ||||
| Not a problem | 77 | 53% | 59% | 82 |
| A bit of a problem | 56 | 39% | 34% | 47 |
| Quite/serious problem | 12 | 9% | 7% | 9 |
| OAB severity score, mean ± SD | 145 | 12.2 ± 12.1 | 10.5 ± 12.2 | 138 |
| OAB impact, n (%) | ||||
| Not a problem | 65 | 45% | 49% | 68 |
| A bit of a problem | 64 | 44% | 43% | 59 |
| Quite/serious problem | 16 | 11% | 8% | 11 |
| Urinary quality of life score, mean ± SD | 145 | 8.7 ± 13.7 | 8.4 ± 14.1 | 138 |
| Pad use for UI, n (%) | ||||
| None | 96 | 66% | 70% | 96 |
| Yes (but no leakage) and occasional use | 30 | 21% | 17% | 23 |
| Most/all the time | 19 | 13% | 14% | 19 |
| UI previously discussed with healthcare provider, n (%) | ||||
| Within past year | 10 | 7% | 4% | 5 |
| Between 1 and 2 years ago | 17 | 12% | 12% | 17 |
| More than 2 years ago | 10 | 7% | 12% | 17 |
| Time unknown | 3 | 2% | 1% | 2 |
| Fecal incontinence characteristics | ||||
| FI past month n (%) | 20 | 14% | 15% | 21 |
| FI with liquids n (%) | 27 | 19% | 18% | 24 |
| FI with solids n (%) | 9 | 6% | 4% | 6 |
| Any FI n (%) | 29 | 20% | 17% | 24 |
| FI severity score, mean ± SD | 141 | 6.9 ± 12 | 6.3 ± 11.2 | 137 |
| FI impact, mean ± SD | ||||
| Not a problem | 102 | 72% | 70% | 96 |
| A bit of a problem | 21 | 15% | 20% | 28 |
| Quite/serious problem | 18 | 12% | 9% | 13 |
| Bowel quality of life score, mean ± SD | 140 | 6.7 ± 14.8 | 5.3 ± 11.9 | 137 |
| Pad use, n (%) | 0 | 0 | 2% | 5 |
| FI previously discussed with healthcare provider n (%) | ||||
| Within past year | 7% | 7% | 4% | 5 |
| Between 1 and 2 years ago | 2% | 2% | 1% | 2 |
| More than 2 years ago | 2% | 2% | 2% | 3 |
| Time unknown | 1% | 1% | 1% | 1 |
| Double incontinence (UI and FI) n (%) | 16 | 11% | 11% | 17 |
FI, fecal incontinence; OAB, overactive bladder; SUI, stress urinary incontinence.
Outcomes and estimation
Urinary incontinence
UI discussion rates as measured by mention in the clinic note (primary outcome) did not significantly differ between the two groups (27% vs. 19%, p = 0.09, Table 3) for the whole sample, but did for the subgroup with UI (42% vs. 25%, p = 0.02, Table 3). Patients in the intervention group had higher participant reported UI discussion rates (33% vs. 22%, p = 0.047), The ePAQ-PF intervention had a strong effect on clinician-initiated UI discussions for the overall group (18% vs. 4%, p = 0.0003) and the subgroup with UI (23% vs. 6%, p = 0.003). In the subgroup with UI, treatment rates were higher in the intervention group compared with the control group, but this was not statistically significant (25% vs. 15%, p = 0.10, Table 3.)
Table 3.
