Abstract
Background: Quantitative assessment of the dynamic relationship between plasma and interstitial fluid (ISF) glucose and the estimation of the plasma-to-ISF delay are of major importance to determine the accuracy of subcutaneous glucose sensors, an essential component of open- and closed-loop therapeutic systems for type 1 diabetes mellitus (T1DM). The goal of this work is to develop a model of plasma-to-ISF glucose kinetics from multitracer plasma and interstitium data, obtained by microdialysis, in healthy and T1DM subjects, under fasting conditions.
Materials and Methods: A specific experimental design, combining administration of multiple tracers with the microdialysis technique, was used to simultaneously frequently collect plasma and ISF data. Linear time-invariant compartmental modeling was used to describe glucose kinetics from the tracer data because the system is in steady state.
Results: A two-compartment model was shown accurate and was identified from both plasma and ISF data. An “equilibration time” between plasma and ISF of 9.1 and 11.0 min (median) in healthy and T1DM subjects, respectively, was calculated.
Conclusions: We have demonstrated that, in steady-state condition, the glucose plasma-to-ISF kinetics can be modeled with a linear two-compartment model and that the “equilibration time” between the two compartments can be estimated with precision. Future studies will assess plasma-to-interstitium glucose kinetics during glucose and insulin perturbations in both healthy and T1DM subjects.
Introduction
Glucose monitoring plays a fundamental role in diabetes management. The most used approach is the self-monitoring of capillary blood glucose, performed before and after meals. However, the few self-monitoring of capillary blood glucose measurements per day cannot give a complete picture of glucose dynamics. To overcome self-monitoring of capillary blood glucose limitations, in the last 10–15 years subcutaneous continuous glucose monitoring (CGM) sensors, measuring glucose concentration in the interstitial fluid (ISF), have been introduced. A quantitative assessment of the dynamic relationship between plasma and ISF glucose is fundamental to determine the accuracy of subcutaneous CGM sensors, which are an essential component of open- and closed-loop system therapy for type 1 diabetes mellitus (T1DM).1,2
Several studies have investigated the temporal relationship between plasma and ISF glucose in subjects with and without diabetes, using different methodologies, such as microdialysis/open-flow microperfusion3–10 or electrochemical11–15 measurement techniques. A wide range of delays from 2 min6 to 45 min7 has been reported, possibly due to the different techniques used for subcutaneous glucose measurement, as well as different experimental conditions and subjects. Uncertainty in the underlying temporal relationship between plasma and ISF glucose has been cited as a reason that closed-loop control of glucose may not be successful.16 In addition, comparison of CGM data time series with temporally matched blood glucose reference measurement time series appeared to show large temporal delays of the CGM signal, but these were later attributed to the filtering used on the CGM signal to suppress noise.17 Finally, most of the studies have also reported a wide range of gradients between the capillary and interstitial glucose measurements, likely due to a temporary local tissue damage and inflammation due to device insertion.8,18,19
In the last few years models were developed to assess the plasma-to-interstitium glucose kinetics by using different techniques for ISF glucose measurement and experimental designs. Most of them assume that glucose exchange between plasma and ISF is driven by diffusion and that an irreversible glucose disposal takes place in the ISF. In Rebrin et al.12 and Steil et al.,14 such a model was identified from plasma glucose and subcutaneous glucose sensor current (a surrogate for ISF glucose), in dogs without diabetes during different hyperglycemic clamps and in humans without diabetes during insulin-induced hypoglycemia. In Regittnig et al.,9 a similar model was identified from plasma and ISF glucose data, sampled with a high flow rate open-flow microperfusion technique to obtain an acceptable time resolution (5 min), in skeletal muscle and adipose tissue of fasted normal subjects after an intravenous infusion of labeled glucose. In Wilinska et al.,10 several models have been postulated and identified from plasma and ISF glucose data, sampled with a low time resolution open-flow microperfusion technique (30 min), in T1DM subjects after a standard meal: the selected model included, at variance with the previous ones, a constant glucose disposal in the ISF and an insulin-stimulated glucose transfer from plasma to ISF.
