Abstract
Objective: In this radiographic and microbiologic split-mouth clinical trial, efficacy of a diode laser as an adjunct to conventional scaling in the nonsurgical treatment of peri-implantitis was investigated. Background data: Eradication of pathogenic bacteria and infected sulcular epithelium presents a significant challenge in the nonsurgical treatment of peri-implantitis. Materials and methods: Ten patients (mean age, 55.1 years; SD, 11.4) with 48 two piece, rough-surface implants and diagnosed with peri-implantitis were recruited (NCT02362854). In addition to conventional scaling and debridement (control group), crevicular sulci and the corresponding surfaces of 24 random implants were lased by a diode laser running at 1.0 W power at the pulsed mode (λ, 810 nm; energy density, 3 J/cm2; time, 1 min; power density, 400 mW/cm2; energy, 1.5 J; and spot diameter, 1 mm); (laser group). Healing was assessed via periodontal indexes (baseline and after 1 and 6 months after the intervention), microbiologic specimens (baseline and after 1 month), and radiographs (baseline and after 6 months). Results: Baseline mean pocket depths (4.71, SD, 0.67; and 4.38, SD 0.42 mm) and marginal bone loss (2.71, SD 0.11; and 2.88, SD 0.18 mm) were similar (p = 0.09 and p = 0.12) between the control and laser groups, respectively. After 6 months, the laser group revealed higher marginal bone loss (2.79, SD 0.48) than the control groups (2.63, SD 0.53) (p < 0.0001). However, in both groups, the microbiota of the implants was found unchanged after 1 month. Conclusions: In this clinical trial, adjunct use of diode laser did not yield any additional positive influence on the peri-implant healing compared with conventional scaling alone.
Introduction
Peri-implantitis is an “inflammatory process” affecting the tissues around an already osseointegrated implant, and it is becoming the most challenging problem in clinical implant dentistry.1 In the early stage, the lesion is isolated within the soft tissues (perimucositis); however, disease progression may result in the loss of supporting alveolar bone characterized by crater-like defects in the marginal crestal zone.2
According to a review, peri-implant mucositis occurs in ∼80% of the patients (50% of the implants), and in 28–56% of patients (12–40% of implants), the disease translates into peri-implantitis.3 Despite various proposed treatment modalities, there are currently no established criteria for the definitive treatment and eradication of perimucositis and peri-implantitis.4 Decontamination of the implant surface and eradication of the biofilm and endotoxins are a major challenge in its treatment.5 In addition to conventional scaling, many adjuvant methods have also been introduced, including citric acid application,6 air flow,7 and laser irradiation.8
Lasers can effortlessly irradiate the entire surface, especially in irregular and rough areas where mechanical instruments cannot easily reach. They not only eliminate bacteria but also inactivate bacterial diffused toxins.9
The aim of this study was to compare the efficacy of an 810 nm diode laser (DL) as an adjunct to conventional scaling in the nonsurgical treatment of mild to moderate peri-implantitis. A split-mouth controlled clinical trial was conducted to test the following null hypothesis: adjunct application of an 810 nm DL in the conventional nonsurgical treatment of peri-implantitis is not associated with a statistically significant difference in microbial counts, marginal bone loss, and peri-implant parameters.
Materials and Methods
The study was approved by the Istanbul University Ethical Committee of human subjects (2010/357-80), conducted according to the Helsinki Declaration of 1975 as revised in 2008, and was registered as a clinical trial (NCT02362854). In order to estimate the required sample size, the results of a previous study10 were referred to, and a minimum of 22.1 subjects (implants) per group were determined to detect a 30% difference of bacterial load with a statistical power of 80% at the level α = 0.05.
