Abstract
Multiple primary malignancies (MPMs) are present when a patient is diagnosed with more than one primary malignancy and when each tumor is histologically unrelated to the others. MPMs are considered synchronous when they present within 6 months of one another. Here, we report the case of a 57-year-old woman with a past medical history significant for melanoma in 1988, who presented in 2014 with 5 distinct tumors within 4 months: malignant melanoma of the right popliteal fossa, invasive lobular breast carcinoma, diffuse large B cell lymphoma, nodular lymphocyte predominant Hodgkin lymphoma, and a giant cell tumor of tendon sheath/pigmented villonodular synovitis. We discuss her treatment and also present a brief review of the literature. The incidence of MPMs appears to be on the rise, which demands an interdisciplinary, multimodal, and personalized approach to care.
Key Words: Multiple malignancies, Melanoma, Breast cancer, Lymphoma, Giant cell tumor
Introduction
Multiple primary malignancies (MPMs) were initially described by Billroth [1] in 1889 and later defined by Warren and Gates [2] as the presence of at least 2 unrelated primary malignancies in a single patient. Occurrences of MPMs are rare and are described mostly as case reports [3,4], although there are reports from single institutions and from the registries of certain countries [5,6]. The mechanism of development of MPMs is unclear and likely multifactorial; identified risk factors include previous cancer treatment, smoking, diet, and genetic mutations [6]. As advances have been made in tumor detection and treatment, cancer survivorship has improved, which may contribute to the increasing likelihood of the development of additional cancers [7]. Here, we present the case of a 57-year-old woman presenting with melanoma, breast cancer, diffuse large B-cell lymphoma (DLBCL), nodular lymphocyte-predominant Hodgkin lymphoma (NLPHL) and a giant cell tumor, with all 5 diagnoses made within 4 months. To our knowledge, this constellation of tumors has never been reported in the literature.
Case Report
A 57-year-old woman was evaluated for a skin lesion in the right popliteal space. Her past medical history is significant for melanoma on her right arm in 1988. She is G1P0, with menarche at age 12 and menopause at age 55. She has used oral contraceptives for 15 years, most recently in her 30s. She has not received infertility therapy or hormone replacement therapy. Her family history is significant for melanoma (paternal grandmother, age unknown) and colon cancer (mother, age 74). She has never smoked. She drinks 2 glasses of wine every night.
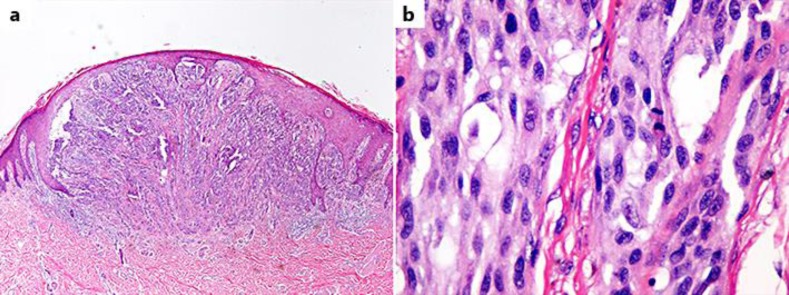
Her skin lesion was diagnosed via excisional biopsy in April 2014 as superficial spreading melanoma. Pathology showed a Breslow thickness of 3.4 mm, no ulceration, and Clark level IV, with no satellite lesions (fig. 1). Surgical margins were widely clear (>2 cm) and inguinal sentinel lymph node biopsy was negative. The final stage was pT3aN0M0.
Fig. 1.
Melanoma. a Invasive malignant melanoma, spindle cell type, low power. b Invasive malignant melanoma, spindle cell type; mitotic figure present in the center right.
The patient had a screening mammogram the same month, which revealed an 8-mm irregular asymmetry in the left breast. The diagnostic mammogram revealed a lymph node in the left axillary tail that appeared highly suggestive of malignancy. Biopsy of the suspicious axillary node was significant for DLBCL as well as Epstein-Barr virus (EBV)-positive NLPHL (fig. 2).
Fig. 2.
Top row: DLBCL. a Low power. b Low power CD20 immunostain; strongly staining uninvolved lymphoid follicles present on the left. Bottom three rows: EBV-positive NLPHL. c Low power. d Medium power. e High power showing Reed-Sternberg cell variants. f CD30+ stain. g EBV-ISH-EBER stain. h EBV-LMP-1 stain.
PET/CT obtained for lymphoma staging in June 2014 showed an area of intense FDG avidity in a left axillary lymph node that was suspicious for lymphoma. The scan also revealed an intense discrete focus of hypermetabolic activity in the proximal left tibia, also suspicious for malignancy.