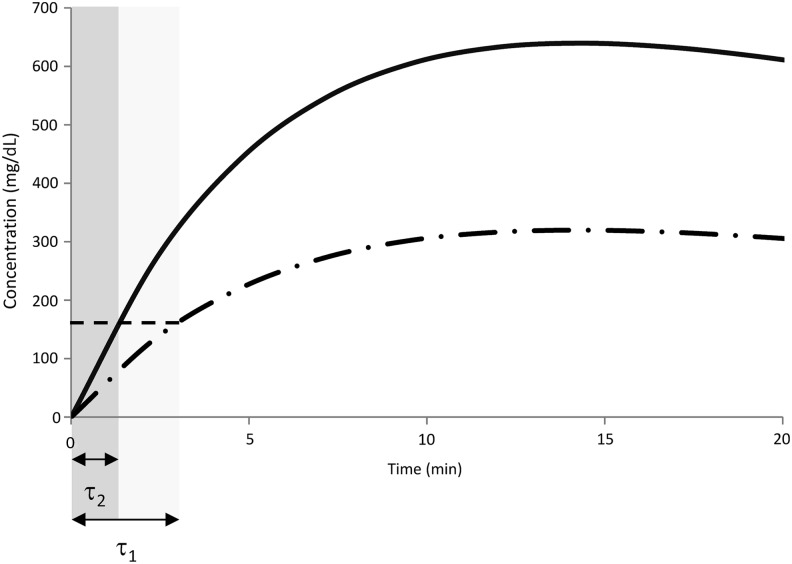
Recently, an innovative multiple tracer experimental design was developed to accurately measure the plasma-to-ISF glucose kinetics3,4: plasma and ISF tracer glucose levels were simultaneously frequently sampled to achieve a fine grid time resolution (1-min sampling), thus overcoming the intrinsic delay of the microdialysis technique. However, this first exploratory data analysis was semiquantitative because the “time lag” between plasma and ISF was defined as the time taken by the tracer itself to reach detectable levels in the ISF. With this definition, the authors found that, in the overnight fasted state, the “time lag” was, on average, 5.8 min in healthy individuals and 6.8 min in those with T1DM. However, this “time lag” is dependent on the experimental condition (e.g., how and how much tracer is infused) (Fig. 1). The only way to rigorously assess such delay in a protocol-independent fashion is, first, to fit the above data with an appropriate model of the plasma to ISF kinetics and, second, to use the model to calculate the “equilibration time,” which is an intrinsic property of the system defined as the time constant characterizing the response of the ISF compartment to a unit step infusion in plasma. This is the purpose of the present work.
FIG. 1.
Comparison between simulated tracer concentrations in interstitial fluid after an injection of a tracer bolus of amount D1 (dotted-dashed line) or D2 = 2 · D1 (continuous line). Defining the “time lag” (τ) as in Basu et al.3 (i.e., the time taken by the tracer itself to reach detectable values in the interstitial fluid), provides a protocol-dependent measurement of τ, in particular, τ1 > τ2.
Experimental Design, Database, and Model
Experimental design
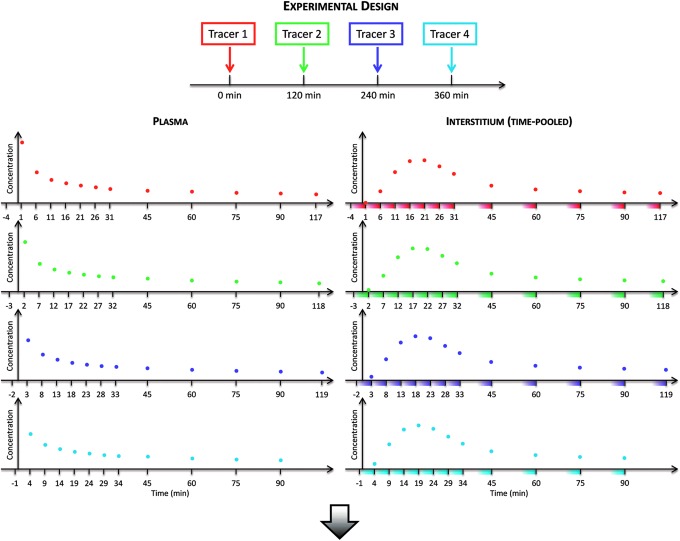
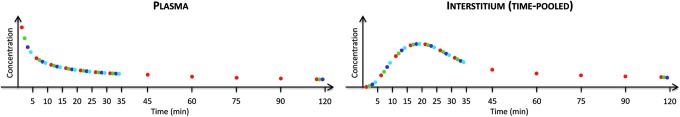
The multitracer and microdialysis experimental design has been recently proposed by Basu et al.3,4 In brief, in order to accurately measure the microdialysis samples, a multitracer technique has been used to overcome the intrinsic delay introduced by the microdialysate catheter itself.18,19 A schematic representation of the experimental design is shown in Figure 2: four glucose tracers were rapidly injected intravenously 2 h apart (Fig. 2, top panel), whereas plasma and time-pooled microdialysate effluent samples were simultaneously frequently collected for glucose and tracer enrichments (Fig. 2, middle panel). Starting with different time intervals prior to each tracer bolus, all tracers samples were collected with a 5-min sampling interval (i.e., the minimum microdialysis sampling period with the optimal microdialysis pump rate used3,4) for the first 30 min and periodically thereafter until the subsequent tracer bolus. Thanks to the steady-state condition of the glucose system, a single tracer curve for both plasma and ISF can be obtained by aggregating all tracers data (Fig. 2, bottom panel).