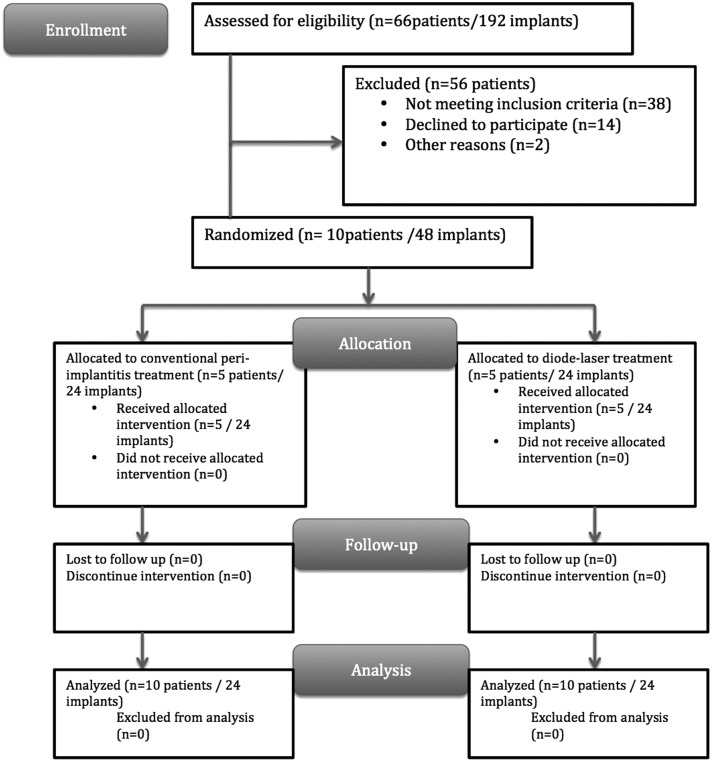
Volunteers were selected from among the patients who applied to the department clinic between February 2010 and May 2013. Initially, all patients were investigated by panoramic radiographs and clinical examination. Patients with at least two functioning bilateral rough-surfaced implants and demonstrating bleeding on probing (BOP), plaque, pain, and/or suppuration, 4–6 mm of periodontal probing depth, and <3 m of marginal bone loss (MBL) were included in the study. To avoid bias, patients with ongoing or a history of periodontitis and patients prescribed any antibiotics 3 months prior to the initiation of the study were excluded. Because implants with an MBL of >3 mm necessitate a surgical approach [cumulative interceptive supportive therapy (CIST) protocol],11 any patients carrying such implants were also excluded. Accordingly, 10 patients (3 males and 7 females) with a total of 48 bilateral implants were recruited consecutively for the study. Written approvals of participation were obtained from all volunteers. The mean age of the patients was 55.1 years (range, 43–76; SD, 12.4). All patients were systemically healthy, and there were no smokers. All of the patients were partially edentulous. Prior to the initiation of the study, proper oral hygiene instructions were given to all patients (Fig. 1). The corresponding implants of the patients were in a tapered root form design and had a rough surface (acid etching and sand blasting) that was marketed by different manufacturers (15 MIS, Seven, Shalomi, Israel; 12 Camlog Biotechnologies, Karlhusen, Germany; 8 Nobel Biocare, Replace, Karloskoga, Sweden; and 7 Biohorizons, Birmingham, AL). The mean function time of the implants was 19.4 months (SD, 3.2; range 12.2–25.2), and all implants were restored by cement-retained fixed metal-ceramic prostheses. Any restorations with overhanging or poor margins were discarded or corrected to establish proper margin contours. An acrylic-based temporary crown was cemented on the treated implants if the permanent restoration was faulty. The occlusal contacts were also checked to ensure the absence of overloading. Abutments were not removed, because plaque-retaining parts or imperfections were not detected. At the beginning of the treatment, the test (laser group) and control implants (control group) were allocated via a coin toss (Fig. 2).
FIG. 1.
Study flow diagram.
FIG. 2.
Localization of implants in the study groups.
All suprastructures were removed, and the peri-implant depth (PD) [measured from four points to the nearest mm using a calibrated manual probe (PQ-OW Hu-Friedy Instrument Co. Chicago, IL)] interproximal plaque index (PI),12 and BOP13 were recorded by a calibrated examiner (V.A.). BOP was evaluated as present if bleeding was evident within 30 sec after probing, or absent if no bleeding was noticed within 30 sec after probing. PD was measured from the mucosal margin to the bottom of the probable peri-implant sulci.