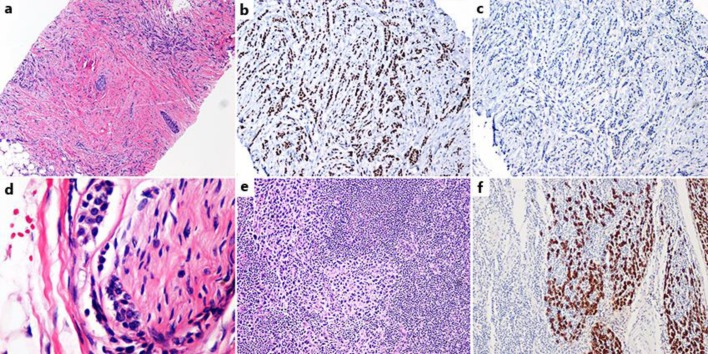
In July 2014, she underwent bilateral mastectomy with right sentinel lymph node biopsy and left axillary lymph node dissection. Pathological analysis revealed stage IIA, T1cN1aM0 grade 1 left breast invasive lobular carcinoma (ILC) with 3 synchronous lesions, 0.5-1.5 cm in diameter (fig. 3). Staining was positive for estrogen receptor (ER) and progesterone receptor but negative for HER2. Left axillary lymph node dissection revealed 2 out of 23 lymph nodes positive for metastatic ILC and 1 lymph node with isolated tumor cells, focal lymphovascular space invasion, and negative margins.
Fig. 3.
Breast carcinoma (3 separate tumors were present, all lobular carcinoma). a One of the 3 lobular tumors, low power. b ER+ stain; all 3 tumors were ER+. c Her2-stain; all 3 tumors were Her2-. d Perineural invasion by lobular carcinoma. e Lymph node metastasis by lobular carcinoma. f Lymph node metastasis by lobular carcinoma, pan-cytokeratin immunostain.
CT-guided core biopsy of the left tibial soft tissue mass revealed CD68/CD45-positive cells, most consistent with giant cell tumor of tendon sheath/pigmented villonodular synovitis.
The management of each malignancy was discussed in a multidisciplinary conference. It was the consensus of the tumor board that, for her excised melanoma, no further therapy was indicated. For a pT3aN0 melanoma with negative margins by at least 2 cm and a negative sentinel lymph node biopsy, adjuvant radiation to the primary site is not indicated [8]. Similarly, in the absence of positive nodes with high-risk features such as ≥3 lymph nodes positive in the inguinal region, bulky lymph node involvement (≥4 cm in the axillary or inguinal region, ≥3 cm for cervical region), or extracapsular extension, nodal irradiation is also not indicated [8].
For her 2 synchronous lymphomas, the consensus was to treat her DLBCL with systemic therapy followed by involved site radiation therapy to the left axilla. The standard of care for her NLPHL would be observation or radiation therapy after surgical excision. In her case, the affected area was planned for radiation due to the proximity of her other tumors.
For her stage IIA breast ILC, the decision was to treat her with chemotherapy followed by postmastectomy radiation therapy due to multiple risk factors, including focal lymphovascular space invasion and 2 positive lymph nodes [9]. Given the overlap in chemotherapy drugs for locally advanced breast cancer and DLBCL, R-CHOP therapy was recommended, as it includes both Adriamycin and cyclophosphamide and covers her lymphoma as well as her breast cancer [10,11].
In terms of radiotherapy, we planned to treat the chest wall, the supraclavicular fossa, and her undissected axillary lymph nodes for her breast cancer. She did not warrant radiation to the dissected axilla (level I) for the management of her breast cancer as she was without gross extracapsular extension [12]. However, for her DLBCL, she did warrant radiation to the site of initial disease, which was the dissected axilla (level I) [11].
The patient completed 6 cycles of R-CHOP and her planned course of radiation therapy, which she tolerated well. She also underwent breast reconstruction with silicone implants placed. She was then started on adjuvant endocrine therapy with anastrozole. A wide local resection with curettage was planned for the giant cell tumor of the left proximal tibia.
Discussion
More patients are living longer after a cancer diagnosis and unfortunately become susceptible to the development of further malignancies, especially if there is an underlying oncogenic predisposition [7]. Indeed, elderly patients are more likely to develop MPMs, underscoring the importance of time to the process [7].
This case is an exceedingly rare instance of 5 simultaneous tumors. Diagnostic criteria require that each cancer must be definitively malignant by histopathology, each must be histopathologically distinct, and the possibility of metastasis among each tumor must be excluded [2]. Moertel [13] defined synchronous tumors as occurring within 6 months of one another. It is conceivable that our patient's melanoma is metastatic from her lesion in 1988, but each of her tumors is histopathologically distinct and came to attention within 4 months.
The data are limited, but Spratt and Hoag [14] found that the reported prevalence of MPMs varies from 0.7 to 11.7%. Bittorf et al. [5] performed a retrospective review of 52,398 patients and found that 3.8% had at least 2 primary malignancies, and that 2.8% of patients with a second malignancy had at least 3 tumors. Interestingly, patients with multiple malignancies demonstrated improved 5-year survival compared to patients with corresponding solitary malignancies. It is unclear whether these patients have some manner of increased tumor resistance or if these survival rates were simply necessary for the development of multiple cancers in the first place.
