Abstract
Varicella zoster virus lies dormant in the dorsal root ganglia after symptomatic chicken pox infection, usually in childhood. If the virus reactivates in the trigeminal ganglia, it can cause varicella zoster ophthalmicus, which can have severe ocular complications. We report a case of a 73-year-old woman in severe immunosuppression due to treatment with mycophenolate mofetil, glucocorticosteroids and a tumor necrosis factor alpha inhibitor. The reactivation caused superior orbital fissure syndrome, which has only rarely been described in relation to varicella zoster virus reactivation. In our case, the syndrome was seen along with severe encephalitis.
Key Words: Varicella zoster virus, Varicella zoster ophthalmicus, Immunosuppression, Tumor necrosis factor alpha inhibitor, Superior orbital fissure syndrome, Ophthalmoplegia, Orbital apex syndrome, Herpes zoster
Background
The incidence of varicella zoster virus (VZV) reactivation is increased in immunosuppressed patients, and reactivation of herpes zoster is a common adverse event reported in clinical trials with tumor necrosis factor alpha inhibitors (TNF-alpha) [1].
Varicella zoster ophthalmicus (VZO) is caused by reactivation of latent virus in the trigeminal ganglion, and ocular complications may include blepharitis, keratoconjunctivitis, iritis, scleritis and acute retinal necrosis [2]. Another ophthalmological complication is ophthalmoplegia, which can be seen in relation to VZO, and there is a slight increase in simultaneous aseptic meningitis when VZO is accompanied by ophthalmoplegia [3].
Superior orbital fissure syndrome (SOFS) is rare in relation to VZO, but a closely related entity named orbital apex syndrome (OAS) has been correlated with the occurrence of VZO; however, only in a few cases [2,4]. In SOFS, there is no lesion to the optic nerve in contrast to OAS, in which the optic nerve is compromised, leading to reduced visual acuity [5].
Case Presentation
A 73-year-old woman with active pyoderma gangrenosum was treated with both mycophenolate mofetil and glucocorticoids for years. Due to worsening of her skin condition, she began a series of treatments with infliximab, a TNF-alpha inhibitor. She received 400 mg intravenous treatment on two occasions 14 days apart. Approximately 4 weeks after her last treatment, she started complaining of severe, right-sided, retrobulbar pain and was evaluated by an ophthalmologist. The examination only revealed slight periorbital edema.