Impact of the ePAQ-PF Intervention on Urinary and Fecal Incontinence Discussion Rates and Treatment in the Full Sample and in Those Reporting Incontinence
| All participants | Past month incontinence | |||||
|---|---|---|---|---|---|---|
| ePAQ-PF intervention | Control | OR [95% CI] | ePAQ-PF intervention | Control | OR [95% CI] | |
| Urinary incontinence | ||||||
| All ages | n = 145 | n = 139 | n = 94 | n = 87 | ||
| Clinic note mentiona | 27% | 19% | 1.6 [0.9–2.8] | 42% | 25% | 2.2 [1.1–4.1]* |
| Discussed (patient report)b | 33% | 22% | 1.7 [1.0–2.9]* | 46% | 33% | 1.7 [0.9–3.1] |
| MD/NP askedc | 18% | 4% | 4.8 [1.9–12.1]*** | 23% | 6% | 4.9 [1.7–13.5]** |
| Treated/referreda | 16% | 11% | 1.7 [0.8–3.2] | 25% | 15% | 1.9 [0.9–4.0] |
| Age 40–59 | n = 101 | n = 90 | n = 64 | n = 54 | ||
| Clinic note mentiona | 26% | 23% | 1.1 [0.6–2.2] | 40% | 30% | 1.6 [0.8–3.4] |
| Discussed (patient report)b | 33% | 27% | 1.4 [0.7–2.6] | 48% | 41% | 1.3 [0.6–2.9] |
| MD/NP askedc | 19% | 7% | 3.2 [1.2–8.3]* | 25% | 9% | 3.2 [1.1–9.5]* |
| Treated/referreda | 20% | 11% | 2.0 [0.9–4.6] | 22% | 19% | 1.6 [0.6–3.9] |
| Age ≥60 years | n = 43 | n = 49 | n = 30 | n = 33 | ||
| Clinic note mention | 33% | 12% | 3.8 [1.2–11.8]* | 47% | 18% | 4.3 [1.3–14.1]* |
| Discussed (patient report) | 33% | 14% | 2.9 [1.0–8.1]* | 43% | 21% | 2.8 [0.9–8.6] |
| MD/NP asked | 16% | 0% | (p = 0.003)**,f | 20% | 0% | (p = 0.007)**,f |
| Treated/referred | 21% | 8% | 3.1 [0.9–10.8] | 30% | 9% | 4.3 [1.0–17.7]* |
| Fecal incontinence | ||||||
| All ages | n = 144 | n = 139 | n = 20 | n = 21 | ||
| Clinic note mentiona | 3% | 2% | 1.3 [0.3–5.9] | 20% | 10% | 2.1 [0.5–8.6] |
| Discussed (patient report)d | 14% | 6% | 2.5 [1.1–6.0]* | 35% | 15% | 3.5 [0.9–13.0] |
| MD/NP askede | 9% | 3% | 3.5 [1.1–11.0]* | 17% | 5% | 4.0 [0.4–42.4] |
| Treated/referreda | 1% | 1% | 1.0 [0.1–6.9] | 10% | 10% | 1.1 [0.1–8.3] |
p < 0.05; **p < 0.01; ***p < 0.001 (Pearson chi-squared).
Missing = 1.
Missing = 4.
Missing = 6.
Missing = 7.
Missing = 8.
Unable to calculate OR since reference is 0.
CI, confidence interval; NP, nurse practitioner; OR, odds ratio.
In the age subgroup analyses (Table 3), the ePAQ intervention had a greater effect on UI discussion rates in the older group (33% vs. 12%, p = 0.02), particularly older participants with UI (47% vs. 18%, p = 0.02). In the older control group there were no clinician-initiated discussions of incontinence compared to a 16% rate in the intervention group (p = 0.003). In older patients with UI, 30% in the intervention group were treated compared to only 9% in the control group (p = 0.03). In the younger group, the main effect of the intervention was on clinician-initiated discussions (19% vs. 7%, p = 0.02).
Of patient reported UI discussions, only 63% of them had mention of UI in their clinic visit note. Of participants with UI and mention of UI in the clinic note, 30% reported not having previously discussed UI with a healthcare provider, a percentage which did not differ between groups. In addition, 47% were assigned a diagnostic code for the visit. The percent of assigned diagnostic codes was also similar between groups. There was no evidence for more complete reporting of incontinence history in the clinic notes of intervention group participants with UI. . Similarly, 61% of participants with UI that was noted in the clinic note received treatment but the treatment rate also did not differ between groups.
Fecal incontinence
FI mention in the clinic note was rare (only 7 instances in total, 2 of which were newly diagnosed FI) and did not differ between the two groups (pre 3% vs. post 2%, p = 0.74, Table 3). However, participants in the pre-visit group were more likely to report any FI discussion (13% vs. 6%, p = 0.05) and clinician-initiated discussion of FI (9% vs. 3%, p = 0.02) than participants in the post-visit group (Table 3). When participants reported discussion of FI, it was documented in the clinic note 23% of the time.