FIG. 2.
(Top panel) Schematic representation of the experimental design showing the sequence of intravenous boluses of labeled glucose during the entire experiment, (left and right middle panels) plasma and time-pooled (shaded colored areas) microdialysate, respectively, samples in the interstitium for each tracer with different sampling times, and (bottom panel) single tracer curve for both plasma and interstitium obtained by aggregating all the data from each tracer experiment.
Given that the kinetics of the four tracers are identical, the aggregation of tracer data allowed us to build a high-resolution (1-min) virtual tracer curve both in plasma and also in the ISF. To the best of our knowledge, this is the first time that a 1-min sampled tracer curve has been made available in the ISF. In fact, in standard single-tracer protocols, the achievement of such a high time resolution in ISF sampling requires very high pump flow rates, which, paradoxically, lead to an incomplete mixing between the perfusate and the ISF (reduced glucose recovery). The sophisticated technique presented here has overcome the intrinsic delay introduced by the low pump rates previously used for an optimal glucose recovery,18,19 without using any calibration technique.9
The development of such a unique experimental design was a fundamental component of this work to solidify the modeling exercise to quantitatively describe the plasma-to-ISF glucose kinetics and the estimation of the plasma-to-ISF delay. In fact, the high-resolution sampling grid is necessary to accurately and precisely estimate the delay between plasma and ISF.
Database
Eight healthy subjects (age, 40 ± 19 years; weight, 73.7 ± 14.9 kg; body mass index, 25.4 ± 3.1 kg/m2)3 and six T1DM subjects (age, 44 ± 14 years; weight, 74.8 ± 10.6 kg; body mass index, 25.2 ± 3.6 kg/m2)4 underwent the experimental design described previously.3,4 All glucose tracers ([1-13C], [6,6-2H2], [3-3H], and [2-13C]) were intravenously infused into four healthy subjects, whereas the remaining healthy and all the T1DM subjects were infused with the three stable isotopes only ([1-13C], [6,6-2H2], and [2-13C]) ([3-3H]glucose was replaced with saline because it was not possible to reliably measure it in the pooled microdialysate samples).
Because the tracee system is in steady-state condition for the entire duration of the experiment (in both healthy and T1DM populations),3,4 the analysis of stable isotopic tracer data can be done applying the same kinetic formalism of radioactive tracer data by using the tracer-to-tracee mass ratio.20 Starting from the measurements of tracer enrichment, for both plasma and ISF, the tracer-to-tracee mass ratios were calculated as:
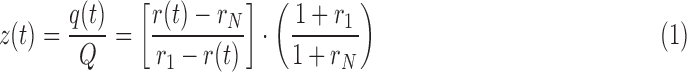 |
where q(t) is the tracer glucose mass, Q is the tracee glucose mass, which is assumed to be constant, r(t) is the ratio of the masses of the labeled and unlabeled species, and rI and rN are isotope ratios in a sample of pure tracer and tracee, respectively. Tracer-to-tracee mass ratio was calculated for each stable isotopic tracer, and the single tracer curve was obtained by aggregating data for all tracers as described above. However, because the [1-13C]glucose level was still above the baseline value, in both plasma and ISF, at the time the [2-13C]glucose was administered, in order to correctly calculate the single tracer curve (Fig. 2, bottom panel), the interference from [1-13C]glucose with [2-13C]glucose was removed by subtracting the residual tail of [1-13C]glucose from [2-13C]glucose.