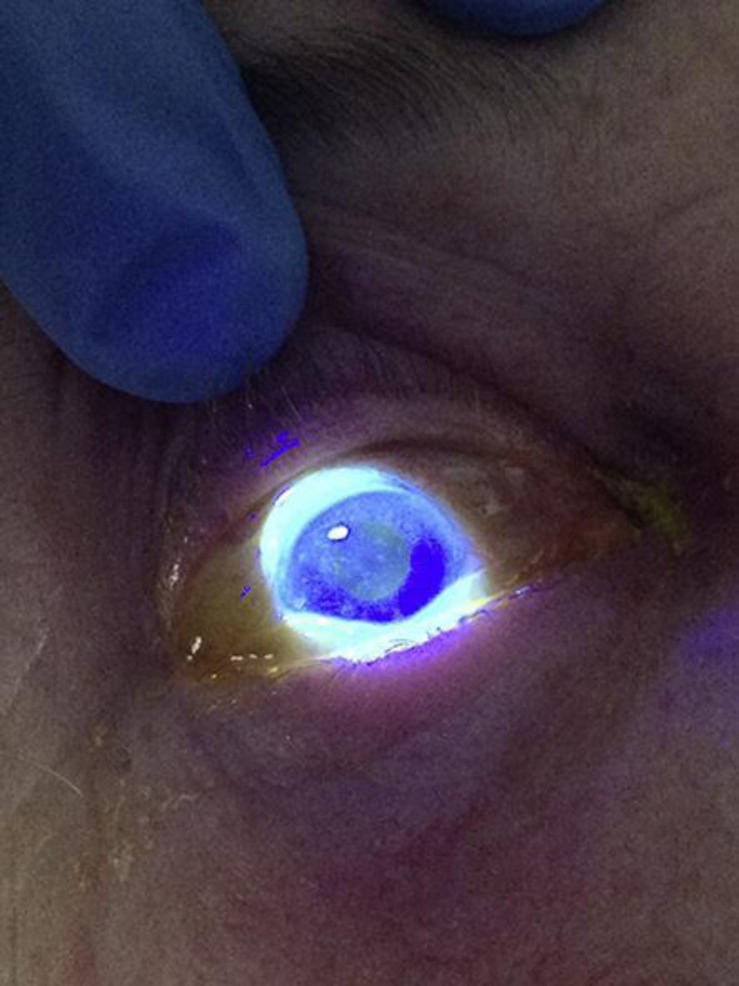
Seven days later, she was admitted with near-complete ophthalmoplegia of her right eye; dilated, fixed pupil; near-complete ptosis; severe, retrobulbar pain; prominent edema of the periorbital surroundings on both sides, and a feeling of altered sensibility in the area of the first trigeminal branch on the right side. The ophthalmoplegia and ptosis became complete within 12 h of admission. She complained of slightly impaired vision on her right eye, but ophthalmological reevaluation revealed full visual acuity in the affected eye. No skin vesicles were noticed, but the fluorescein test of her right eye showed diffuse uptake (fig. 1).
Fig. 1.
Diffuse uptake of fluorescein in the eye with complete ophthalmoplegia.
On the 7th day of admission, the patient became febrile and fluctuated in consciousness. Only a few days later, her left eye also became ophthalmoplegic with fixed, dilated pupil. Ophthalmological examination revealed normal tension, clear anterior chamber and bilateral well-defined optic discs.
During the admission, the patient's condition deteriorated. She became progressively encephalopathic and then unconscious. The patient died during assessment of complications related to her encephalopathy.
Differential Diagnosis and Tests
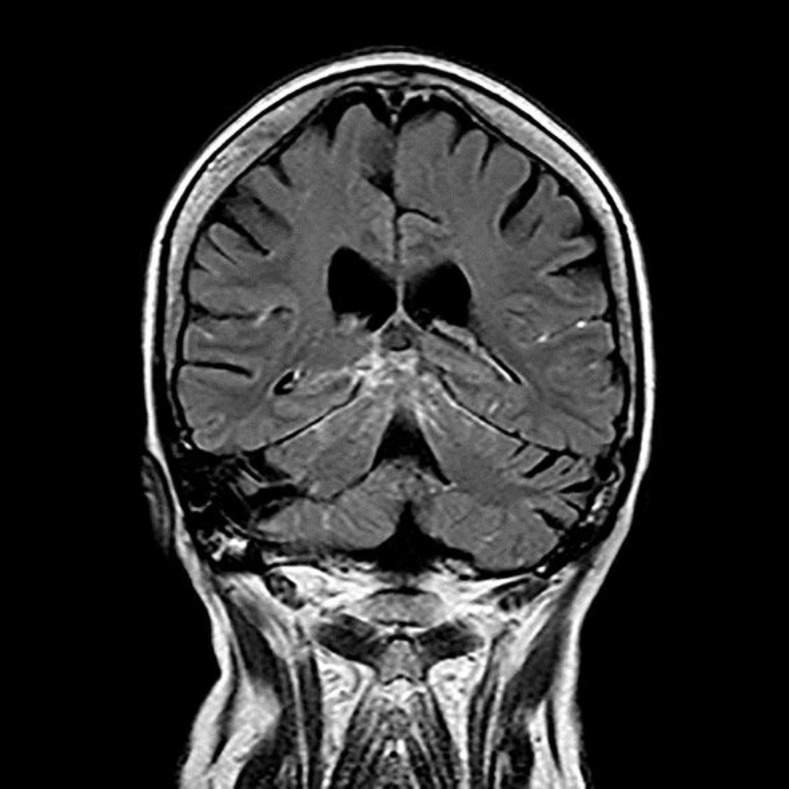
At the initial workup, a computed tomography scan of the cerebrum including cerebral angiography was performed and found to be normal. A magnetic resonance imaging (MRI) scan with intravenous contrast and MRI venous angiography was carried out, revealing meningeal enhancement in the posterior fossa and the area around the cerebellopontine angle on the right side (fig. 2). This was strongly suspicious of basal meningitis, and a lumbar puncture showed 114 white blood cells with 96% being of lymphocytic origin. The cerebrospinal fluid was considered free of malignant cells and bacteria, but positive for VZV DNA on polymerase chain reaction (PCR). A PCR of the fluid from the right eye was also positive for VZV.