There are many potential causes of MPMs, including genetic factors, prior cancer therapy, smoking, alcohol consumption, and diet [7,15]. However, it may be important to consider detection bias when identifying certain types of malignancy and attributing causality to prior cancer treatment or other factors. Patients with an initial cancer diagnosis may be followed more closely and may have other malignancies detected that otherwise may not have come to attention. Regardless, random chance clearly also seems to be a factor, especially given our patient's lack of established risk factors. Her family history is significant for melanoma and colon cancer, but there is no evidence suggestive of a hereditary cancer syndrome and she is a never-smoker. She was seen by a genetics counselor but declined testing at that time.
It appears that the incidence of MPMs is increasing [7], which implies that clinicians should be vigilant to the possibility that patients are at continued risk for new and separate malignancies even after an initial diagnosis. More studies are needed to further elucidate the contemporary incidence of MPMs and to investigate risk factors.
There is no universal protocol for the treatment of multiple malignancies. Each case must be considered individually, ideally by a multidisciplinary team, accounting for the type and stage of each tumor, response to treatment, and the patient's overall health status. Radical therapy is indicated for curable cancers, and more conservative approaches are appropriate if radical therapy is not feasible for one or more malignancies. Interdisciplinary treatment is essential given the diversity of disease combinations and treatment strategies.
Statement of Ethics
Informed consent was obtained from the patient.
Disclosure Statement
The authors have no potential conflicts of interest to disclose.
References
- 1.Billroth T. Die allgemeine chirurgische Pathologie and Therapie. In: Reimer G, editor. 51 Vorlesungen – Ein Handbuch für Studierende and Ärzte. ed 14. Berlin: Reimer; 1889. [Google Scholar]
- 2.Warren S, Gates O. Multiple primary malignant tumors: a survey of the literature and a statistical study. Am J Cancer. 1932;16:1358–1414. [Google Scholar]
- 3.Angurana SL, Kapoor R, Kumar P, et al. Quadruple malignancy in a single patient: a case report and comprehensive review of literature. J Cancer Res Ther. 2010;6:230–232. doi: 10.4103/0973-1482.65237. [DOI] [PubMed] [Google Scholar]
- 4.Demandante CG, Troyer DA, Miles TP. Multiple primary malignant neoplasms: case report and a comprehensive review of the literature. Am J Clin Oncol. 2003;26:79–83. doi: 10.1097/00000421-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Bittorf B, Kessler H, Merkel S, et al. Multiple primary malignancies: an epidemiological and pedigree analysis of 57 patients with at least three tumours. Eur J Surg Oncol. 2001;27:302–313. doi: 10.1053/ejso.2001.1112. [DOI] [PubMed] [Google Scholar]
- 6.Coleman MP. Multiple primary malignant neoplasms in England and Wales, 1971-1981. Yale J Biol Med. 1986;59:517–531. [PMC free article] [PubMed] [Google Scholar]
- 7.Wood ME, Vogel V, Ng A, et al. Second malignant neoplasms: assessment and strategies for risk reduction. J Clin Oncol. 2012;30:3734–3745. doi: 10.1200/JCO.2012.41.8681. [DOI] [PubMed] [Google Scholar]
- 8.Burmeister BH, Henderson MA, Ainslie J, et al. Adjuvant radiotherapy versus observation alone for patients at risk of lymph-node field relapse after therapeutic lymphadenectomy for melanoma: a randomised trial. Lancet Oncol. 2012;13:589–597. doi: 10.1016/S1470-2045(12)70138-9. [DOI] [PubMed] [Google Scholar]
- 9.Ebctcg. McGale P, Taylor C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8,135 women in 22 randomised trials. Lancet. 2014;383:2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network Breast Cancer (Version 2.2015). http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed May 26, 2015).
- 11.National Comprehensive Cancer Network Non-Hodgkin's Lymphomas (Version 2.2015). http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf (accessed May 26, 2015).
- 12.Strom EA, Woodward WA, Katz A, et al. Clinical investigation: regional nodal failure patterns in breast cancer patients treated with mastectomy without radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:1508–1513. doi: 10.1016/j.ijrobp.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Moertel CG. Multiple primary malignant neoplasms: historical perspectives. Cancer. 1977;40:1786–1792. doi: 10.1002/1097-0142(197710)40:4+<1786::aid-cncr2820400803>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Spratt JS, Jr, Hoag MG. Incidence of multiple primary cancers per man-year of follow up: 20-year review from the Ellis Fischel State Cancer Hospital. Ann Surg. 1966;164:775–784. doi: 10.1097/00000658-196611000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schatzkin A, Baranovsky A, Kessler LG. Diet and cancer. Evidence from associations of multiple primary cancers in the SEER program. Cancer. 1988;62:1451–1457. doi: 10.1002/1097-0142(19881001)62:7<1451::aid-cncr2820620734>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]