Abstract
Changes of intracellular and extracellular pH are involved in a variety of physiological and pathological processes, in which regulation of the Ca2+ release activated Ca2+ channel (ICRAC) by pH has been implicated. Ca2+ entry mediated by ICRAC has been shown to be regulated by acidic or alkaline pH. Whereas several amino acid residues have been shown to contribute to extracellular pH (pHo) sensitivity, the molecular mechanism for intracellular pH (pHi) sensitivity of Orai1/STIM1 is not fully understood. By investigating a series of mutations, we find that the previously identified residue E106 is responsible for pHo sensitivity when Ca2+ is the charge carrier. Unexpectedly, we identify that the residue E190 is responsible for pHo sensitivity when Na+ is the charge carrier. Furthermore, the intracellular mutant H155F markedly diminishes the response to acidic and alkaline pHi, suggesting that H155 is responsible for pHi sensitivity of Orai1/STIM1. Our results indicate that, whereas H155 is the intracellular pH sensor of Orai1/STIM1, the molecular mechanism of external pH sensitivity varies depending on the permeant cations. As changes of pH are involved in various physiological/pathological functions, Orai/STIM channels may be an important mediator for various physiological and pathological processes associated with acidosis and alkalinization.
A variety of physiological and pathological processes are regulated by alterations in intracellular and extracellular pH1,2. For example, intracellular alkalinization is associated with physiological functions such as activity-dependent membrane depolarization3, oocyte maturation4, sperm activation5,6,7, and growth factor induced cell proliferation, differentiation, migration, and chemotaxis1. Pathologically, intracellular alkalinization and extracellular acidosis are hallmarks of malignant cells and are associated with tumor progression8,9, and intracellular acidic pH (pHi) has been shown to promote apoptosis10. Extracellular low pH, which occurs under injury and ischemia conditions, inhibits a number of cellular responses, including cytosolic- and membrane-associated enzyme activities, and ion transport as well as ion channel activities2. Many cases of clinical acidosis are also accompanied by immunodeficiency2.
Considerable evidence has accumulated that Ca2+ signaling is involved in various physiological/pathological processes associated with acidosis and alkalinization. Notably, it has been demonstrated that Ca2+ entry through Ca2+ release activated Ca2+ channel (ICRAC) plays an essential role in mediating acidosis- and alkalinization-induced physiological/pathological functional changes. It was demonstrated that platelet stimulation results in cytoplasmic alkalinization and increased cytosolic Ca2+ concentration, which is essential for platelet aggregation in response to thrombin11. Similarly, extracellular acidosis-induced inhibition, as well as alkalosis-induced promotion of platelet aggregation is mediated by the changes of store-operated Ca2+ entry12. Moreover, store-operated Ca2+ entry was shown to mediate intracellular alkalinization in neutrophils13, and extracellular low pH was reported to inhibit ICRAC in macrophages14. In Jurkat T-lymphocytes, cytosolic alkalinization induces Ca2+ release and Ca2+ entry15, and acidic internal and external pH inhibit ICRAC16. In SH-SY5Y neuroblastoma cells, however, store-operated Ca2+ entry was not affected by changes of intracellular pH, even though it was attenuated by low extracellular pH and potentiated by high extracellular pH17. In smooth muscle cells, extracellular acidosis decreases store operated Ca2+ entry, whereas extracellular alkalosis potentiates it18. Thus, it seems that changes of both intracellular and extracellular pH regulate ICRAC activity or store-operated Ca2+ entry, albeit there are some discrepancies among different studies.
Since regulation of ICRAC seems to play a critical role in acidosis- and alkalosis-associated physiological and pathological processes, it is essential to understand the molecular basis underlying pH regulation of ICRAC. As activation of ICRAC requires coupling of Orai and STIM as well as gating of Orai19,20,21,22,23,24, alterations of either the coupling of Orai/STIM or gating properties of the pore-forming subunit Orai may cause functional changes of ICRAC. Indeed, it was demonstrated that intracellular low pH caused by oxidative stress induces uncoupling of Orai1 and STIM1, thereby inhibiting ICRAC25, and that intracellular high pH causes store depletion, thereby activating ICRAC. Moreover, mutation of the Ca2+ selective filter residue E106 in the channel pore (E106D) has been shown to alter acidic pH-dependent inhibition of ICRAC26. Furthermore, mutation of D110 and D112 (D110/112A) leads to reduced external pH sensitivity of Orai1/STIM127. Whereas it is known that regulation of pore-forming subunit Orai1 by protons contributes to external pH sensitivity of Orai1/STIM1, the molecular mechanisms by which ICRAC is regulated by internal pH is not fully understood.
Here we show that internal acidosis and alkalosis, as well as external acidosis and alkalosis markedly change Orai1/STIM1 channel functions. By investigating a series of mutants generated on residues located in the channel pore region, intracellular and extracellular loops, N- and C-termini, as well as transmembrane domains (TM3), we found that, in agreement with a previous report26, E106 is responsible for pHo sensitivity when Ca2+ is the permeant cation. However, we found that E106 has no influence on pHo sensitivity when Na+ is the charge carrier. Unexpectedly, we identified that the amino acid residue E190 located in TM3 of Orai1 is the major sensor of pHo when Na+ is the charge carrier. Furthermore, we found that H155 located in the intracellular loop is responsible for intracellular pH sensitivity. Our results indicate that internal and external pH can regulate Orai1/STIM1 channel function by modulating the pore-forming subunit Orai1. Interestingly, our results suggest that the molecular basis for pH sensitivity when Ca2+ is the charge carrier is different from that of when Na+ is the charge carrier, an experimental condition which has been used for investigating pH regulation on Ca2+-selective and Ca2+-permeable channels. Thus, caution needs to be taken when extrapolating the mechanisms of pH sensitivity obtained using Na+ as the permeant cation to the physiological conditions when Ca2+ is the charge carrier. As E106, E190, and H115 are conserved residues in all the three isoforms of Orai, it is conceivable that they are the common external and internal pH sensors of different isoforms of Orai/STIM channels.
Results
Effects of extracellular pH on Orai1/STIM1 currents
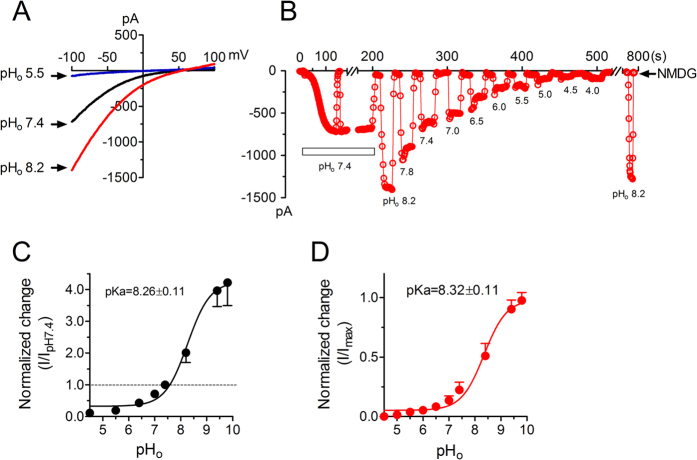
Orai1/STIM1 currents were recorded by including high EGTA concentration in the pipette solution to passively induce store depletion. The effects of extracellular pH on Orai1/STIM1 were evaluated by perfusing the cells with external divalent free solutions (DVF) at various pHs after Orai1/STIM1 activation reached a steady-state. As shown in Fig. 1A, Orai1/STIM1 currents were elicited by a ramp protocol ranging from −100 to +100 mV in the DVF extracellular solutions. Current amplitude was significantly increased when the cell was exposed to high pHo. Without store depletion, Orai1/STIM1 currents were not able to be induced by high pHo, indicating that basic pHo potentiates but does not activate Orai1/STIM1 channels (Fig. S1). In contrary to the effects of alkaline pHo, acidic pHo markedly inhibited current amplitude. A concentration dependent effect of external pH on Orai1/STIM1 is shown in Fig. 1B. Current amplitude was enhanced 3- to 4-fold at pHo 9, and was inhibited to a minimal level at pHo 4.5. The effects of pH were reversible as shown in Fig. 1B. The changes of current amplitude at various pH normalized to the current amplitude at pH 7.4 are shown in Fig. 1C. The best fit of the dose-response curve yielded a pKa of 8.26 ± 0.11 (Fig. 1C). Similar pKa (8.32 ± 0.11) was also obtained by the best fit of the normalized currents in reference to the maximal current amplitude (Fig. 1D).
Figure 1. Effects of acidic and basic pHo on Orai1/STIM1 channels heterologously expressed in HEK-293 cells.
(A) Orai1/Stim1 currents elicited by a ramp protocol ranging from −100 to +100 mV at pHo 7.4, 5.5 and 8.2. Note the significant increase by pHo 8.2 and marked inhibition by pHo 5.5. (B) Concentration-dependent effects of pHo on Orai1/STIM1. Inward current was measured at −100 mV. The effects of pHo was evaluated by perfusing the cells with different pHo in DVF after activation of Orai1/STIM1 in pHo 7.4 DVF, and NMDG solution was perfused to ensure that there was no leak current during the experiment. (C) Changes of current amplitude at each pHo normalized to the current amplitude at pHo 7.4. The best fit of the dose-response curve yielded pKa of 8.26 ± 0.11 (n = 8). (D) Dose-response curve analyzed by normalizing current amplitude at each pHo to the maximal current amplitude. Best fit of the dose-response curve produced similar pKa (8.32 ± 0.11, n = 8) to that shown in (C).