Fig. 2.
T1 sequence of an MRI scan showing basal enhancement of the leptomeninges.
Treatment
From the time of admission, the patient received intravenous treatment with acyclovir initially 750 mg three times per day, but the dosage of acyclovir was increased to 1,000 mg three times per day once the PCR for herpes virus family came back positive for VZV. She continued on an unaltered oral dosage of prednisone at 15 mg per day.
Discussion
Reactivation of herpes zoster is more likely to happen in patients with compromised cell-mediated immunity as a result of i.e. common aging, the use of immunosuppressive therapies, or concomitant illness. The severity of the zoster outbreak is correlated with the degree of immunoincompetence [6].
McDonald et al. [7] found a significantly higher risk of herpes zoster in patients with rheumatoid arthritis treated with TNF-alpha inhibitors compared with those treated with, i.e., sulfasalazine. The patients treated with TNF-alpha inhibitors, who developed herpes zoster, were subdivided into three groups, and a significantly higher risk was associated with the use of infliximab compared to etanercept and adalimumab. Another retrospective study showed significantly greater risk when using infliximab and adalimumab compared with no TNF-alpha inhibitor treatment [8].
A case report by Allorent et al. [9] described a patient treated with mycophenolate mofetil who developed hemi-cauda equina syndrome due to varicella zoster meningoradiculitis. However, this patient also had renal function impairment, which is known to increase the risk of VZV reactivation [10]. The study by Koo et al. [11] on the incidence of herpes zoster reactivation in patients after heart transplantation showed treatment with mycophenolate mofetil as an independent risk factor for herpes zoster. Using glucocorticoids has also been shown to have a significant association with varicella zoster reactivation [12].
SOFS consists of dysfunction of the oculomotor nerve (III), the trochlear nerve (IV), the abducens nerve (VI) and the ophthalmic division of the trigeminal nerve (V1). If there is involvement of the optical nerve (II) as well, it is called OAS. The causes of both SOFS and OAS are many, i.e. inflammation, infection, neoplasia, iatrogenic, trauma and vascular conditions [5].
The ophthalmic branch of the trigeminal nerve is purely sensory. It supplies the cornea, the skin of the forehead, eyelid and nose, and it gives off branches to the tentorium cerebelli, dura mater and the posterior area of the falx cerebri [4], possibly explaining a way for the virus to reach the brain causing encephalitis and meningitis.
The pathogenesis of OAS in relation to VZO is thought to be related to immune complexes, direct tissue infection with the virus and/or secondary vasculitis [4]. The same is likely to be true for the SOFS when it occurs in relation to VZO.
Following reactivation of VZV in the ganglion and the spread along the first branch of the trigeminal nerve, direct tissue infection and an immune response may take place within the orbit. The immune response is both humoral and cellular, resulting in inflammation with clinical manifestations of pain, hyperemia, edema, heat and loss of function. It is mediated by the release of lipid mediators, cytokines and altered vascular permeability. The involvement of orbital tissue ipsilateral to cutaneous lesions supports the notion that the pathogenesis is likely to be due to direct viral infection in the orbital cells and a consequent immune system reaction. Circulating immune complex-mediated mechanisms could, however, potentially involve the contralateral orbital tissue resulting in the same symptoms [4], explaining the involvement of the other eye in our patient.
The viral replication of VZV is of short duration and confined to the early stage of the disease. Antiviral therapies should be administered early to have an effect. In our patient, the first symptom of retrobulbar pain was likely due to the onset of viral replication, and the antiviral therapy was administered at a late stage of the disease. The lack of vesicles or other types of apparent skin rashes delayed the diagnosis, but the patient was administered acyclovir intravenously immediately after the first results of the lumbar puncture with 114 white blood cells.