Any supragingival plaque or debris was cleaned by sterile gauze, and all subsequent procedures were performed aseptically. Two dedicated sterile paper point tips (Greiner Bio-One, Frickenhausen, Germany) were gently inserted into the peri-implant sulci, and care was taken to avoid trauma-related bleeding. After 20 sec, the paper points were removed and placed into a dedicated eppendorf tube (Greiner Bio-One, Frickenhausen, Germany). For each sample, DNA was extracted and purified with a Bacterial Genomic DNA Kit (GenElute, Sigma-Aldrich Co, St. Louis, MO) according to the manufacturer's instructions. PCR amplification and hybridization procedures were performed according to the manufacturer's instructions (ParoCheck®, Greiner Bio-One GmbH, Frickenhausen, Germany). Results were generated using a scanner (CheckScanner™, Greiner Bio-One GmbH) and a semiquantitative labeling scheme was processed by its software [ParoReport (supplied with the ParoCheck® Kit), based on Gene Pixt, Axon Instruments Inc. Frickenhausen, Germany]. The results were estimated according to the labeling scheme corresponding to different signal levels: no detection (absent), low, moderate and high. The following 20 species can principally be identified by the dedicated microarray detection system: Actinomyces odontolyticus, Actinomyces viscosus, Aggregatibacter actinomycetemcomitans, Campylobacter concisus, Campylobacter gracilis, Campylobacter rectus/showae, Capnocytophaga gingivalis/sputigena/ochracea, Eikenella corrodens, Eubacterium nodatum, Fusobacterium nucleatum, Peptostreptococcus micros, Porphyromonas gingivalis, Prevotella intermedia, Prevotella nigrescens, Streptococcus constellatus group, Streptococcus gordonii group, Streptococcus mitis group, Tannerella forsythia (Bacteroides forsythus; Tannerella forsythensis), Treponema denticola, and Veillonella parvula.
To clean the infected implant surface, local infiltrative anesthesia (Ultracain, Articain Hydroclorure, 20 mg/mL, Aventis Farma, Istanbul, Turkey) was administered, and the peri-implant sulci and the corresponding implant surface were mechanically debrided in accordance with the scaling technique via a dedicated plastic implant curette (W&H, Dentalwerk Bürmoos GmbH, Salzburg, Austria). Additionally, the peri-implant sulci of the implant(s) in the laser group was lased by a Diode Laser (Denlase 810/7, Beijing, China) with a wavelength of 810 nm (energy density, 3 J/cm2; power density, 400 mW/cm2; energy, 1.5 J; and spot diameter, 1 mm) for a duration of 1 min in pulsed mode with a power level of 1 W using a standard 400 μm delivery optical fiber tip. The uninitiated tip was inserted parallel to the long axis of the implant ∼1 mm from the most apical level of the peri-implant sulci, and moved in a mesiodistal and apicocoronal direction around the implant. Care was taken to prevent any coagulation and subsequent temperature increase in the application tip by regular cleaning via saline-dampened sterile gauze. Irradiation was performed by the same operator (S.V.A.) in all patients. The peri-implant sulci of all implants were washed with sterile saline solution to remove any remaining debris (Fig. 3). After the treatment, all superstructures were re-cemented by a polycarboxylate cement (Adhesor Carbonfine, Spofa Dental, Jicin Czech Republic). A thin layer of cement was applied via a bonding brush, and care was taken to avoid any cement excess around the treated peri-implant sulcus, by careful cleaning using the dental explorer and a dental floss.
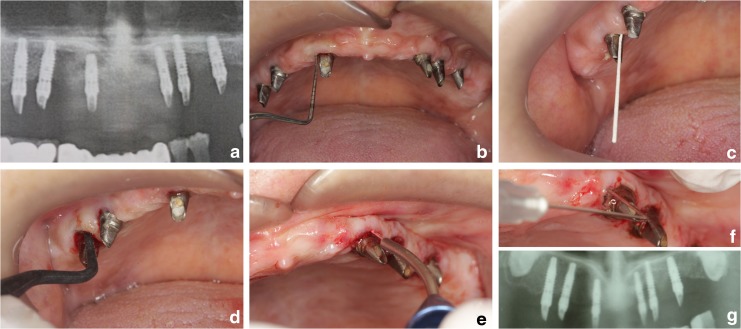
FIG. 3.
(a) Panoramic radiography of a patient in the study group. (b) Baseline marginal bone loss (MBL) was measured using special software. (c) Periodontal indexes were measured. (d) Microbial sampling used dedicated paper points. (e) Scaling used dedicated plastic curettes in the control group. (f) Additional diode laser (DL) application in the laser group. (g) Panoramic graph taken after 6 months.