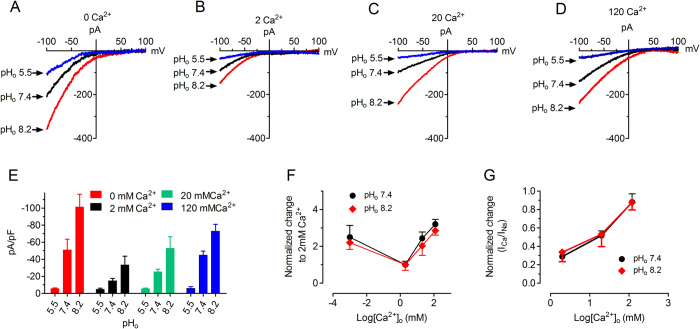
The results shown in Fig. 1 were obtained using DVF solution because DVF solution produces larger current amplitude. We next tested the effects of various pHs on Orai1/STIM1 currents recorded in Tyrode’s solutions containing 2, 20 and 120 mM Ca2+, respectively. As show in Fig. 2A–D, pHo 5.5 inhibited and pHo 8.2 enhanced Orai1/STIM1 inward current independent of extracellular Ca2+ concentrations. In non-transfected control cells, high or low pHo did not induce any current (Fig. S2). The averaged current amplitude under different conditions is shown in Fig. 2E. Two-way ANOVA analysis indicated that the effect of pHo on Orai1/STIM1 was independent of extracellular Ca2+ concentrations. The ratios of the current amplitude at various extracellular Ca2+ concentrations versus the current amplitude recorded in 2 mM Ca2+ Tyrode solution at pHo 8.2 were similar to those at pHo 7.4 (Fig. 2F), suggesting that potentiation of Orai1/STIM1 by basic pH was not significantly influenced by extracellular Ca2+ concentrations. Similarly, the normalized ratios of Ca2+ current at different Ca2+ concentrations versus Na+ current in DVF at pHo 8.2 are well superimposed with the ratios at pHo 7.4 (Fig. 2G), further suggesting that modulation of Orai1/STIM1 channel activity by protons is not dependent on the charge carrier. Thus, we first used DVF extracellular solution to investigate the effects of pHo on Orai1/STIM1 channels.
Figure 2. Effects of pHo on Orai1/STIM1 at various extracellular Ca2+ concentrations.
(A–D) Representative currents recorded at pHo 5.5, 7.4 and 8.2 with extracellular Ca2+ concentrations of 0, 2, 20 and 120 mM. E, Averaged current amplitude measured at −100 mV at pHo 5.5, 7.4, and 8.2 in various extracellular Ca2+ concentrations. Acidic pHo 5.5 significantly inhibited current amplitude (p < 0.01; n = 8 ~ 17) and basic pHo 8.2 remarkably potentiated current amplitude at each extracellular Ca2+ concentration (p < 0.01; n = 8 ~ 17). F, Normalized current amplitude at each Ca2+ concentration to the current amplitude at 2 mM Ca2+. G, Normalized Ca2+ current amplitude at each Ca2+ concentration to the Na+ current amplitude at 0 mM Ca2+. Analysis with two-way ANOVA indicated that the effects of pHo 8.2 on Orai1/STIM1 was independent of extracellular Ca2+ concentration (F,G).
Effects of external pH on Orai2/STIM1 and Orai3/STIM1 currents
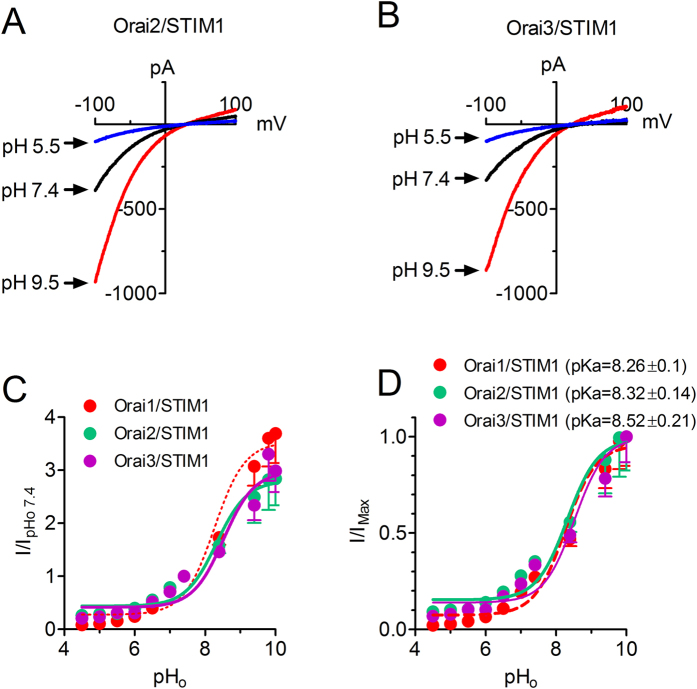
Before we went on to investigate the molecular mechanism of pHo regulation on Orai1/STIM1, we tested if external pH regulates channel activity of Orai2/STIM1 and Orai3/STIM1. As shown in Fig. 3, Orai2/STIM1 and Orai3/STIM1 currents were significantly potentiated by basic pHo and inhibited by acidic pHo (Fig. 3A,B). The fold changes of current amplitude by normalizing current amplitude at each pH to that of pHo 7.4 are shown in Fig. 3C. The maximal increases in Orai2/STIM1 and Orai3/Sim1 at high pH are about 3 fold, similar to the maximal increase of Orai1. The dose-response curves obtained by normalizing current amplitude at each pH to the maximal current amplitude are shown in Fig. 3D. The dose-response curves of Orai1/STIM1, Orai2/STIM1, and Orai3/STIM1 are well superimposed. The pKa obtained from the best fit of the dose-response curves are 8.32 ± 0.14 and 8.52 ± 0.21 for Orai2/STIM1 and Orai3/STIM1 respectively, similar to the pKa of Orai1/STIM1 shown in Fig. 1 (dotted lines in Fig. 3). These results indicate that Orai1/STIM1, Orai2/STIM1 and Orai3/STIM1 have similar pHo sensitivity.
Figure 3. Effects of extracellular protons on Orai2/STIM1 and Orai3/STIM1.
(A,B) Representative recordings at pHo 5.5, 7.4 and 9.5. (C) Concentration-dependent effects of external protons on Orai2/STIM1 and Orai3/STIM1 in comparison with Orai1/STIM1. Current amplitude was normalized to that at pHo 7.4. (D) Dose-response curve obtained by normalizing to maximal current amplitude. Best-fit of the dose-response curves yielded pKa of 8.32 ± 0.14 for Orai2/STIM1 and 8.52 ± 0.21 for Orai3/STIM1, identical to the pKa obtained in (C). Note that the dashed lines in (C,D) represent the data of Orai1/STIM1 taken from Fig. 1C,D.
Mechanisms of external pH regulation on Orai1/STIM1
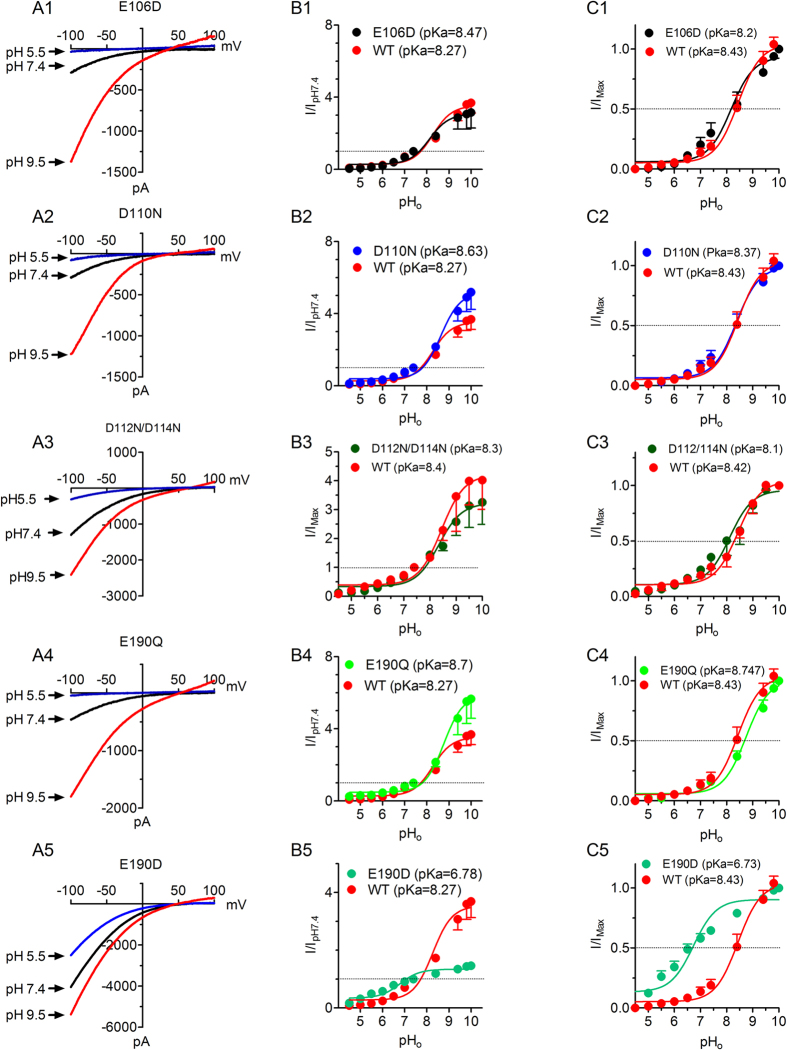
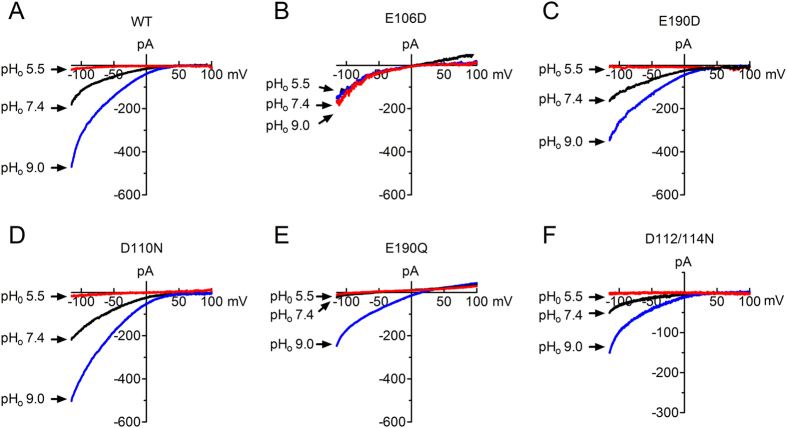
Activation of Orai1/STIM1 involves coupling of Orai1 and STIM1 as well as gating of Orai1. Since the external pH enhanced Orai1/STIM1 current amplitude after the channel was fully activated, we reasoned that protons may directly modulate the pore-forming subunit Orai1. To understand the mechanism by which external protons regulate Orai1/STIM1, we generated mutations by neutralizing a series of negatively charged residues located on the external site of the channel or along the channel pore, including E106Q, D110N, D112/114N, and E190Q. We also generated the mutations E106D and E190D. The negatively charged residues E106, D110, and E190 are conserved residues in all three isoforms of Orai, whereas the negatively charged residues of D112 and D114 are only conserved in Orai1 and Orai3 (Fig. S3). The mutant E106Q produced minimal current, consistent with the inability of E106Q to produce Ca2+ influx20 and the dominant-negative effects of E106Q reported previously28,29. Thus, we did not investigate E106Q in detail. For all the other mutants, representative recordings at pHo 5.5, 7.4 and 9.5 are shown in Fig. 4A1–A5. Changes of current amplitude at each pHo are shown in Fig. 4B1–B5, and the dose-response curves obtained by normalizing current amplitude at each pHo to the maximal current amplitude are shown in Fig. 4C1–C5. Whereas the majority of mutants were inhibited by pHo 5.5 and enhanced by pHo 8.4 to a similar degree in comparison to WT, the mutant E190D displayed much smaller inhibition at pHo 5.5 and significantly reduced potentiation at