To account for the time to cover the catheter dead space (6.2 min), the timing of microdialysate sample collection was re-indexed.3,4 Moreover, because a 5-min period (Δt) was necessary to collect an adequate sample volume to reliably measure glucose tracers from the microdialysate effluent, the measurement equation for the microdialysate samples of ISF (zI) was averaged over a 5-min period:
 |
where tk is the time at which the sample is drawn.
Of note is that one healthy subject has been excluded from the analysis (hence, n = 7) because of an interference in [2-13C]glucose chromatography in the microdialysate data.
The model
Model structure
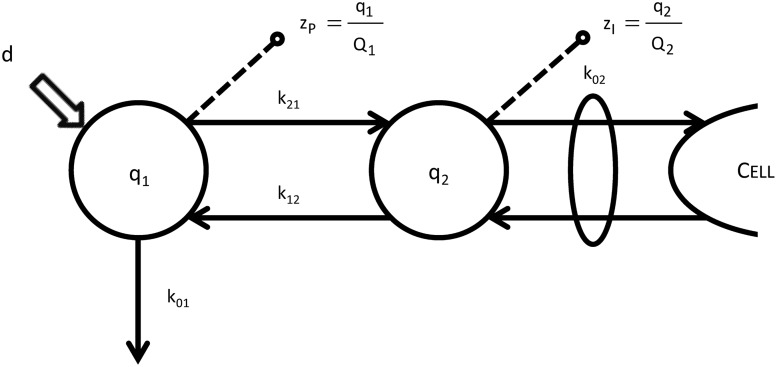
The constant steady-state condition of the tracee system allows the use of a linear and time-invariant description of the kinetics of the tracer system.21 A linear two-compartment model is used to describe glucose kinetics between plasma and ISF compartments as shown in Figure 3. The model is described by the following equations:
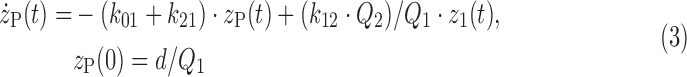 |
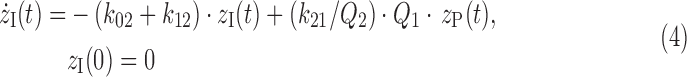 |
FIG. 3.
The plasma–interstitial fluid model used in both healthy and type 1 diabetes mellitus subjects. See Eqs. 3 and 4 for the model parameterization details; q1 and q2 denote labeled glucose mass, whereas Q1 and Q2 denote the tracee glucose mass in the first (plasma) and second (interstitial fluid) compartments, respectively (in mg/kg). Of note is that to account for time-pooled microdialysate samples, interstitial fluid tracer-to-tracee model prediction is averaged over the time-pooled collection period (Δt = 5 min).
where zP and zI are the glucose tracer-to-tracee mass ratio in the plasma and ISF compartments, respectively (dimensionless), Q1 and Q2 are the tracee mass in the plasma and ISF compartments, respectively (in mg/kg), k21 and k12 are the rate constants describing plasma-to-ISF glucose exchange (in min–1), k01 is the rate constant describing glucose disposal in plasma (in min–1), k02 is the rate constant describing the net ISF–cell transmembrane transport (in min−1), and d is the bolus amount of labeled glucose (in mg/kg).
A fundamental parameter describing the delay between the plasma and ISF glucose is the “equilibration time” constant (τ). It is defined as the time constant characterizing the response of the ISF compartment to a unit step input in plasma and can be easily derived from Eq. 4 as:
 |
Model identification
The model in Eqs. 3 and 4 is a priori nonidentifiable21 but can be put in its uniquely identifiable reparameterization by defining the new parameter vector (θ):
 |
It is notable that, even with this reparameterization, τ is still calculable by θ (Eqs. 5 and 6).