ePAQ-PF evaluation
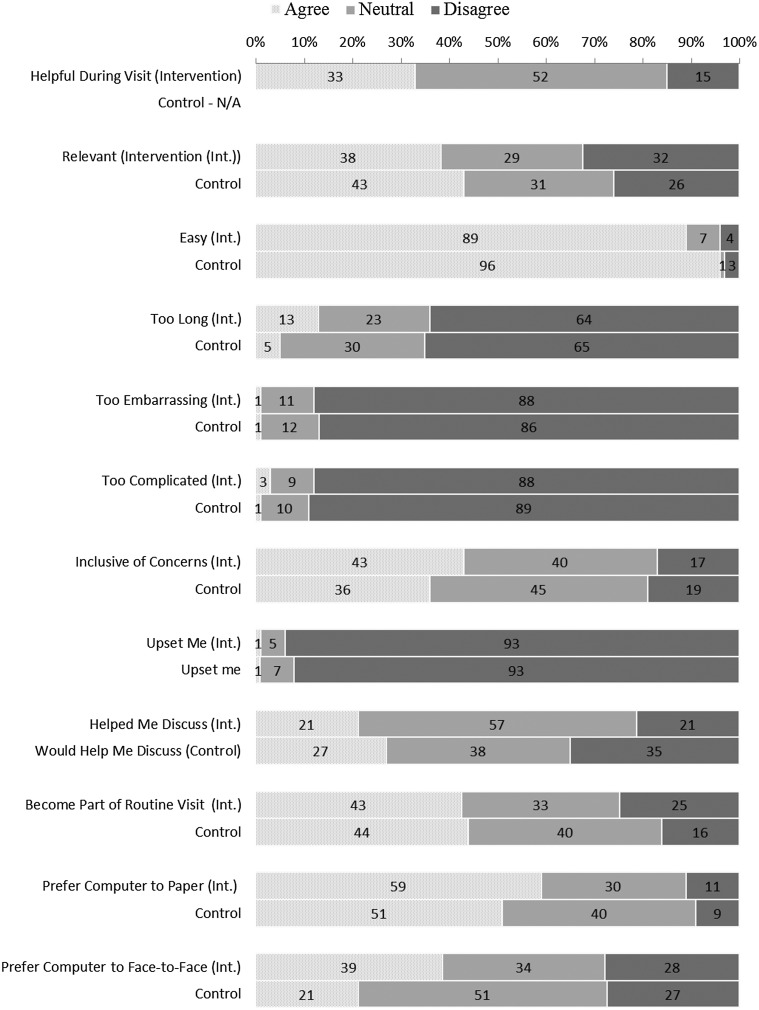
Results of the post-visit ePAQ-PF evaluation questionnaire are presented in Figure 3. About a third of intervention patients thought the ePAQ-PF was helpful during their visit. Most participants found the ePAQ-PF easy to complete and very few found it too complicated. The intervention group was slightly more likely to think the ePAQ was too long (13% pre-visit, 5% post-visit). Hardly any thought the ePAQ-PF was too embarrassing or too upsetting (1%). Almost half of participants in both groups agreed that the ePAQ-PF should be routinely used for primary care visits. The majority preferred computer to paper and the intervention group was more likely to prefer computer to face-to-face interview. Some participants commented that the ePAQ-PF helped them to discuss their problems with their clinician, whereas others commented that their clinician did not look at the results. The majority of clinicians found the ePAQ-PF helpful during the visit. One clinician did think it would need to be streamlined to be useful in primary care.
FIG. 3.
Participant evaluation of the ePAQ-PF.
Contamination and potential confounding
Nine (6%) of participants in the post-visit group reported that participation in the study influenced what they chose to discuss with their clinician. Out of these 9 participants, 6 had discussed UI with their clinician by self-report and 6 had mention of UI in the clinic note. None of these participants had discussed FI by either self-report or clinic note. We performed a sensitivity analysis excluding the 9 participants. After excluding them, there was an increased effect size for our primary outcome of UI discussion per clinic note (27% vs. 15%; OR 2.0, 95%CI 1.1–3.7) and our secondary outcomes of participant report of UI discussion (33% vs. 19%; OR 2.1, 95% CI 1.2–3.6), and clinician-initiated discussion (OR 6.8, 95% CI 2.3–20). There was no impact on FI discussion outcomes.
Results did not substantially change when controlling for potential confounders using multivariate logistic regression. They also were not affected by controlling for clinician impact using the Mantel-Haenszel test.
Discussion
The major finding of our study is that administration of a computerized pelvic floor questionnaire prior to the visit increased rates of UI discussion compared to standard clinic procedures which included a paper health form which asked about UI. The strongest effect was on clinician-initiated discussions. Our primary outcome, however, discussion as measured by mention in the clinic note, was only significantly different in the subgroup with UI and in the older subgroup. Greater discussion of UI (as measured by clinic note mention) led to increased treatment and/or referral of participants with UI who were 60 and older. For fecal incontinence, our results suggest participants in the ePAQ-PF intervention group were more likely to report FI discussion and that the clinician was more likely to initiate the conversation. However, despite a strong effect size, our study lacked statistical power to demonstrate this definitively due to low numbers of participants with FI.