For the assessment of healing and the microbiological outcome, the aforementioned peri-implant health indexes were measured after 1 month. To determine the effect of the treatment variables on the marginal bone levels, an additional measurement, including MBL and peri-implant health indexes, were repeated 6 months after the intervention.
MBL was measured using a previously established method.14 Baseline and the final panoramic radiographs (taken 6 months after the intervention) were scanned and visualized using dedicated software (ImageJ, National Institutes of Health, Bethesda, MD). The measuring tool was calibrated using the known implant length. Then, the distance between the implant shoulder and the marginal peri-implant crestal bone was measured in millimeters. This was repeated in the distal and mesial aspect of all implants. The measurements were repeated twice, and the results were averaged to yield final values.
Statistical analysis
All statistical testing was performed using a commercial software package (Graphpad Prism 6.0, Graphpad Software Inc. CA). Descriptive statistics of measured values consisting of the mean, standard deviation, range, and 95% confidence interval (CI) were calculated. The distribution of the data was analyzed using the D'Agastino Pearson Omnibus Normality test. MBL and PD values were analyzed using two way repeated measures analysis of variance (ANOVA). Sidak's test was used for post-hoc multiple comparisons. BOP scores in the measurement intervals were analyzed by the Fisher's exact test. Comparison of BOP scores and the microbial load in the given intervals were analyzed using the McNemar Test.
Results
All patients completed the study. There were no complications or negative outcomes. No residual or excess cement was detected in any peri-implant sulcus.
The mean and the deepest PD measurements in the measurement intervals were similar between the groups (p = 0.56, p = 0.86, and p = 0.88 for the mean, and p = 0.108, p = 0.066, and 0.065 for the deepest PD values measured at baseline, and after 1 and 6 months, respectively). The change in the mean and the deepest PD values in the measurement intervals were statistically significant in both groups (p < 0.001). In both groups, the mean and the deepest measured PD values demonstrated a decrease after 1 month and increased again after 6 months. However, there was no statistically significant difference in any of the measurement intervals between the laser and control groups (Table 1).
Table 1.
Mean and the Deepest Peri-Implant Depth (PD) Measurements (mm) and Corresponding Statistics in the Measurement Intervals
| Mean (SD) | Deepest (SD) | |||
|---|---|---|---|---|
| Laserd | Controle | Laserd | Controle | |
| Baselinea | 4.71 (0.67) | 4.38 (0.42) | 5.37 (0.49) | 5.17 (0.38) |
| After 1 monthb | 4.25 (0.61) | 3.99 (0.35) | 4.79 (0.72) | 4.41 (0.50) |
| After 6 monthsc | 4.54 (0.74) | 4.17 (0.41) | 5.08 (0.72) | 4.71 (0.62) |
vs; bp < 0.001; cp < 0.001; cp < 0.008 for the mean PD scores; cp < 0.002; cp < 0.001 for the mean PD scores; dvs; ep > 0.05 for the mean and deepest PD values.
At the beginning of the study, interproximal plaque was detected in both groups (91.7%). After 1 month, the PI scores revealed a statistically significant decrease (29.2 and 25% in the laser and control groups, respectively; p = 0.001 for both groups). However, the differences between the groups were not statistically significant (p = 0.745). At the final visit, the PI scores showed an increase, but the differences were not statistically significant (29.2% and 25.0%; p = 0.070 and p = 0.086 in the laser and control groups, respectively). The final PI scores were still below the initial PI scores, and the differences were statistically significant (54.2% and 41.7%; p = 0.012 and p = 0.002 in laser and control groups, respectively).
BOP that was present around all implants (100% in both groups) at the beginning of the study showed a statistically significant decrease in both groups after 1 month (58.3%; p = 0.002 in both groups). However, it reversed almost back to the initial situation after 6 months (95.8%; p = 0.004 and 100%; p = 0.002 for the laser and control groups, respectively).