The model was numerically identified from both plasma and ISF glucose tracer-to-tracee mass ratio data, in its uniquely identifiable parameterization (Eq. 6), by nonlinear weighted least squares for multiple datasets.22 In particular, measurement error was assumed to be independent Gaussian, with zero mean and known SD up to a proportionally constant a posteriori estimated for each dataset.22 Precision of parameter estimates was obtained from the inverse of the Fisher information matrix.23
To account for the time-pooled microdialysate samples, ISF tracer-to-tracee model prediction (zI) was averaged over a 5-min period:
 |
In this way, estimation of model parameters is not biased by time-pooled microdialysate measurement technique.
Statistical analysis
Data are presented as median ± interquartile range values. Two-sample comparisons were done by the Mann–Whitney U test.
Results
Model fit
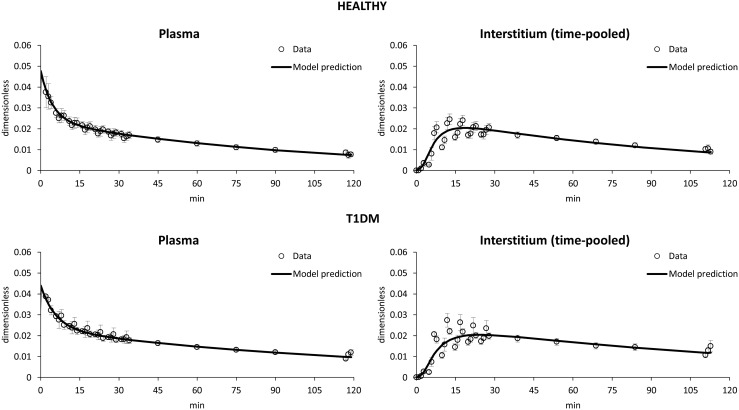
The model was identified in both healthy and T1DM subjects (Fig. 4). The model reproduced well both plasma and ISF data: after an intravenous bolus of labeled glucose, a rapid fall in plasma level occurred (Fig. 4, left), whereas a slower rise and decay in ISF (Fig. 4, right) glucose tracer-to-tracee mass ratio were predicted in both healthy (Fig. 4, top) and T1DM (Fig. 4, bottom) subjects.
FIG. 4.
Average data versus model prediction of (left) plasma and (right) interstitium tracer-to-tracee mass ratio in (top) healthy and (bottom) type 1 diabetes mellitus (T1DM) subjects.
Parameter estimates
All model parameters were numerically identified with good precision. In Table 1 median values of model parameters and their precision, expressed as coefficient of variation, for healthy and T1DM subjects are reported. Median values of parameters k01 + k21, k12 · Q2, and k02 + k12 are quite similar between the two populations (0.10 vs. 0.09 min−1, 6.6 vs. 9.7 mg/kg/min, and 0.11 vs. 0.09 min−1, respectively), whereas parameters Q1 and k21/Q2 are statistically different between the two populations (96 vs. 150 mg/kg and 0.0013 vs. 0.0007 kg/mg/min, respectively) (P < 0.05). This reflects the higher average plasma glucose concentrations measured in T1DM versus healthy subjects in Basu et al.3,4
Table 1.
Values of Model Parameters and Their Precision
| Parameter | Subjects | ||
|---|---|---|---|
| Definition | Unit | Healthy | T1DM |
| k01 + k21 | min−1 | 0.10 ± 0.08 (17 ± 5) | 0.09 ± 0.03 (15 ± 10) |
| k12 · Q2 | min−1·mg/kg | 6.6 ± 5.4 (15 ± 5) | 9.7 ± 5.5 (14 ± 8) |
| Q1 | mg/kg | 96 ± 21 (5 ± 2) | 150 ± 28 (6 ± 3) |
| k02 + k12 | min−1 | 0.11 ± 0.03 (10 ± 2) | 0.09 ± 0.03 (11 ± 4) |
| k21/Q2 | min−1·kg/mg | 0.0013 ± 0.0006 (13 ± 4) | 0.0007 ± 0.0001 (12 ± 5) |
| τ | min | 9.1 ± 2.3 | 11.0 ± 3.3 |
Data are median ± interquartile range values, with their precision in parentheses (% coefficient of variation).