pHo ≥8.4 in comparison to WT Orai1/STIM1 channels (Fig. 4B1–B5, , , , ,C1–C5). In fact, E190D largely diminished the sensitivity to both acidic pHo and alkaline pHo, whereas E106D displayed similar pH sensitivity to WT Orai1/STIM1 (Fig. 4A1–C1, and A5–C5). The pKa of each mutant obtained by the best fit of the concentration-dependent changes of current amplitude (Fig. 4B1-B5) is similar or identical to the pKa obtained by the best fit of the normalized dose-response curve (Fig. 4C1–C5). The dose-response curves of E106D, D110N, and D112N/D114N were well superimposed with that of WT Orai1/STIM1 (Fig. 4C1–C3; p > 0.05). At much higher pHo such as pHo 9.0, 9.4 and 10.0, the potentiation of D110N was greater than that of WT but did not reach statistical significance (Fig. 4B2), and the potentiation of D112N/D114N was smaller than that of WT (Fig. 4B3) without reaching statistical differences. The dose-response curve of E190Q was slightly right-shifted without causing significant changes in pKa (p > 0.05; Fig. 4C4), albeit the increase of E190Q current amplitude by high pHo was greater than that of WT Orai1/STIM1 (Fig. 4B4). By contrast, the dose-response curve of E190D was significantly shifted to the left, resulting in a pKa that is almost two pH units lower than that of WT Orai1/STIM1 (p < 0.05; Fig. 4C5). The significant change in pKa in the E190D indicates that E190 is essential for the extracellular pH sensitivity of Orai1/STIM1 channels in DVF solution. Since E190Q only generated a small change in pKa, whereas E190D shifted the dose-response curve significantly, it is likely that the length of the side chain rather than the charge of E190 plays a key role in pHo modulation of Orai1/STIM1. Taken together, although D110N and D112N/D114N produce some changes in potentiation of current amplitude at very high pHo, it seems that the conserved residue E190 plays a major role in pHo sensitivity of Orai1/STIM1 when Na+ is the charge carrier.
Figure 4. Concentration-dependent effects of protons on Orai1/STIM1 WT channel and mutants.
(A1–A5), Typical recordings of each mutant at pHo 5.5, 7.4 and 9.5. (B1–B5) Concentration-dependent effects of various pHs on current amplitude of each mutant in comparison with WT. The changes of current amplitude were obtained by normalizing current amplitude at each pHo to the value at pHo 7.4. The pKa of each mutant was obtained by best fit of the dose-response curves. (C1–C5) Dose-response curves constructed using the normalized current amplitude to the maximal current amplitude. The best fit of the dose-response curves generated pKa of 8.43 ± 0.1 (n = 8) for WT, 8.17 ± 0.13 (n = 9, p > 0.05) for E106D, 8.37 ± 0.09 (p > 0.05, n = 8) for D110N, 8.10 ± 0.18 (n = 12, p > 0.05) for D112/114N, 8.74 ± 0.08 (n = 4 ~ 6, P > 0.05) for E190Q, and 6.73 ± 0.08 (n = 8, p < 0.05) for E190D.
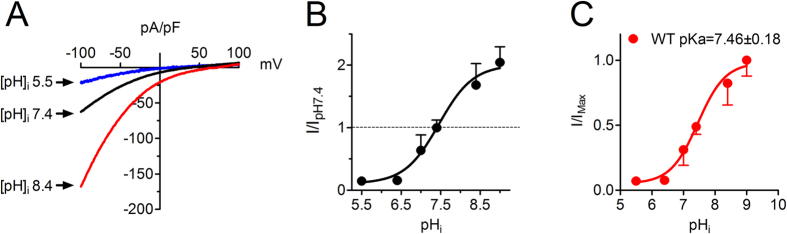
Figure 5. Effects of internal pH on Orai1/STIM1.
(A) Representative Orai1/Stm1 currents elicited by a ramp protocol ranging from −100 to +100 mV in cells with pipette solution at pHi 5.5, 7.4 and 8.4, respectively. (B) Concentration-dependent effects of pHi on Orai1/STIM1 currents. Current amplitude measured at −100 mV at each pHi was normalized to the current amplitude at pHi 7.4. Current amplitude was almost completely inhibited at pHi 5, and was enhanced by about 2-fold at pHi 9 (n = 12, 5, 6, 44, and 6 at pHi 5.5, 6.5, 7.4, 8.4, and 9, respectively). (C) Dose-response curve analyzed by normalizing current amplitude at each pHi to the maximal current amplitude at pHi 9. Best fit of the dose-response curve produced same pKa (7.46 ± 0.18, n = 12, 5, 6, 44 at pHi 5.5, 6.5, 7.4, 8.4, and 9, respectively).
Effects of internal pHi on Orai1/STIM1 channels
To study whether internal pH (pHi) also influences Orai1/STIM1 channel activity, we titrated the pipette solution at various pHs. At acidic pHi 5.5, Orai1/STIM1 current amplitude was only about 10% of the current amplitude at pHi 7.4 (Fig. 5A), whereas at basic pHi 8.4, the current amplitude was almost two-fold greater than the current amplitude at pHi 7.4 (Fig. 5A). The internal pH regulates Orai1/STIM1 channel activity in a concentration-dependent manner (Fig. 5B), with the maximal increase of current amplitude by 2-fold at pHi 9 (Fig. 5B). The best fit of the dose-response curve yielded pKa of 7.46 (Fig. 5C). Thus, similar to the effects of pHo on Orai1/STIM1, internal acidic pH inhibits and alkaline pH potentiates Orai1/STIM1 channel activities.
Molecular mechanisms of internal pH sensitivity of Orai1/STIM1
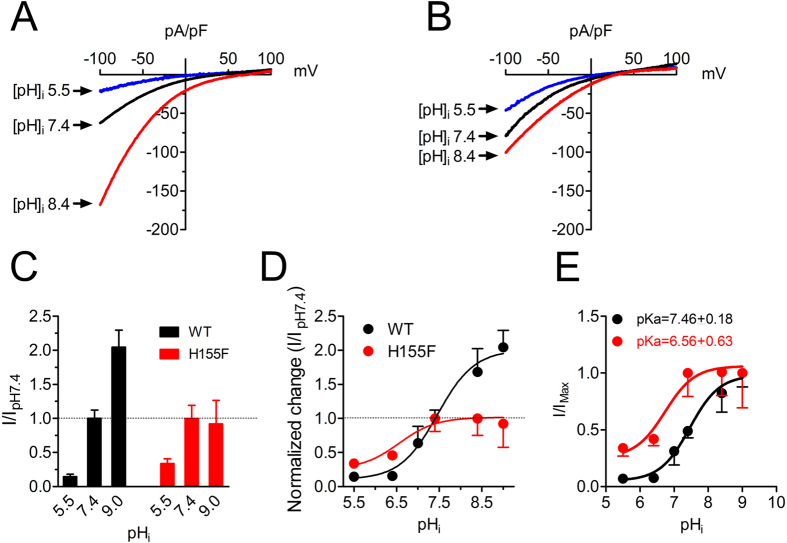
To understand the mechanism by which Orai1/STIM1 channels are regulated by pHi changes, we made a series of mutations at the N- and C- termini, and at the loop between TM2 and TM3 (Fig. 6A). The mutations were made on the titratable residues including histidine (His) and glutamic acid (Glu), as well as cysteine (Cys) residues which have been shown to be involved in internal pH sensing in other channels30,31,32. We first tested current amplitude of each mutant at pHi 5.5 and 9.0 in comparison with the current amplitude at pHi 7.4. Representative recordings at pHi 5.5, 7.4, and 9.0 of each mutant are shown in Fig. S4. The normalized current amplitude of each single or double mutant is shown in Fig. 6B. Similar to WT Orai1/STIM1, current amplitude of all the mutants was markedly inhibited at pHi 5.5 and significantly enhanced at pHi 9.0 except for H155F. For H155F, current amplitude at pHi 9.0 was even smaller than that at pHi 7.4, whereas the current amplitude of other mutants was enhanced 1.5- to 2-fold at pHi 9.0, which was similar to the changes in WT Orai1/STIM1. At pHi 5.5, current amplitude of H155F was significantly larger than WT (p < 0.05). These results indicate that H155F is less sensitive to both acidic and basic internal pH.