It is known that neurological complications may occur even in the absence of a skin rash – zoster sine herpete [13]. Our patient did not have vesicular skin eruption but clearly had fluorescein uptake in the cornea.
Conclusion
Patients treated with TNF-alpha inhibitors, mycophenolate mofetil and glucocorticoids are at greater risk of VZV reactivation compared to patients with no such treatment. This case report highlights that herpes zoster does not always have a benign course, and caution must be taken for this category of patients. Immunologically incompetent patients with VZO should be treated with antiviral drugs at the first signs of reactivation in order to try to prevent secondary ocular complications. Oral antiviral medication should be switched to intravenous treatment in addition to corticosteroids if there is any kind of cranial nerve palsy, SOFS or OAS.
The patient in this case report was heavily immunosuppressed with three medications that all increase the risk of VZV reactivation. The treatment and management of VZO should be twofold: (1) systemic treatment with corticosteroids based on their anti-inflammatory effects and (2) systemic treatment with antiviral therapies. Treatment within 72 h is the most commonly suggested guideline, and fast treatment should reduce the risk of ocular complications. It seems reasonable to believe that early treatment might also prevent the occurrence of SOFS and spread to the contralateral side.
Statement of Ethics
Written informed consent was obtained from the next of kin of the patient before publication of this case report.
Disclosure Statement
No conflict of interest is present for any of the authors.
References
- 1.Strangfeld A, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301:737–744. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 2.Lee C-Y, Tsai H-C, Lee S, Chen Y-S. Orbital apex syndrome: an unusual complication of herpes zoster ophthalmicus. BMC Infect Dis. 2015;15:33. doi: 10.1186/s12879-015-0760-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanjay S, Chan EW, Gopal L, Hegde SR, Chang BC-M. Complete unilateral ophthalmoplegia in herpes zoster ophthalmicus. J Neuroophthalmol. 2009;29:325–337. doi: 10.1097/WNO.0b013e3181c2d07e. [DOI] [PubMed] [Google Scholar]
- 4.Ugarte M, Dey S, Jones CA. Ophthalmoplegia secondary to herpes zoster ophthalmicus. BMJ Case Rep. 2010;2010 doi: 10.1136/bcr.12.2009.2532. pii: bcr1220092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aryasit O, Preechawai P, Aui-Aree N. Clinical presentation, aetiology and prognosis of orbital apex syndrome. Orbit. 2013;32:91–94. doi: 10.3109/01676830.2013.764439. [DOI] [PubMed] [Google Scholar]
- 6.Kim SY, Solomon DH. Tumor necrosis factor blockade and the risk of viral infection. Nat Rev Rheumatol. 2010;6:165–174. doi: 10.1038/nrrheum.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDonald JR, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis. 2009;48:1364–1371. doi: 10.1086/598331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wendling D, Streit G, Toussirot E, Prati C. Herpes zoster in patients taking TNFalpha antagonists for chronic inflammatory joint disease. Joint Bone Spine. 2008;75:540–543. doi: 10.1016/j.jbspin.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Allorent J, Cozic C, Guimard T, Tanguy G, Cormier G. Sciatica with motor loss and hemi-cauda equina syndrome due to varicella-zoster virus meningoradiculitis. Joint Bone Spine. 2013;80:436–437. doi: 10.1016/j.jbspin.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Wung PK, et al. Herpes zoster in immunocompromised patients: incidence, timing, and risk factors. Am J Med. 2005;118:1416.e9–1416.e18. doi: 10.1016/j.amjmed.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Koo S, et al. Incidence and risk factors for herpes zoster following heart transplantation. Transpl Infect Dis. 2014;16:17–25. doi: 10.1111/tid.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun H, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease-modifying therapy. Arthritis Care Res (Hoboken) 2015;67:731–736. doi: 10.1002/acr.22470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koskiniemi M, et al. Acute central nervous system complications in varicella zoster virus infections. J Clin Virol. 2002;25:293–301. doi: 10.1016/s1386-6532(02)00020-3. [DOI] [PubMed] [Google Scholar]