It is notable that our intervention was more effective in increasing discussion rates (per clinic note mention) in the older age group which had lower incontinence discussion rates in the control group than those aged 40–60 years. It is particularly striking that none of the older control group participants reported clinician-initiated discussion. It may be particularly important for clinicians to inquire about incontinence in older women who may not see incontinence as a medical problem because of the misconception that incontinence is a normal part of aging. The higher rates of clinic note mention of UI in the older subgroup with UI translated to a significant difference in treatment rates between the two groups in this subgroup. That our intervention led to these improvements is important since other studies have shown that active treatment of older women with urinary incontinence leads to greater improvement of UI symptoms, including severity and number of episodes compared to standard care.33
Our study is a single site study and our study participants were predominantly white and well educated which may limit the generalizability of our results to other populations. Furthermore, all our clinicians were female, and it is not known whether our results generalize to male clinicians.
Given the high prevalence of incontinence in our study population, it is likely that we had an enriched sample since it is logical that women with incontinence would be more likely to volunteer for a study on assessing incontinence. This does not affect the internal validity of our results. It does explain to a large extent why our estimations for UI mention in the entire sample is higher than predicted given that the prevalence of UI in our sample was 2.6× the conservative estimate we used. In addition, if our participants were more comfortable discussing incontinence than nonparticipants; this may have reduced the effect of our intervention.
A small percentage of control participants answered affirmatively to the question of whether participation in the study affected whether they discussed with their physician. Those that did and who had UI mentioned in the clinic note comprised of 22% of control UI discussants and this reduced our effect size and further contributed to our elevated UI mention rate in control patients (compared with what we estimated).
Clinician contamination is unlikely given the small number of study participants compared to their entire patient load. Moreover, clinicians were blinded to which of their patients were control participants. There was no evidence that the performance of the study in the clinic led to increased incontinence screening in general or that discussion rates increased over time. Any clinician contamination effect would reduce the effect of our intervention.
The strongest effect was seen on clinician initiation of discussion, although the strength of this association varied among clinicians. Given the low number of clinicians in our study, we could not fully characterize clinician characteristics associated with greater responsiveness to the questionnaire. In questioning clinicians about their use of the results, one-third looked at them more than half the time, one-third only when the patient asked, and one-third less than half the time or never.
While our intervention significantly increased the rates of incontinence discussion among participants with UI, we still observed that slightly less than half of participants who rated their UI to be a problem discussed it with their clinician. This may in part be because our intervention cannot fully address the problem of clinic visit time constraints as well as the issue of competing health problems and how incontinence is prioritized in relationship to them. More research needs to be done to more fully understand these barriers and how to optimally address them.
Use of a computerized pelvic floor questionnaire may integrate well with other quality improvement initiatives. In the United States, urinary incontinence in women 65 years and older is one of the Medicare Physician Quality Reporting Systems measures.34 Since the ePAQ-PF does save clinician time in history taking, it has the potential to be an important tool to meet these performance goals. In addition to the clinic version evaluated in our study, there is now a web version that patients can access at home. For those patients with internet access but inability to spend extra time at the clinic because of time constraints (a common reason for nonparticipation in our study), the web version may improve access to the questionnaire and further reduce clinic burden. Evaluating the effectiveness of the web version to increase discussion rates is another important direction for future study. More work needs to be done, however, on how best to integrate such a questionnaire into primary care. This could include selected screening such as women over 65 years or women after childbirth; using it as part of assessing incontinence or for primary care follow-up after pelvic floor procedures and making it available to patients (perhaps as one of number of assessments a patient could choose from based on their concerns).
Conclusions
Our study took a novel approach of evaluating a computerized electronic pelvic floor questionnaire through a randomized controlled trial. Administration of the ePAQ-PF and provision of the results to both the clinician and patient increased rates of clinician-initiated incontinence discussion. The effect of the intervention on participants with incontinence was strongest in older women and translated to increased treatment rates in this subgroup. Use of a computerized questionnaire to gather history about incontinence has the potential to be a helpful tool for clinicians who wish to improve the recognition and treatment of incontinence in their practice.