Figure 6. Effects of neutralization of internal residues on pHi sensitivity of Orai1/STIM1.
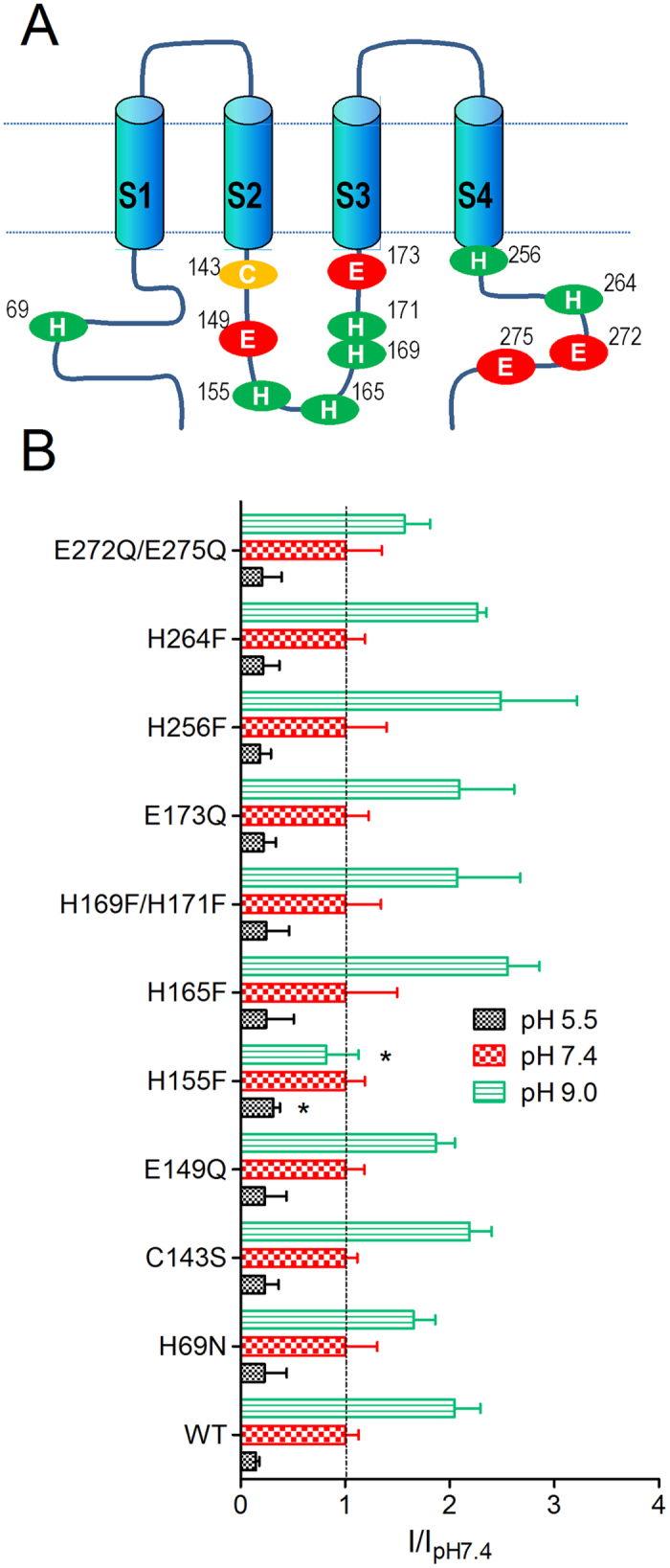
(A) Schematic diagram of Orai1 showing the position of the substituted residues. There were 8 single mutants and two double mutants. (B) Averaged changes of current amplitude by pHi 5.5 and 9.0 in the mutants in comparison with the WT Orai1/STIM1. Current amplitude at pHi 5.5 and 9.0 was normalized to that at pHi 7.4. Data were analyzed by ANOVA followed by t-test with Bonferroni correction.
We further investigated the effects of different pHi on H155F in comparison with WT Orai1/STIM1. Original recordings of WT and H155F at pHi 5.5, 7.4, and 8.4 are shown in Fig. 7A,B, and the average current amplitude is shown in Fig. 7C. The concentration-dependent changes of H155F current amplitude normalized to IpHi 7.4 are shown in Fig. 7D. It is noticeable that H155F lost response to basic pHi, and the response to acidic pHi was also significantly diminished. The pKa obtained by best fit of the dose-response curves constructed using I/IMax was 6.56 ± 0.63 for H155F and 7.47 ± 0.18 for WT Orai1/STIM1 (Fig. 7E). The dose-response curve of H155F was left-shifted by almost one pH unit (p < 0.01). These results suggest that H155 is responsible for intracellular pH sensitivity of Orai1/STIM1.
Figure 7. H155 is the intracellular pH sensor of Orai1/STIM1 channels.
(A,B) effects of pHi 5.5 and pHi 8.4 on H155F in comparison with WT Orai1/STIM1. The original recordings of WT Orai1/STIM1 in A are from the Fig. 5A. Note that pHi 5.5 and pHi 8.4 produced much smaller change on H155F (B) compared with WT Orai1/STIM1 (A). (C) Averaged changes of current amplitude by pHi 5.5 and 9.0. (D) Concentration-dependent effects of pHi on H155F compared with WT Orai1/STIM1. Note that alkaline pHi failed to potentiate H155F current amplitude, whereas acidic pHi produced much smaller inhibition in comparison with WT Orai1/STIM1. E, Dose-response curves constructed using current amplitude normalized to the maximal current amplitude. The pKa was 7.46 ± 0.18 (n = 6–44) and 6.56 ± 0.63 (n = 6–11) for WT and H155F mutant.
Endogenous ICRAC channel exhibits similar pH sensitivity to Orai1/STIM1
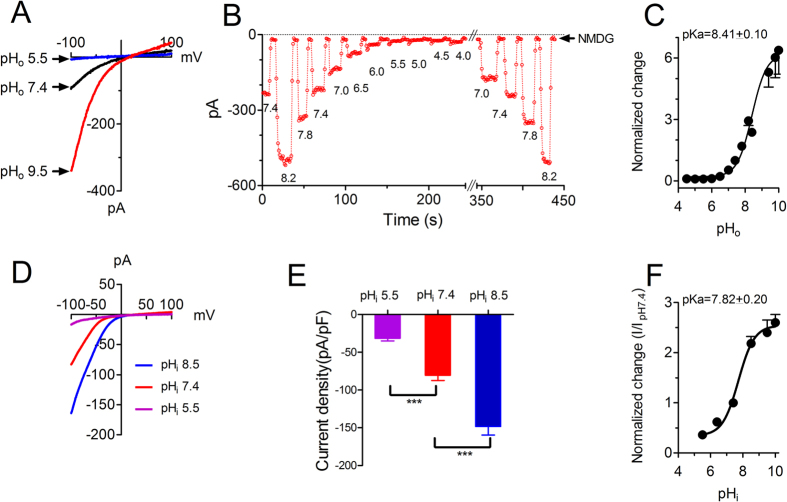
To investigate whether endogenous ICRAC channels are also sensitive to pH changes, we used RBL cells for current recording in DVF solution under various pHo conditions. ICRAC was activated by passive store depletion, and then cells were perfused with different external pH solutions. As shown in Fig. 8A, ICRAC was completely inhibited by pHo 5.5, and significantly increased by pHo 9.5. The effect of pHo on ICRAC was concentration dependent and reversible (Fig. 8B). The pKa of ICRAC was 8.41 ± 0.10, similar to the pKa of Orai1/STIM1 shown in Fig. 1. Moreover, the ICRAC current in RBL cells was also regulated by internal acidic and alkaline pH. Current amplitude was inhibited by about 80% at pHi 5.5, and potentiated by about 1.5–2 fold at pHi 8.5 (Fig. 8D,E). The pKa (Fig. 8F) of ICRAC was also similar to that of Orai1/STIM1 shown in Fig. 7D.
Figure 8. Effects of pHo and pHi on endogenous ICRAC currents in RBL cells.
(A) Representative recording of ICRAC in RBL cells. Currents were elicited by a ramp protocol ranging from −100 to +100 mV in DVF solutions. After ICRAC activation reached a steady-state, the cell was exposed to external solutions with different pH. (B) Concentration-dependent effects of pHo on ICRAC. NMDG was used to test leak current. The inhibition and potentiation effects of pHo were reversible. (C) Dose-response curve constructed using current normalized to the current amplitude at pHo 7.4. The best fit of the dose-response curve yielded pKa of 8.41 ± 0.10 (Mean ± SEM, n = 8–11). (D) Representative traces of ICRAC at pHi 5.5, 7.4, and 8.5. (E) Average current amplitude of ICRAC at pHi 5.5, 7.4 and 8.5. (F) Dose-response curve constructed using current normalized to the current amplitude at pHi 7.4. The best fit of the dose-response curve yielded pKa of 7.82 ± 0.2 (Mean ± SEM, n = 7–13).