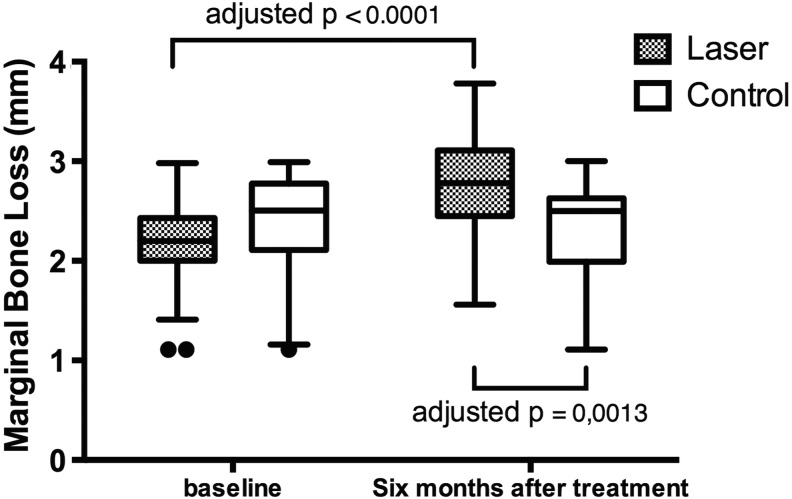
Baseline and 6 months post-treatment MBL values are illustrated in Table 2. Prior to the initiation of the treatment, MBL was similar between the groups (mean 2.13 and 2.35 mm for the laser and control groups, respectively; p = 0.44). The change in the MBL values over time was statistically significant (F = 38.34, p < 0.0001), and after 6 months, the difference in MBL values was also significant in the laser group [mean difference 0.6521 mm; corrected p < 0.0001; two-way ANOVA, interaction F (1.46) = 67.35; p < 0.0001; column factor (time) F (1.46) = 38.34; p < 0.0001; row factor (treatment type) F (1.46) = 1.193; p = 0.2804; subjects (matching) F (46.46) = 9.872; p < 0.0001]. The differences in MBL values measured after 6 months in the laser and control groups was statistically significant (mean difference 0.5227 mm; adjusted p = 0.0013) (Fig. 4).
Table 2.
Marginal Bone Loss (MBL) in the Laser and Control Groups
| Laser | Control | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | 95% CI | Mean (SD) | Range | 95% CI | |
| Baseline | 2.13 (0.47) | 1.11–2.98 | 1.93–2.34 | 2.35 (0.56) | 1.04–3 | 2.11–2.59 |
| After 6 months | 2.79 (0.48) | 1.56–3.78 | 2.58–2.993 | 2.63 (0.53) | 1.12–3.09 | 2.03–2.48 |
FIG. 4.
Box-plot graphs of marginal bone loss (MBL) in the laser and control groups.
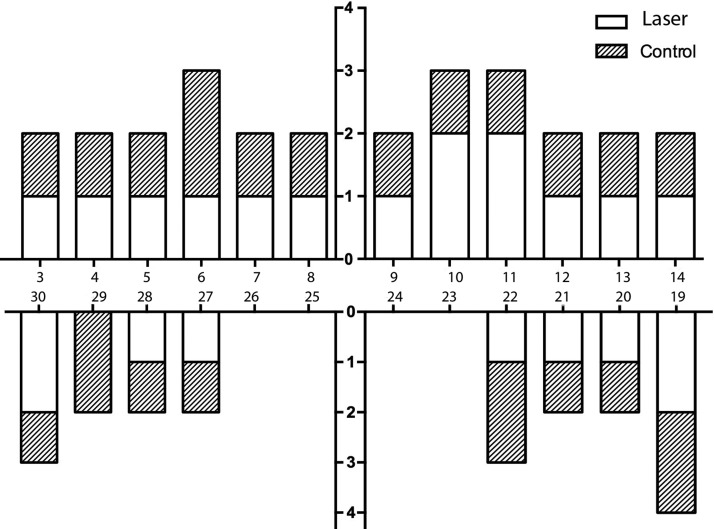
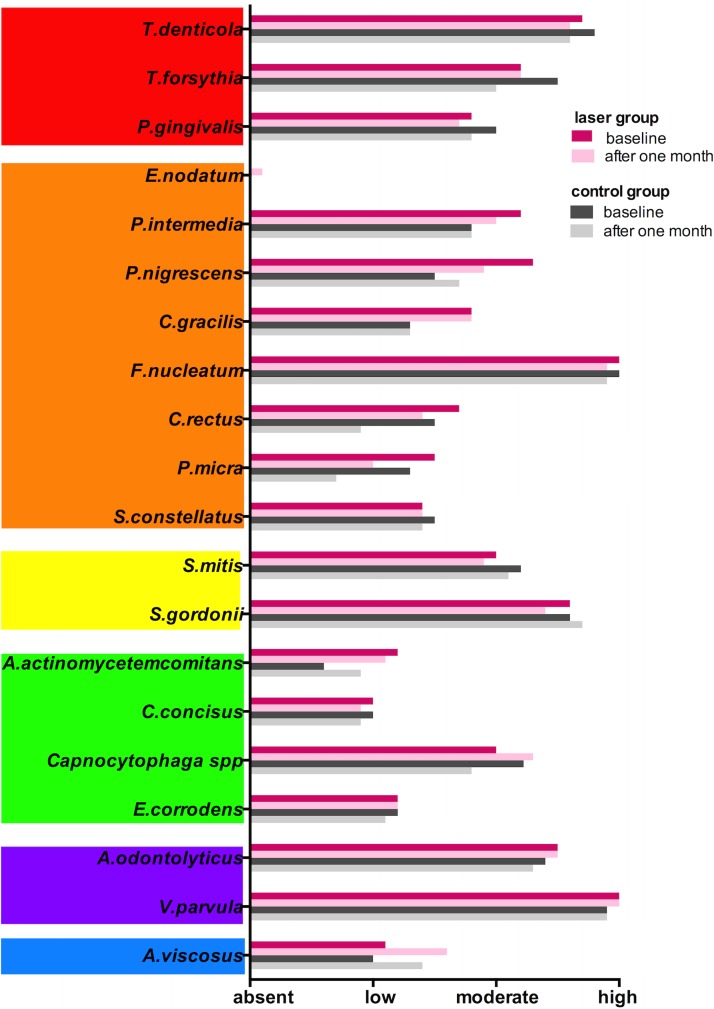
Both methods seem to be able to diminish the total bacterial counts on affected peri-implant sulcus. However, there was no statistically significant difference in any of the microbial complexes15 (Tables 3 and 4, and Fig. 5).
Table 3.