Acknowledgments
We thank Dr. Nancy Fuller for her assistance with implementing the study in the clinic. We thank Dr. Stephen Radley for generously providing us the information on the ePAQ-PF including ePAQ-PF scoring algorithms and the ePAQ-PF evaluation questionnaire used in his validation studies. We also thank all the participants for generously giving their time for the study.
An Agency for Healthcare Research and Quality dissertation grant (R36 HS017028) and a Sigma Xi Society Grant-in-Aid of Research (G20079191130187097) to Dr. Sophia Miryam Schüssler-Fiorenza Rose provided financial support for the study. Revision of the manuscript was supported by the Office of Academic Affiliations Advanced Fellowship Program in Spinal Cord Injury Medicine and the Spinal Cord Injury Service of the Veteran Affairs Palo Alto Health Care System. The funders had no role in the design and conduct of the study, collection, management, analysis, interpretation of the data, or preparation, review, or approval of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fultz N, Girts T, Kinchen K, Nygaard I, Pohl G, Sternfeld B. Prevalence, management and impact of urinary incontinence in the workplace. Occup Med 2005;55:552–557 [DOI] [PubMed] [Google Scholar]
- 2.Melville JL, Katon W, Delaney K, Newton K. Urinary Incontinence in us women: A population-based study. Arch Intern Med. 2005;165:537–542 [DOI] [PubMed] [Google Scholar]
- 3.Thom DH, Nygaard IE, Calhoun EA. Urologic diseases in America project: Urinary incontinence in women-national trends in hospitalizations, office visits, treatment and economic impact. J Urol 2005;173:1295–1301 [DOI] [PubMed] [Google Scholar]
- 4.Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int 2011;108:1132–1138 [DOI] [PubMed] [Google Scholar]
- 5.Shaw C, Gupta RD, Bushnell DM, et al. The extent and severity of urinary incontinence amongst women in UK GP waiting rooms. Fam Pract 2006;23:497–506 [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein MM, Skelly J, Kaczorowski J, Swanson G. The incontinence quality of life instrument in a survey of primary care patients. J Fam Pract 2002;51:952. [PubMed] [Google Scholar]
- 7.Kinchen KS, Lee J, Fireman B, Hunkeler E, Nehemiah JL, Curtice TG. The prevalence, burden, and treatment of urinary incontinence among women in a managed care plan. J Womens Health 2007;16:415–422 [DOI] [PubMed] [Google Scholar]
- 8.Bharucha AE, Zinsmeister AR, Locke GR, et al. Prevalence and burden of fecal incontinence: A population-based study in women. Gastroenterology 2005;129:42–49 [DOI] [PubMed] [Google Scholar]
- 9.Melville JL, Fan MY, Newton K, Fenner D. Fecal incontinence in US women: A population-based study. Am J Obstet Gynecol 2005;193:2071–2076 [DOI] [PubMed] [Google Scholar]
- 10.Bharucha AE, Dunivan G, Goode PS, et al. Epidemiology, pathophysiology, and classification of fecal incontinence: State of the science summary for the National Institute of Diabetes and Digestive and Kidney Diseases (WIDDK) workshop. Am J Gastroenterol 2015;110:127–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts RO, Jacobsen SJ, Reilly WT, Pemberton JH, Lieber MM, Talley NJ. Prevalence of combined fecal and urinary incontinence: A community-based study. J Am Geriatr Soc 1999;47:837–841 [DOI] [PubMed] [Google Scholar]
- 12.Brown S, Gartland D, Perlen S, Mcdonald E, Macarthur C. Consultation about urinary and faecal incontinence in the year after childbirth: A cohort study. BJOG 2014:122:954–962 [DOI] [PubMed] [Google Scholar]
- 13.Sampselle CM, Harlow SD, Skurnick J, Brubaker L, Bondarenko I. Urinary incontinence predictors and life impact in ethnically diverse perimenopausal women. Obstet Gynecol 2002;100:1230–1238 [DOI] [PubMed] [Google Scholar]
- 14.Burgio KL, Ives DG, Locher JL, Arena VC, Kuller LH. Treatment seeking for urinary-incontinence in older adults. J Am Geriatr Soc 1994;42:208–212 [DOI] [PubMed] [Google Scholar]
- 15.Diokno AC, Sand PK, Macdiarmid S, Shah R, Armstrong RB. Perceptions and behaviours of women with bladder control problems. Fam Pract 2006;23:568–577 [DOI] [PubMed] [Google Scholar]