Effects of external Ca2+ on pH sensitivity of Orai1/STIM1 mutants
DVF solution has been commonly used for investigating pH sensitivity of different ion channels33,34,35,36,37,38,39. Although the pH sensitivity of WT Orai1/STIM1 is not dependent on charge carrier (Fig. 2), we tested pH sensitivity of the Orai1 mutants when Ca2+ is the permeant cation for Orai1/STIM1 channels, since changing the relative permeability and/or selectivity may serve as a mechanism by which protons regulate Ca2+-permeable channels35,40,41 including ICRAC26,27. We used Ca2+ concentration (20 mM) higher than physiological Ca2+ (2mM) in order to get larger currents. Similarly high Ca2+ concentration for ICRAC recording has been used previously26,27. As shown in Fig. 9, in the presence of 20 mM external Ca2+, the mutants D110N and D112/114N displayed similar pH sensitivity to that of WT Orai1/STIM1, indicating that the residues D110 and D112/114 do not contribute to pH sensitivity of Orai1/STIM1, which is consistent with the results obtained in DVF solution (Fig. 4). However, the mutant E106D was insensitive to pH changes in 20 mM Ca2+ Tyrode’s solution, a feature which presumably manifests the reduced Ca2+ permeability and selectivity as previously reported26. Similarly, the mutant E190Q, which largely loses Ca2+ selectivity20, conducted very minimal currents and was insensitive to low pH (Fig. 9E), albeit a large inward current was elicited at pH 9.0 with an unknown mechanism which is worthy of further investigation in the future. Interestingly, the mutant E190D has similar pH sensitivity as the WT Orai1/STIM1 in 20 mM Ca2+ solution (Fig. 9F). This is not surprising given that E190D preserves similar Ca2+ selectivity to that of WT Orai1/STIM1 channels20.
Figure 9. Effects of external pH on Orai1/STIM1 channel and its mutants in external solutions with 20 mM Ca2+.
(A–F) Original recordings in 20 mM Ca2+ Tyrode’s solution at pHo 5.5, 7.4 and 9.0. Please note that E106D, especially E190Q, conducted very small currents. E106D is insensitive to both pHo 5.5 and pHo 9.0, whereas E190Q is insensitive to pHo 5.5.
Using normal Tyrode’s solution containing 2 mM Ca2+ as the external solution, we also tested whether external Ca2+ influences pH sensitivity of the internal pH sensor of Orai1/STIM1, H155F. We found that the pH sensitivity of H155F was not altered by external Ca2+ concentration. For example, the ratios of current amplitude at pHi 5.5 and pHi 8.4 versus pHi 7.4 were 0.34 ± 0.07 (n = 6) and 1.1 ± 0.3 (n = 6) respectively in 2 mM Ca2+ Tyrode’s solution, similar to the ratios of 0.36 ± 0.05 and 1.0 ± 0.24 (n = 6) obtained in DVF solution as shown in Fig. 7.
Discussion
In this study, we demonstrate that Orai1/STIM1 channels are regulated by both internal and external pH. Whereas acidic internal and external pH inhibit channel activity, alkaline intra- and extracellular pH dramatically increase channel activity. We identify a new residue H155 which is responsible for intracellular acidic and alkaline pH sensitivity. For extracellular pH sensitivity, we find that even though pH regulation on Orai1/STIM1 channels is independent of the charge carrier, the mechanisms underlying pH sensitivity are different with different charge carriers. While the residue E106 is responsible for the pH sensitivity when Ca2+ is the charge carrier, we find that E190 is responsible for pH sensitivity when Na+ is the charge carrier. Furthermore, we show that endogenous ICRAC in RBL cells is also inhibited by acidic pHi and pHo, and potentiated by basic pHi and pHo. As ICRAC plays an important role in various physiological functions, and given that acidosis and alkalinization are involved in a variety of physiological/pathological processes, ICRAC may play a crucial role in mediating various physiological/pathological functions associated with acidosis and alkalinization.
Orai1/STIM1 channel activity is potentiated by intracellular and extracellular alkalinization, and inhibited by internal and external acidosis
Regulation of the endogenous ICRAC by acidic pH in Jurkat T-lymphocytes and by external alkaline pH in macrophages has been previously reported14,16. Moreover, two recent studies have shown that heterologously expressed Orai1/STIM1 currents can be inhibited by external low pH26,27 and internal low pH27, and enhanced by external high pH but not by internal high pH27. We find that Orai1/STIM1 channels expressed in HEK-293 cells are not only inhibited by intracellular and extracellular acidic pH, but also enhanced by both intracellular and extracellular alkaline pH. The reason for the different regulation by internal high pH obtained in this study and in Beck’s study is currently unknown, and will need further investigation. However, previous studies demonstrated that in Jurkat T-lymphocytes and neutrophils, cytosolic alkalinization induces Ca2+ release and store-operated Ca2+ entry13,15, consistent with the notion that alkaline internal pH potentiates Orai1/STIM1. Similar to the regulation of over-expressed ICRAC currents, the native ICRAC is inhibited by external low pH and enhanced by high pH, with the pKa of 8.4 (Fig. 8). We show that pH regulates Orai1/STIM1 channel activities independent of charge carriers. For example, acidic pHo inhibits ICRAC currents carried by Ca2+ ions to the same degree as it inhibits the ICRAC currents carried by Na+ ions. Likewise, basic pHo enhances ICRAC currents carried by both Ca2+ ions and Na+ ions (Fig. 2). Similar results have been reported for the regulation of native ICRAC currents by pH16. Kerschbaum and colleagues demonstrated that ICRAC currents in Jurkat T lymphocytes are inhibited by acidic extracellular and intracellular pH, and that both Ca2+ currents and monovalent Na+ currents of ICRAC can be equally inhibited by acidic pH in a voltage independent manner16. The authors showed that acidic intracellular pH blocks ICRAC with a pKa of 6.8, whereas acidic extracellular pH inhibits ICRAC with a pKa of 8.216. Alkaline external pH was also previously shown to enhance ICRAC currents in macrophages with a pKa of 8.214. Our pKa for the external proton effects on Orai1/STIM1 is similar to that previously reported14,16, but our pKa (7.46) for internal pH effects is higher than that obtained by Kerschbaum and colleagues16. We do not yet know the reason for the different results, but it is conceivable that this discrepancy could be due to the fact that we used heterologously expressed Orai1/STIM1 channels, whereas the native ICRAC may be contributed by different isoforms of Orai and STIM42. Nonetheless, regulation of endogenous ICRAC and heterologously expressed Orai/STIM currents by pH indicates that ICRAC may be an important mediator of altered Ca2+ signaling under acidic and alkaline conditions.
Mechanisms of external pH sensitivity of Orai1/STIM1 channels
By mutating a series of titratable amino acid residues located in the channel pore, in the loop between TM1 and TM2, and within TM343,44, we find that the amino acid residues which are responsible for extracellular pH sensitivity are different with different permeant cations. Whereas E106D loses pH sensitivity when Ca2+ is the charge carrier, which is consistent with the previous report26, E106D shows similar pH sensitivity to that of WT Orai1/STIM1 in the absence of Ca2+ (in DVF solution) when Na+ is the charge carrier. E190D, however, exhibits markedly reduced pH sensitivity in DVF solution when Na+ is the permeant cation, albeit displaying similar pH sensitivity to that of WT Orai1/STIM1 in the Tyrode’s solution containing Ca2+. Thus, it appears that E190 is responsible for external pH sensitivity in the absence of Ca2+ when Na+ is the charge carrier, whereas E106 is responsible for external pH sensitivity when Ca2+ is the charge carrier. Although using monovalent Na+ cation in DVF solution as the charge carrier is a non-physiological condition, Na+ in DVF solution has been commonly used as the permeant cations for investigating pH sensitivity of different ion channels including Ca2+ channels and Ca2+-permeable channels33,34,35,36,37,38,39. Thus, our results provide important insights suggesting that the molecular basis for pH sensitivity can be different when using Ca2+ as the charge carrier or the monovalent cation Na+ as the charge carrier, and that precautions need to be taken when extrapolating the mechanism of pH sensitivity obtained from experimental conditions to physiological conditions.
External protons can regulate ion channel functions via changing gating properties and/or influencing channel permeation33,34,35,36,40. Many Ca2+ permeable channels are regulated by protons via changing Ca2+ selectivity and permeability. For the voltage-gated Ca2+ channels (VGCC), the Ca2+ selectivity and pH sensitivity are conferred by the Glu residues in the pore-forming region35,36. Mutation of Glu by glutamine (Gln) substitution in repeats I or III produces similar low conductance single channel currents, mimicking the protonated state35,36. Thus, the permeant divalent cations and protons compete for the same Glu binding sites35. The similar competing mechanism also underlies pH modulation on TRPM6 and TRPM7 channels41,45,46. Yet, for TRPM6 and TRPM7 channels, binding of protons to the E1024 (TRPM6) and E1047 (TRPM7) in the channel pore releases Ca2+ block on monovalent currents. Therefore, low pHo potentiates TRPM6 and TRPM7 currents by enhancing the monovalent current amplitude41. Similar to VGCCs, the Ca2+ selective TRPV5 channels are inhibited by acidic pHo34. However, the pHo sensing site E522 is independent of the Ca2+ selectivity site D64834. Protons modulate TRPV1 channel activity via influencing both gating and permeation properties37,38,39,40. Activation of TRPV1 by protons is mediated by the extracellular E600 and E648 residues37, whereas the divalent permeability of TRPV1 is conferred by D64647. TRPM2 has also been shown to be inhibited by external protons48,49,50,51, yet the mechanism is more complicated48,49,50. Although one study suggested that external protons permeate through TRPM2 and inhibit channel activity intracellularly49, mutagenesis results in other studies demonstrated that external protons bind to external residues around the channel pore and block the channel activity extracellularly48,50,51. Whereas neutralization of H958, D964, and E994 at the outer vestibule of the channel pore enhances pH sensitivity of TRPM2 by reducing external Ca2+ sensitivity48, Yang and colleagues demonstrated that external protons inhibit TRPM2 in a voltage- and state-dependent manner50,51, and that substitution of the residues K952 and D1002 by alanine (Ala) significantly reduces inhibition of open TRPM2 channels by external protons50. Furthermore, the residue Q992 in the outer pore of mouse TRPM2 (mTRPM2) is crucial for the reduced sensitivity to pHo 6.0 in comparison with human TRPM2 (hTRPM2)51. Nonetheless, it appears that the pH sensing residues are usually located either in the channel pore, or at the vestibule of the channel pore for many Ca2+-selective and Ca2+-permeable channels39. For Orai1, however, we found that neutralizing the negatively charged residues D110, D112 and D114 at the vestibule did not significantly change pHo sensitivity regardless of permeant cations (Figs 4 and 9). At very high pHo (pHo 9.0, 9.4, and 10.0), D110N and D112/114N exhibited larger current than WT but without statistical significance (Fig. 4) when Na+ is the charge carrier. Interestingly, when Ca2+ is the permeant cation, substitution of the Ca2+ selective filter residue E106 by Asp (E106D) eliminates pHo sensitivity, presumably through changes of Ca2+ permeability and selectivity, a similar mechanism by which VGCCs and some other Ca-permeable channels are regulated by protons, as previously reported26. However, when Na+ is the permeant cation, E106D did not produce any change in pH sensitivity, and the pH dose-response curve of E106D was well superimposed with that of WT channels. Unexpectedly, we found that when Na+ is the permeant cation, mutant E190D significantly altered the pH sensitivity of Orai1/STIM1, and shifted the pH dose-response curve by almost two pH units.