Presence of the Red Complex Bacteria in the Measurement İntervals
| Laser (n = 24) n (%) | Control (n = 24) n (%) | |
|---|---|---|
| Tannerella forsythia | ||
| Baseline | 22 (91.7) | 22 (91.7) |
| After 1 month | 20 (83.3) | 22 (91.7) |
| p | 0.500 | 1.000 |
| Treponema denticola | ||
| Baseline | 24 (100) | 24 (100) |
| After 1 month | 22 (91.7) | 24 (100) |
| p | 0.500 | 1.000 |
| Porphyromonas gingivalis | ||
| Baseline | 19 (79.2) | 18 (75.0) |
| After 1 month | 12 (50.0) | 18 (75.0) |
| p | 0.092 | 1.000 |
Table 4.
Presence of the Orange Complex Bacteria in the Measurement İntervals
| Laser (n = 24) n (%) | Control (n = 24) n (%) | |
|---|---|---|
| Campylobacter rectus | ||
| Baseline | 20 (83.3) | 20 (83.3) |
| After 1 month | 20 (83.3) | 18 (75.0) |
| p | 1.000 | 0.727 |
| Prevotella intermedia | ||
| Baseline | 20 (83.3) | 20 (83.3) |
| After 1 month | 20 (83.3) | 22 (91.7) |
| p | 1.000 | 0.500 |
| Peptostreptococcus micros | ||
| Baseline | 24 (100) | 20 (83.3) |
| After 1 month | 19 (79.2) | 15 (62.5) |
| p | 0.063 | 0.125 |
| Fusobacterium nucleatum | ||
| Baseline | 24 (100) | 24 (100) |
| After 1 month | 24 (100) | 24 (100) |
| p | 1.000 | 1.000 |
| Eubacterium nodatum | ||
| Baseline | 0 (0) | 0 (0) |
| After 1 month | 3 (12.5) | 0 (0) |
| p | 0.250 | - |
| Streptococcus constellatus group | ||
| Baseline | 23 (95.8) | 23 (95.8) |
| After 1 month | 20 (83.3) | 20 (83.3) |
| p | 0,250 | 0.250 |
| Campylobacter gracilis | ||
| Baseline | 22 (91.7) | 19 (79.2) |
| After 1 month | 22 (91.7) | 16 (66.7) |
| p | 1.000 | 0,250 |
| Prevotella nigrescens | ||
| Baseline | 23 (95.8) | 19 (79.2) |
| After 1 month | 22 (91.7) | 16 (66.7) |
| p | 1.000 | 0.508 |
FIG. 5.