T1DM, type 1 diabetes mellitus.
The “equilibration time” τ is slightly lower in healthy versus T1DM subjects (9.1 vs. 11.0 min), but no statistical significance is observed.
Discussion
There is an emerging and renewed interest in the accuracy of the subcutaneous CGM systems because of their vital role in contemporary open- and closed-loop systems for T1DM.1 Improvements in sensor accuracy have been cited as a major factor in the acceleration of clinical research on closed-loop systems.2 CGM sensors allow a minimally invasive quasicontinuous glucose measurement in the ISF rather than the more invasive few self-monitoring of capillary blood glucose measurements per day in blood. However, the intrinsic physiological delay between plasma and ISF glucose has been considered to be challenging for the development of safe and effective closed-loop control algorithms based on CGM data.
Different definitions of delay have been proposed in the literature, and this, together with the variety of experimental protocols used, may in part explain the wide range of values reported in different studies. For instance, Basu et al.3 defined the “time lag” as the time taken by the tracer, injected in plasma, to reach detectable levels in the ISF. This definition is easy to grasp, but it is protocol-dependent (i.e., it does not capture the intrinsic feature of the system) (Fig. 1).
The proper way to accurately assess the plasma-to-ISF delay in the adipose tissue is through a modeling approach. Thus, the aim of this work was to exploit the unique data of Basu et al.3,4 to build a model of the plasma-to-ISF kinetics in healthy and T1DM subjects under fasting conditions. A linear two-compartment model was simultaneously identified from both plasma and ISF glucose tracer-to-tracee mass ratio data.
Because a high variability of microdialysate data is observed, likely due to the complexity of the experimental protocol, which may restrain the assumption that glucose concentration in the interstitium is exactly the same during the administration of the three tracers, one could question the validity of parameter estimates, including the equilibration time. On the contrary, the precision of estimated model parameters, including τ, are satisfactory (within 7–18%). Hence, we can conclude that, despite the large scatter in the data, the model provides good estimates of the equilibration time (coefficient of variation, 10–11% of the median).
Despite the large variability of the plasma-to-ISF delays reported in literature, most investigators reported a range from 5 to 10 min.3–5,9,10,12,14,15 In this work, a model-derived “equilibration time” of 9.1 and 11.0 min in healthy and T1DM subjects, respectively, was calculated in the steady-state condition. No significant difference was observed between the two populations, but the sample size does not permit a definitive conclusion. This is certainly a limitation of this study. However, the slightly increased distribution in T1DM may be due to well-established local anatomical differences of the abdominal subcutaneous space that could exist in this population.
Results were comparable with other model-derived plasma-to-ISF “equilibration time” constants reported in the literature. Ranges of delays of 3–14 min and 6–8 min were reported in dogs without diabetes during different hyperglycemic clamps12 and in humans during insulin-induced hypoglycemia,14 respectively, using the subcutaneous glucose sensor current as a surrogate for ISF glucose level. In Regittnig et al.,9 the measurement of the “equilibration time” derived from the appearance of an intravenously infused glucose tracer in the ISF reported an average value of 9–10 min in adipose tissue and skeletal muscle of healthy subjects during fasting conditions. In Wilinska et al.,10 an average “equilibration time” of 10.7 min was reported in ISF glucose in T1DM subjects after a standard meal. Moreover, given that studies of glucose rate of change have shown rates of change of 2 mg/dL/min or less 90% of the time in T1DM subjects, the inherent inaccuracy of ISF glucose sensors can be bounded at approximately 15–20 mg/dL.24
Finally, these results are built on those reported by Basu et al.3,4 for the “time lag.” The model-derived “equilibration time” (τ) represents the time it takes the ISF compartment's response to a unit step input in plasma to reach 63% of its asymptotic value, whereas the “time lag” calculated by Basu et al.3,4 represents the time it takes the tracer to reach detectable levels in the ISF. The “equilibration time” is an intrinsic feature of the system, is protocol-independent, and better represents the plasma-to-ISF glucose kinetics than the “time lag” defined by Basu et al.3,4 However, despite the different meaning of the two parameters, this study agrees with Basu et al.3,4 in finding that the delay is slightly, but not significantly, higher in T1DM than in healthy subjects.