How might E190 influence pH sensitivity of Orai1/STIM1 channels when Na+ is the charge carrier? The E190 residue in the TM3 is not facing the channel pore or involved in the pore formation43,52, even though E190, W176 and G183 in the TM3 have been shown to be involved in Ca2+ selectivity, channel gating and fast inactivation53,54. Since E190 is not facing the channel pore, the mechanisms by which E190 affects both Ca2+ selectivity and/or the size of channel pore are not understood from a structural point of view55. Amcheslavsky and colleagues recently demonstrated that E165 in Orai3, the residue equivalent to E190 in Orai1, is involved in 2-APB induced gating of Orai3 channels55. The authors proposed that E165 of TM3 is directly behind and in between two TM1 domains, and when 2-APB activates Orai3, E165 moves toward the central axis of the channel, therefore affecting channel permeation and pore formation55. Similarly, it is conceivable that E190 in Orai1 becomes accessible when the channel opens. Since E190Q did not significantly change the pHo sensitivity, whereas E190D exhibited markedly reduced sensitivity to both acidic and basic pHo resulting in a significant shift of the pHo dose-response curve by almost two pH units, it is likely that the side chain but not the charge of E190 plays an essential role in pHo sensitivity. Thus, a plausible model is that when Orai1/STIM1 channel opens, E190 becomes accessible to the channel pore. Changing the length of the side chain of E190 may alter the size of the ion-permeation pathway, thereby influencing ion permeation when Na+ is the permeant cation, resulting in changed pH sensitivity. Nonetheless, further investigation such as mutating E190 to different residues, measuring single channel conductance by noise analysis, and evaluating changes of permeability will provide more evidence in order to fully understand the underlying mechanisms.
In agreement with our result that mutant E106D eliminates pH sensitivity at high and low pH (Fig. 9) when Ca2+ is the permeant cation, Scrimgeour and colleagues showed that E106D changes Ca2+ selectivity as well as fast Ca2+-dependent inactivation44,45, and eliminates acidic pH inhibition of Orai1/STIM126. The authors demonstrated that acidic pH inhibits Orai1/STIM1 with a pKa of 7.826. Similar to our results, the authors described that Na+ conductance of the WT Orai1/STIM1 channels in the presence and absence of external Ca2+ displayed the same pH dependence26, though whether E106 is responsible for the pH sensitivity in the absence of Ca2+ was not investigated26. Since E106D alters Ca2+ selectivity and Ca2+ affinity (25 μM for WT and 490 μM for E106D)53, we believe that the changes in pH sensitivity in E106D is caused by altered Ca2+ selectivity and permeability as previously demonstrated26. Indeed, when we used Na+ as the charge carrier, E106D is no longer sensitive to high and low pHo. Instead, we found that in the absence of Ca2+, mutant E190D markedly changed pHo sensitivity in comparison with the WT Orai1/STIM1 channels. Thus, our results indicate that E190 is the pHo sensor of Orai1/STIM1 when Na+ is the permeant cation.
Although we found that the mutants D110N and D112/114N did not significantly alter pHo sensitivity, a recent study by Beck and colleagues demonstrated that D110/D112A shows reduced sensitivity to external pH 8.4 and pH 6.027. As we neutralized the aspartic acid (Asp) at D110 and D112 to asparagine (Asn), whereas Beck and colleagues mutated Asp residues to Ala residues, it is plausible that the size of the residue at D110 and D112 plays a role in sensing external pH. Alternatively, mutating D110 and D112 simultaneously (D110/D112A) may be crucial for altering pHo sensitivity, which may explain the discrepancy between our and their results. However, since Orai2/STIM1 and Orai3/STIM1 display similar pHo sensitivity to that of Orai1/STIM1 (Fig. 3), and the negatively charged residue D112 is not conserved in all the three isoforms of Orai (Fig. S4), it would be interesting to test whether D110/112A also influences pHo sensitivity of Orai2/STIM1.
Taken together, we found that different amino acid residues are responsible for pHo sensitivity of Orai1/STIM1 under different ionic conditions. Under physiological conditions when Ca2+ is the permeant cation, it appears that E10626 and likely D110/D11227 are responsible for pHo sensitivity, whereas E190 contributes to pH sensitivity when Na+ is the charge carrier. Although changes of Ca2+ and Na+ selectivity and permeability are proposed as the underlying mechanism for Orai1/STIM1 regulation by external protons, we and others26,27 have not provided direct experimental evidence yet. Indeed, it seems that the outward currents are much larger and the reversal potential is not as positive as expected at high pHo (Figs 3 and 4), suggesting that alkaline pH changes ionic selectivity and permeability. Future studies focusing on how different pHo can change Ca2+ selectivity and permeability of WT Orai1 and its mutants, how single channel conductance of the WT and mutated channels is influenced by various pHo, and whether mutating E106 and E190 to different amino acid residues will alter the pHo sensitivity are required to fully understand the molecular mechanisms underlying pHo sensitivity of Orai1/STIM1.
Mechanisms of intracellular pH sensitivity of Orai1/STIM1 channels
Previous studies showed that native ICRAC can be blocked by internal acidic pH16, but the effect of basic pH was not evaluated. We demonstrate that Orai1/STIM1 channel activity is not only inhibited by acidic pHi, but also enhanced by alkaline pHi. To further understand mechanisms of internal pH regulation of Orai1/STIM1, we generated a series of mutations on the Cys, His and Glu residues located in the N- and C-termini, as well as the intracellular loop between TM2 and TM3. Among the twelve mutants, H155F displayed markedly reduced sensitivity to both acidic and alkaline pHi, indicating that H155 at the intracellular loop of TM2 and TM3 is responsible for sensing internal pH changes.
It is remarkable that neutralizing one residue H155 diminishes the sensitivity of Orail1/STIM1 to both acidic and alkaline pHi. Since the side chains of His and phenylalanine (Phe) are similar, it appears that the charge of H155 plays an essential role in sensing the internal pH changes. Although the exact mechanism is not clear yet, it is plausible that protonation of H155 under acidic pHi conditions affects intra- or intermolecular interactions of the channel leading to conformational changes that favor channel closing, whereas deprotonation of H155 under alkaline pHi conditions causes conformational changes that favor channel opening. In a previous study, a His residue in the N-terminus has been reported to be responsible for the alkaline pHi induced activation of TRPV130. TRPA1 is also activated by alkaline pHi32. However, unlike TRPV1, the mechanism by which alkaline pHi activates TRPA1 is through the modulation of two N-terminal Cys residues32. Internal pH regulation of HCN2 channel is also mediated by a His residue located in the cytosolic S4-S5 linker31. Protonation of H321 causes a leftward shift of the activation curve therefore reducing HCN2 current amplitude, whereas deprotonation of H321 elicits a rightward shift of the activation curve therefore enhancing HCN2 current amplitude31. Several other channels are also regulated by internal pH changes. For example, TRPM2 and TRPM7 are inhibited by acidic pHi48,56. An intracellular residue D933 located at the TM4 and TM5 linker was found to serve as the internal pH sensor of TRPM248. The residue D933 contributes to TRPM2 pHi sensitivity by influencing TRPM2 channel gating as well as Ca2+ and ADPR sensitivity48. Different from the mechanism by which TRPM2 is regulated by pHi, intracellular protons inhibit TRPM7 channel activity by screening the negative charges of PIP256.
How might H155 located in the loop of TM2 and TM3 sense the changes of internal pH and translate the pH sensitivity to channel gating? The TM2-TM3 loop has previously been shown to play an essential role in fast inactivation of Orai1/STIM157. Mutation of the four residues in the middle of the loop abolished fast inactivation of Orai1, and addition of a 37-amino acid peptide derived from the loop blocked Orai1 currents57. It was proposed that the intracellular loop between TM2 and TM3 of Orai1 may function as an inactivation particle which mediates fast inactivation of Orai1/STIM157. Therefore, it is conceivable that protonation and deprotonation of H155 cause conformational changes of the loop between TM2 and TM3, leading to changes of Orai1/STIM1 channel activity.