- 16.Melville JL, Newton K, Fan MY, Katon W. Health care discussions and treatment for urinary incontinence in US women. Am J Obstet Gynecol 2006;194:729–737 [DOI] [PubMed] [Google Scholar]
- 17.Roberts RO, Jacobsen SJ, Rhodes T, et al. Urinary incontinence in a community-based cohort: prevalence and healthcare-seeking. J Am Geriatr Soc 1998;46:467–472 [DOI] [PubMed] [Google Scholar]
- 18.Johnson TM, Kincade JE, Bernard SL, Busby-Whitehead J, Defriese GH. Self-care practices used by older men and women to manage urinary incontinence: Results from the National Follow-up Survey on Self-Care and Aging. J Am Geriatr Soc 2000;48:894–902 [DOI] [PubMed] [Google Scholar]
- 19.Johanson JF, Lafferty J. Epidemiology of fecal incontinence: The silent affliction. Am J Gastroenterol 1996;91:33–36 [PubMed] [Google Scholar]
- 20.Leigh RJ, Turnberg LA. Fecal incontinence—The unvoiced symptom. Lancet 1982;1:1349–1351 [DOI] [PubMed] [Google Scholar]
- 21.Cohen SJ, Robinson D, Dugan E, et al. Communication between older adults and their physicians about urinary incontinence. J Gerontol A Biol Sci Med Sci 1999;54:M34–M37 [DOI] [PubMed] [Google Scholar]
- 22.Van Eijken M, Wensing M, De Konink M, et al. Health education on self-management and seeking health care in older adults: A randomised trial. Patient Educ Couns 2004;55:48–54 [DOI] [PubMed] [Google Scholar]
- 23.Bland DR, Dugan E, Coben SJ, et al. The effects of implementation of the agency for health care policy and research urinary incontinence guidelines in primary care practices. J Am Geriatr Soc 2003;51:979–984 [DOI] [PubMed] [Google Scholar]
- 24.Khullar V, Damiano R, Toozs-Hobson P, Cardozo L. Prevalence of faecal incontinence among women with urinary incontinence. Br J Obstet Gynaecol 1998;105:1211–1213 [DOI] [PubMed] [Google Scholar]
- 25.Buckley B. It's the way you ask that matters: Comparison of data relating to prevalence of incontinence aid use from two surveys of people with multiple sclerosis. J Wound Ostomy Continence Nurs 2006;33:26–29 [DOI] [PubMed] [Google Scholar]
- 26.Bachman JW. The patient-computer interview: A neglected tool that can aid the clinician. Mayo Clin Proc 2003;78:67–78 [DOI] [PubMed] [Google Scholar]
- 27.Feigelson ME, Dwight SA. Can asking questions by computer improve the candidness of responding? A meta-analytic perspective. Consult Psychol J 2000;52:248–255 [Google Scholar]
- 28.Charles C, Gafni A, Whelan T. Decision-making in the physician-patient encounter: Revisiting the shared treatment decision-making model. Soc Sci Med 1999;49:651–661 [DOI] [PubMed] [Google Scholar]
- 29.Murray E, Charles C, Gafni A. Shared decision-making in primary care: tailoring the Charles et al. model to fit the context of general practice. Patient Educ Couns 2006;62:205–211 [DOI] [PubMed] [Google Scholar]
- 30.Radley SC, Jones GL, Tanguy EA, Stevens VG, Nelson C, Mathers NJ. Computer interviewing in urogynaecology: Concept, development and psychometric testing of an electronic pelvic floor assessment questionnaire in primary and secondary care. Br J Obstet Gynaecol 2006;113:231–238 [DOI] [PubMed] [Google Scholar]
- 31.Jones GL, Radley SC, Lumb J, Jha S. Electronic pelvic floor symptoms assessment: Tests of data quality of EPAQ-PF. Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1337–1347 [DOI] [PubMed] [Google Scholar]
- 32.Dallal GE. Randomization plans: Randomizing subjects to a single treatment. Available at www.tufts.edu/∼gdallal/assign.htm Accessed January12, 2015
- 33.Visser E, De Bock GH, Messelink EJ, et al. Active encouragement of older women with urinary incontinence in primary care to undergo diagnosis and treatment: A matched-pair cluster randomized controlled trial. Maturitas 2015;80:212–219 [DOI] [PubMed] [Google Scholar]
- 34.Centers for Medicare and Medicaid Services. 2015 PQRS Measures List. 2015. Available www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/Downloads/PQRS_2015_Measure-List_111014.zip Accessed January12, 2015