At variance with other studies,9,10 in the current study it was not possible to estimate the plasma-to-ISF glucose gradient by a combination of model parameters as reported by Rebrin et al.12 This was due to the fact that the model was identified on plasma and ISF tracer-to-tracee mass ratio rather than glucose tracer concentration data, thus not allowing the calculation of the ratio between the plasma and ISF labeled glucose concentration. Finally, these experimental conditions did not allow detection of the stimulatory effect of insulin on glucose disposal, the so-called “push–pull” phenomenon, which has been widely discussed in the literature.25 However, current studies evaluating the effect of non–steady-state conditions and stimuli (i.e., meals and exercise) on “equilibration time” are currently being conducted to advance our knowledge in this area.
Conclusions
In this work a modeling analysis of plasma-to-ISF glucose kinetics was accurately assessed from multitracer plasma and microdialysis data in healthy and T1DM subjects under fasting conditions. A multitracer protocol was specifically developed to achieve a high-resolution time grid to overcome the intrinsic delay of the microdialysis technique. A linear two-compartment model was identified from both plasma and ISF glucose data, and a median “equilibration time” between the two compartments of 9.1 and 11.0 min in healthy and T1DM subjects, respectively, was calculated. The results reported here from the multitracer study and modeling analysis can be used to further improve the accuracy of subcutaneous CGM sensors for T1DM diabetes management. Future studies under dynamic conditions, including meals and exercise, will be addressed to complete the picture of plasma-to-ISF glucose kinetics.
Acknowledgments
This work was supported by funding from the Helmsley Charitable Trust (grant 2012PG-T1D005) and Dexcom Inc. to R.B., National Institutes of Health grant DK29953 to R.B., National Institutes of Health grants DK085516 and DK DP3 094331 to A.B. and Y.C.K., and grant UL1 TR000135 from the National Center for Advancing Translational Science, a component of the National Institutes of Health. C.C. and C.D.M. are partially funded by the Italian Ministero dell'Istruzione, dell'Università e della Ricerca (Progetto FIRB 2008). M. Schiavon was funded by a Fellowship of the University of Padova.
Author Disclosure Statement
T.P. is an employee of IDDM Consulting. M. Schiavon, C.D.M., S.D., M. Slama, Y.C.K., A.B., R.B., and C.C. declare no competing financial interests exist.
M. Schiavon, C.D.M., and C.C. developed the model, analyzed the results, and drafted the manuscript. S.D., M. Slama, Y.C.K., A.B., and R.B. performed the experiment. Y.C.K., T.P., AB., R.B., and C.C. developed the protocol and experimental design. Y.C.K., T.P., A.B., and R.B. contributed to the discussion of the results, commented, and edited the manuscript.
References
- 1.Cobelli C, Renard E, Kovatchev BP: Artificial pancreas: past, present, future. Diabetes 2011;60:2672–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peyser T, Dassau E, Breton M, et al. : The artificial pancreas: current status and future prospects in the management of diabetes. Ann N Y Acad Sci 2014;1311:102–123 [DOI] [PubMed] [Google Scholar]
- 3.Basu A, Dube S, Slama M, et al. : Time lag of glucose from intravascular to interstitial compartment in humans. Diabetes 2013;62:4083–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basu A, Dube A, Veettil S, et al. : Time lag of glucose from intravascular to interstitial compartment in type 1 diabetes. J Diabetes Sci Technol 2015;9:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bantle JP, Thomas W: Glucose measurement in patients with diabetes mellitus with dermal interstitial fluid. J Lab Clin Med 1997;130:436–441 [DOI] [PubMed] [Google Scholar]