Since ICRAC activation requires coupling of Orai and STIM, as well as gating of Orai1, it was previous demonstrated that acidic pH under hypoxia conditions causes uncoupling of STIM1 and Orai1 and thereby reduces current amplitude25. Moreover, intracellular alkalinization has been shown to inhibit Ca2+-ATPase (SERCA), leading to store depletion and Orai1/STIM1 activation58. Using high EGTA buffering condition to passively delete the store, we demonstrate that changes in internal pH directly influence pore-forming subunit Orai1 through protonation and deprotonation of H155 in the loop of TM2 and TM3, a previously unknown mechanism by which Orai1/STIM1 channel activity is regulated by pHi.
Conclusions
We demonstrate that the Orai1/STIM1 channel is regulated by changes of both intracellular and extracellular pH. Acidic internal and external pH reduce Orai1/STIM1 channel activity, whereas alkaline intracellular and extracellular pH enhance Orai1/STIM1 channel activity. Whereas E106 is responsible for external pH sensitivity when Ca2+ is the charge carrier as previously reported, we find that the residue E190 in TM3 is the major extracellular pH sensor when Na+ is the permeant cation. Moreover, we demonstrate that H155 in the intracellular TM2-TM3 loop is the intracellular pH sensor of Orai1/STIM1 channels. Similar to the pH sensitivity of over-expressed Orai1/STIM1 channels, the endogenous ICRAC is also regulated by changes of internal and external pH. Given the important roles of intracellular and extracellular pH in a variety of cellular functions, our results suggest that Orai1/STIM1 channels could be an essential mediator for pH induced modulation of physiological/pathological functions.
Materials and Methods
Molecular Biology
The constructs Orai 1–3 and STIM1 from Dr. Rao’s group20 were purchased from Addgene (IDs: 12199, 16369, 16370 and 19754 for Orai1, Orai2, Orai3, and STIM1, respectively). Mutations of Orai1 were generated by site-directed mutagenesis (QuickChange, Stratagene) following the manufacturer’s instruction. The predicted mutations were verified by sequencing analysis.
Cell culture and functional expression of Orai1/STIM1 and the mutants
HEK-293 cells were grown in DMEM/F12 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 mg/ml streptomycin at 37 °C in a humidity-controlled incubator with 5% CO2. Cells were transiently transfected with wild-type (WT) Orai1/STIM1 or its mutants by Lipofectamine 2000 (Invitrogen). We used 5 μl Lipofectamine 2000 for trasfection of the cells in a 35 mm culture dish. The GFP-containing pEGFP vector was transfected to the HEK293 cells as mock controls. Successfully transfected cells can be identified by their green fluorescence when illuminated at 480 nm. Electrophysiological recordings were conducted between 24 to 36 h after transfection. All patch-clamp experiments were performed at room temperature (20–25 °C).
RBL-2H3 cells were provided by Dr. D. Clapham (Harvard Medical School, Boston, MA). Cells were cultured in DMEM supplemented with 10% FBS and 100 U/ml penicillin and 100 mg/ml streptomycin. For electrophysiological experiments, cells were plated onto glass coverslips and used 12 h thereafter59.
Electrophysiology
Whole-cell currents were recorded using an Axopatch 200B amplifier. Data were digitized at 10 or 20 kHz, and digitally filtered off-line at 1 kHz. Patch electrodes were pulled from borosilicate glass and fire-polished to a resistance of ~3 MΩ when filled with internal solutions. Series resistance (Rs) was compensated up to 90% to reduce series resistance errors to <5 mV. Cells with Rs > 10 MΩ were discarded60.
For whole cell current recording, voltage stimuli lasting 250 ms were delivered at 1 s intervals with voltage ramps or voltage steps ranging from −120 to +100 mV. A holding potential of 0 mV was used for most experiments, unless otherwise stated. A fast perfusion system was used to exchange extracellular solutions, with a complete solution exchange achieved in about 1 to 3 s45. Original traces without leak subtraction were used in most figures unless otherwise stated. For the recording traces with leak subtraction (Figs 1, 2 and 4), leak currents were determined by taking an initial ramp current before Orai1/STIM1 activated61 or after Orai1/STIM1 currents were blocked by 10 μM La3+ 20,62.
The internal pipette solution for whole cell current recordings contained (in mM) 145 Cs-methanesulfonate (CsSO3CH3), 8 NaCl, 10 EGTA, and 20 HEPES, with pH adjusted to 7.2 with CsOH. MgCl2 (3 mM) was included in the pipette solution to block potential endogenous TRPM7 currents in the cells63,64. Acidic pHi was adjusted by citrate acid with 5 MES, and alkaline pHi was adjusted by CsOH.
The standard extracellular Tyrode’s solution for whole cell current recordings contained (mM): 145 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 10 glucose; pH was adjusted to 7.4 with NaOH. Extracellular solution with acidic pH was prepared by adjusting pH with HCl and 5 mM MES; and extracellular solution with basic pH was adjusted by NMDG. Divalent-free solution (DVF) contained (mM) 145 Na-SO3CH3, 20 HEPES, 5 EGTA, 2 EDTA and 10 glucose, with estimated free [Ca2+]<1 nM and free [Mg2+] ≈10 nM at pH 7.4. The acidic pH was adjusted with citric acid and 5 mM Mes; and basic pH was adjusted using NMDG. MaxChelator was used to calculate free Ca2+ and free Mg2+ concentrations (http://www.stanford.edu/~cpatton/webmaxcS.htm). The solutions containing 2, 20, and 120 mM Ca2+ concentrations were prepared by reducing Na+ concentrations in Tyrode’s solution to keep the same osmolarity. The acidic pH was adjusted with citric acid and 5 mM Mes; and basic pH was adjusted using NMDG as previously reported62. The amount of NMDG used for adjusting pH in different solutions did not influence Orai1/STIM1 current amplitude. For example, the average current amplitude in pHo 9.0 DVF solution adjusted with NMDG was 185.7 ± 20.3 pA/pF, and the current amplitude of Orai1/STIM1 recorded in pHo 9.0 DVF solution adjusted with NaOH was 187.4 ± 19.6 pA/pF (n = 4), and there was no statistical difference (p > 0.05). All the chemicals used in electrophysiological experiments were from Sigma-Aldrich. Solutions were titrated to their nominal pH at room temperature (20–25 °C).
Data Analysis
Pooled data are presented as mean ± SEM. Dose–response curves were fitted by an equation of the form: E = Emax{1/[1+(EC50/C)n]}, where E is the effect at concentration C, Emax is maximal effect, EC50 is the concentration for half-maximal effect and n is the Hill coefficient65. EC50 is replaced by IC50 if the effect is an inhibitory effect. Statistical comparisons were made using analysis of variance (ANOVA) and two-tailed t-test with Bonferroni correction. P < 0.05 indicated statistical significance.
Additional Information
How to cite this article: Tsujikawa, H. et al. Identification of key amino acid residues responsible for internal and external pH sensitivity of Orai1/stim1 channels. Sci. Rep. 5, 16747; doi: 10.1038/srep16747 (2015).
Supplementary Material
Acknowledgments
We would like to thank Dr. Stefan Feske for the Orai1 and STIM1 plasmids which were used in the beginning of the study. We would like to thank all the support from current lab members and previous lab members. We would also like to thank Yi Feng at Boston University School of Medicine for editing and proof-reading the manuscript. This work was generously supported by the National Institutes of Health, National Heart, Lung and Blood Institute (NHLBI, 2R01HL078960) and American Heart Association (AHA, 12GRNT12050683) to LY.
Footnotes
Author Contributions H.T. and A.Y. carried out most of the electrophysiological experiments and J.X. and Z.Y. designed and carried out mutagenesis experiments. H.T., J.X. and A.Y. analyzed data of majority of experiments. Z.Y., W.Y. and Y.H. performed some mutation and electrophysiological experiments. L.Y. conceived and supervised the work and wrote the paper.