- 6.Sternberg F, Meyerhoff C, Mennel FJ, et al. : Does fall in tissue glucose precede fall in blood glucose? Diabetologia 1996;39:609–612 [DOI] [PubMed] [Google Scholar]
- 7.Pfeiffer EF, Meyerhoff F, Bischof F, et al. : On line glucose monitoring of subcutaneous tissue glucose is feasible by combining portable glucosensor with microdialysis. Horm Metab Res 1993;25:121–124 [DOI] [PubMed] [Google Scholar]
- 8.Wientjes KJ, Schoonen AJ: Determination of time delay between blood and interstitial adipose tissue glucose concentration change by microdialysis in healthy volunteers. Int J Artif Organs 2001;24:884–889 [PubMed] [Google Scholar]
- 9.Regittnig W, Ellmerer M, Fauler G, et al. : Assessment of transcapillary glucose exchange in human skeletal muscle and adipose tissue. Am J Physiol Endocrinol Metab 2003;285:E241–E251 [DOI] [PubMed] [Google Scholar]
- 10.Wilinska ME, Bodenlenz M, Chassin LJ, et al. : Interstitial glucose kinetics in subjects with type 1 diabetes under physiologic conditions. Metabolism 2004;53:1484–1491 [DOI] [PubMed] [Google Scholar]
- 11.Pickup JC, Shaw GW, Claremont DJ: In vivo molecular sensing in diabetes mellitus: an implantable glucose sensor with direct electron transfer. Diabetologia 1989;32:213–217 [DOI] [PubMed] [Google Scholar]
- 12.Rebrin K, Steil GM, Van Antwerp WP, et al. : Subcutaneous glucose predicts plasma glucose independent of insulin: implications for continuous monitoring. Am J Physiol 1999;277:E561–E571 [DOI] [PubMed] [Google Scholar]
- 13.Boyne MS, Silver DM, Kaplan J, et al. : Timing of changes in interstitial and venous blood glucose measured with a continuous subcutaneous glucose sensor. Diabetes 2003;52:2790–2794 [DOI] [PubMed] [Google Scholar]
- 14.Steil GM, Rebrin K, Hariri F, et al. : Interstitial fluid glucose dynamics during insulin-induced hypoglycemia. Diabetologia 2005;48:1833–1840 [DOI] [PubMed] [Google Scholar]
- 15.Kamath A, Mahalingam A, Brauker J: Analysis of time lags and other sources of error of the DexCom SEVEN continuous glucose monitor. Diabetes Technol Ther 2009;11:689–695 [DOI] [PubMed] [Google Scholar]
- 16.Winikoff J, Drexler A: Who needs an artificial pancreas? J Diabetes 2013;5:254–257 [DOI] [PubMed] [Google Scholar]
- 17.Rebrin K, Sheppard NF, Jr, Steil GM: Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol 2010;4:1087–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bolinder J, Ungerstedt U, Arner P: Microdialysis measurement of the absolute glucose concentration in subcutaneous adipose tissue allowing glucose monitoring in diabetic patients. Diabetologia 1992;35:1177–1180 [DOI] [PubMed] [Google Scholar]
- 19.Bolinder J, Ungerstedt U, Arner P: Long-term continuous glucose monitoring with microdialysis in ambulatory insulin-dependent diabetic patients. Lancet 1993;342:1080–1085 [DOI] [PubMed] [Google Scholar]
- 20.Cobelli C, Toffolo GM, Foster DM: Tracer-to-tracee ratio for analysis of stable isotope tracer data: link with radioactive kinetic formalism. Am J Physiol 1992;262:E968–E974 [DOI] [PubMed] [Google Scholar]
- 21.Carson ER, Cobelli C: Modelling Methodology for Physiology and Medicine. San Diego, CA: Academic Press, 2001 [Google Scholar]
- 22.Cobelli C, Foster DM, Toffolo GM: Tracer Kinetics in Biomedical Research: From Data to Model. New York: Kluwer Academic/Plenum, 2000 [Google Scholar]
- 23.Carson ER, Cobelli C, Finkelstein L: The Mathematical Modeling of Endocrine-Metabolic Systems. Model Formulation, Identification and Validation. New York: Wiley, 1983 [Google Scholar]
- 24.Weinstein RL, Schwartz SL, Brazg RL, et al. : Accuracy of the 5-Day FreeStyle Navigator continuous glucose monitoring system. Diabetes Care 2007;30:1125-–130 [DOI] [PubMed] [Google Scholar]
- 25.Aussedat B, Dupire-Angel M, Gifford R, et al. : Interstitial glucose concentration and glycemia: implications for continuous subcutaneous glucose monitoring. Am J Physiol Endocrinol Metab 2000;278:E716–E728 [DOI] [PubMed] [Google Scholar]