References
- Srivastava J., Barber D. L. & Jacobson M. P. Intracellular pH sensors: design principles and functional significance. Physiology (Bethesda) 22, 30–39 (2007). [DOI] [PubMed] [Google Scholar]
- Lardner A. The effects of extracellular pH on immune function. J Leukoc Biol 69, 522–530 (2001). [PubMed] [Google Scholar]
- Lyall V. & Biber T. U. Potential-induced changes in intracellular pH. Am J Physiol 266, F685–696 (1994). [DOI] [PubMed] [Google Scholar]
- Mahnensmith R. L. & Aronson P. S. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res 56, 773–788 (1985). [DOI] [PubMed] [Google Scholar]
- Lishko P. V. & Kirichok Y. The role of Hv1 and CatSper channels in sperm activation. J Physiol 588, 4667–4672 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. J. & Nuccitelli R. Direct measurement of intracellular pH changes in Xenopus eggs at fertilization and cleavage. J Cell Biol 91, 562–567 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccitelli R., Webb D. J., Lagier S. T. & Matson G. B. 31P NMR reveals increased intracellular pH after fertilization in Xenopus eggs. Proc Natl Acad Sci USA 78, 4421–4425 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reshkin S. J. et al. Na+/H+ exchanger-dependent intracellular alkalinization is an early event in malignant transformation and plays an essential role in the development of subsequent transformation-associated phenotypes. FASEB J 14, 2185–2197 (2000). [DOI] [PubMed] [Google Scholar]
- Lindner D. & Raghavan D. Intra-tumoural extra-cellular pH: a useful parameter of response to chemotherapy in syngeneic tumour lines. Br J Cancer 100, 1287–1291 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagadic-Gossmann D., Huc L. & Lecureur V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ 11, 953–961 (2004). [DOI] [PubMed] [Google Scholar]
- Siffert W. & Akkerman J. W. Activation of sodium-proton exchange is a prerequisite for Ca2+ mobilization in human platelets. Nature 325, 456–458 (1987). [DOI] [PubMed] [Google Scholar]
- Marumo M., Suehiro A., Kakishita E., Groschner K. & Wakabayashi I. Extracellular pH affects platelet aggregation associated with modulation of store-operated Ca2+ entry. Thromb Res 104, 353–360 (2001). [DOI] [PubMed] [Google Scholar]
- Sandoval A. J. et al. Store-operated calcium entry mediates intracellular alkalinization, ERK1/2, and Akt/PKB phosphorylation in bovine neutrophils. J Leukoc Biol 82, 1266–1277 (2007). [DOI] [PubMed] [Google Scholar]
- Malayev A. & Nelson D. J. Extracellular pH modulates the Ca2+ current activated by depletion of intracellular Ca2+ stores in human macrophages. J Membr Biol 146, 101–111 (1995). [DOI] [PubMed] [Google Scholar]
- Guse A. H., Roth E. & Emmrich F. Ca2+ release and Ca2+ entry induced by rapid cytosolic alkalinization in Jurkat T-lymphocytes. Biochem J 301 (Pt 1), 83–88 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerschbaum H. H. & Cahalan M. D. Monovalent permeability, rectification, and ionic block of store-operated calcium channels in Jurkat T lymphocytes. J Gen Physiol 111, 521–537 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskay G., Kalman K., Van Kerkhove E., Steels P. & Ameloot M. Store-operated Ca2+-channels are sensitive to changes in extracellular pH. Biochem Biophys Res Commun 337, 571–579 (2005). [DOI] [PubMed] [Google Scholar]
- Iwasawa K. et al. Effects of extracellular pH on receptor-mediated Ca2+ influx in A7r5 rat smooth muscle cells: involvement of two different types of channel. J Physiol 503 (Pt 2), 237–251 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeromin A. V. et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakriya M. et al. Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233 (2006). [DOI] [PubMed] [Google Scholar]
- Vig M. et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C. Y. et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J. P. et al. SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat Cell Biol 11, 337–343 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. et al. Distinct Orai-coupling domains in STIM1 and STIM2 define the Orai-activating site. Nat Commun 5, 3183 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarella S. et al. Hypoxia-induced acidosis uncouples the STIM-Orai calcium signaling complex. J Biol Chem 286, 44788–44798 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimgeour N. R., Wilson D. P. & Rychkov G. Y. Glu¹106 in the Orai1 pore contributes to fast Ca2+-dependent inactivation and pH dependence of Ca2+ release-activated Ca2+ (CRAC) current. Biochem J 441, 743–753 (2012). [DOI] [PubMed] [Google Scholar]
- Beck A., Fleig A., Penner R. & Peinelt C. Regulation of endogenous and heterologous Ca release-activated Ca currents by pH. Cell Calcium 56, 235–243 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwack Y. et al. Biochemical and Functional Characterization of Orai Proteins. J Biol Chem 282, 16232–16243 (2007). [DOI] [PubMed] [Google Scholar]
- Ashmole I., Duffy S. M., Leyland M. L. & Bradding P. The contribution of Orai(CRACM)1 and Orai(CRACM)2 channels in store-operated Ca2+ entry and mediator release in human lung mast cells. PLoS One 8, e74895 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A. et al. TRPV1 is activated by both acidic and basic pH. J Neurosci 29, 153–158 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X., Stieber J., Ludwig A., Hofmann F. & Biel M. A single histidine residue determines the pH sensitivity of the pacemaker channel HCN2. J Biol Chem 276, 6313–6319 (2001). [DOI] [PubMed] [Google Scholar]
- Fujita F. et al. Intracellular alkalization causes pain sensation through activation of TRPA1 in mice. J Clin Invest 118, 4049–4057 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh B. I., Kim Y. K., Jabbar W. & Huang C. L. Conformational changes of pore helix coupled to gating of TRPV5 by protons. EMBO J 24, 3224–3234 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh B.-I., Sun T.-J., Lee J. Z., Chen H.-H. & Huang C.-L. Mechanism and Molecular Determinant for Regulation of Rabbit Transient Receptor Potential Type 5 (TRPV5) Channel by Extracellular pH. J Biol Chem 278, 51044–51052 (2003). [DOI] [PubMed] [Google Scholar]
- Chen X. H., Bezprozvanny I. & Tsien R. W. Molecular basis of proton block of L-type Ca2+ channels. J Gen Physiol 108, 363–374 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X.-H. & Tsien R. W. Aspartate Substitutions Establish the Concerted Action of P-region Glutamates in Repeats I and III in Forming the Protonation Site of L-type Ca2+ Channels. J Biol Chem. 272, 30002–30008 (1997). [DOI] [PubMed] [Google Scholar]
- Jordt S. E., Tominaga M. & Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA 97, 8134–8139 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S., Liu B. & Qin F. Low pH potentiates both capsaicin binding and channel gating of VR1 receptors. J Gen Physiol 122, 45–61 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu S., Liu B., Yao J., Fu Q. & Qin F. Uncoupling proton activation of vanilloid receptor TRPV1. J Neurosci 27, 12797–12807 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. H. & Zheng J. Proton block of proton-activated TRPV1 current. J Gen Physiol 146, 147–159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. et al. Molecular Determinants of Mg2+ and Ca2+ Permeability and pH Sensitivity in TRPM6 and TRPM7. J Biol Chem 282, 25817–25830 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. J. & Feske S. Regulation of lymphocyte function by ORAI and STIM proteins in infection and autoimmunity. J Physiol 590, 4157–4167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Pedi L., Diver M. M. & Long S. B. Crystal Structure of the Calcium Release–Activated Calcium Channel Orai. Science 338, 1308–1313 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg B. S., Wang Y. & Gill D. L. Orai channel pore properties and gating by STIM: implications from the Orai crystal structure. Sci Signal 6, pe9 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J., Li M. & Yue L. Potentiation of TRPM7 Inward Currents by Protons. J. Gen. Physiol. 126, 137–150 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Jiang J. & Yue L. Functional Characterization of Homo- and Heteromeric Channel Kinases TRPM6 and TRPM7. J Gen Physiol 127, 525–537 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez C., Morenilla-Palao C., Planells-Cases R., Merino J. M. & Ferrer-Montiel A. Identification of an Aspartic Residue in the P-loop of the Vanilloid Receptor That Modulates Pore Properties. J Biol Chem 275, 32552–32558 (2000). [DOI] [PubMed] [Google Scholar]
- Du J., Xie J. & Yue L. Modulation of TRPM2 by acidic pH and the underlying mechanisms for pH sensitivity. J. Gen. Physiol. 134, 471–488 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus J. G., Fleig A. & Penner R. The calcium-permeable non-selective cation channel TRPM2 is modulated by cellular acidification. J Physiol 588, 1227–1240 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W. et al. State-dependent inhibition of TRPM2 channel by acidic pH. J Biol Chem 285, 30411–30418 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J., Yang W., Beech D. J. & Jiang L. H. A residue in the TRPM2 channel outer pore is crucial in determining species-dependent sensitivity to extracellular acidic pH. Pflugers Arch 462, 293–302 (2011). [DOI] [PubMed] [Google Scholar]
- McNally B. A., Yamashita M., Engh A. & Prakriya M. Structural determinants of ion permeation in CRAC channels. Proc Natl Acad Sci USA 106, 22516–22521 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M., Navarro-Borelly L., McNally B. A. & Prakriya M. Orai1 mutations alter ion permeation and Ca2+-dependent fast inactivation of CRAC channels: evidence for coupling of permeation and gating. J Gen Physiol 130, 525–540 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S., Yee M. K., Gwack Y. & Ribalet B. The third transmembrane segment of orai1 protein modulates Ca2+ release-activated Ca2+ (CRAC) channel gating and permeation properties. J Biol Chem 286, 35318–35328 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amcheslavsky A., Safrina O. & Cahalan M. D. State-dependent block of Orai3 TM1 and TM3 cysteine mutants: insights into 2-APB activation. J Gen Physiol 143, 621–631 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak J. A., Matsushita M., Nairn A. C. & Cahalan M. D. Charge Screening by Internal pH and Polyvalent Cations as a Mechanism for Activation, Inhibition, and Rundown of TRPM7/MIC Channels. J Gen Physiol 126, 499–514 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S., Jung H.-J., Ribalet B. & Gwack Y. The Intracellular Loop of Orai1 Plays a Central Role in Fast Inactivation of Ca2+ Release-activated Ca2+ Channels. Journal of Biological Chemistry 285, 5066–5075 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. et al. Intracellular alkalinization induces cytosolic Ca2+ increases by inhibiting sarco/endoplasmic reticulum Ca2+-ATPase (SERCA). PLoS One 7, e31905 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Peng J.-B., Hediger M. A. & Clapham D. E. CaT1 manifests the pore properties of the calcium-release-activated calcium channel. Nature 410, 705–709 (2001). [DOI] [PubMed] [Google Scholar]
- Yue L., Navarro B., Ren D., Ramos A. & Clapham D. The Cation Selectivity Filter of the Bacterial Sodium Channel, NaChBac. J Gen Physiol 160, 845–853 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven W. I., Smyth J. T., Boyles R. R., Bird G. S. & Putney J. W. Complex Actions of 2-Aminoethyldiphenyl Borate on Store-operated Calcium Entry. J Biol Chem 283, 19265–19273 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M. & Prakriya M. Divergence of Ca2+ selectivity and equilibrium Ca2+ blockade in a Ca2+ release-activated Ca2+ channel. J Gen Physiol 143, 325–343 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runnels L. W., Yue L. & Clapham D. E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 291, 1043–1047 (2001). [DOI] [PubMed] [Google Scholar]
- Du J. et al. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res 106, 992–1003 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue L., Feng J., Wang Z. & Nattel S. Effects of ambasilide, quinidine, flecainide and verapamil on ultra-rapid delayed rectifier potassium currents in canine atrial myocytes. Cardiovasc Res 46, 151–161 (2000). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.