Microbial load in the laser and control groups in the measurement intervals. Bacteria were grouped according to the bacterial complexes described by Socransky and co-workers.15
Discussion
In this study, the efficacy of an adjunct 810 nm DL in the nonsurgical treatment of mild to moderate peri-implantitis was investigated via microbial, radiographic, and peri-implant measures. The split-mouth design of the study yielded objective screening of all parameters throughout the study interval.
The similarity of the pathologic microbiota around the diseased implants and teeth16 led to assignment of similar treatment approaches for periodontitis and peri-implantitis, which mainly consisted of mechanical debridement accompanied by an adjunctive therapy aiming to eliminate the biofilm attached on the implant surface.16 Metallic instruments scratch the implant surface, whereas the plastic ones do not perform equivalently. Although many different types of lasers show promising results, currently, there is no consensus on a particular type. In addition to the demonstrated bactericidal effects in the peri-implant sulcus, many of the lasers have been shown to alter the titanium surface.17 Because of the variability of the power settings in the higher powered lasers (i.e., Nd:YAG, Er:YAG, and Er,Cr:YSGG), the clinician should pay particular attention to exposure and distance setting, so that the implant surface and the peri-implant tissues remain stable.18 Such a risk was eliminated in this investigation by the preference of a low-powered 810 nm DL, which was shown to be innocuous on the implant surface.19 It should also be emphasized that the strategies suggested for the treatment of peri-implantitis depend on empirical findings, and that lasers would be used in combination with the suggested conventional treatment methods.6
The similarity of the biofilm response in diseased teeth and implants has been previously shown. A high proportion of gram-negative organisms consisting of anaerobic bacteria and mobile rods and spirochetes were reported in this infection.20 Healthy peri-implant sulci are characterized by dominating coccoid bacteria that mainly consist of aerobic gram-positive bacteria.21,22 It has been shown that a decrease in the bacterial count in the diseased peri-implant sulci may result in the re-establishment of a healthy equilibrium between the microflora and the host defense, thereby improving the healing potential of the site.23 Therefore, many studies were focused on the photo elimination (laser irradiation) of the pathologic bacteria; Fontana and co-workers24 investigated the bactericidal efficacy of the power intensity (0.4, 0.6, 0.8, 1.0, and 1.2 W) of a DL in an experiment on rats. Bacterial reduction was evident in all cycles irrespective of the power intensity. Moritz and co-workers10 applied DL with a 2.5 W power into periodontal pockets, following mechanical scaling and debridement. DL yielded significantly more reduction of the pathologic bacteria than scaling alone, and the elimination of A. actinomycetemcomitans was clearly noted. In almost all of the previous studies, DL was especially effective in the elimination of pigmented bacteria such as A. actinomycetemcomitans.25,26 This can be explained by the optical affinity of the laser beam to the darker pigmentation on such bacteria.27
Although the clinical applications of DL lead to an immediate microbial decrease, the results were unfortunately not sustained in the long term.18,26,28–30 This finding was also evident in the present investigation, as no significant change was found for any of the investigated bacteria after 1 month. Except for minor differences, any substantial change of the bacterial load was hardly noticeable in the laser and control groups. Hence, DL application for the elimination of pathologic bacterium did not perform better than scaling alone. PD, deepest PD, and PI parameters yielded improvements in both groups without any statistically significant differences. BOP, which is accepted as the main indicator of peri-implant health,31 showed a decrease after 1 month, but returned back to the initial level. Similar outcomes were reported in a series of clinical studies by Schwarz and co-workers using the Er:YAG lasers. Serious bacterial reduction obtained immediately after the application of the laser was no longer discernable after ≥1 month.32–35 The success of peri-implantitis treatment appeared to be related to factors other than bacterial load reduction.35
MBL was used for the assessment of outcome in various treatment approaches used in implantology.36 In the present study, the MBL values that were similar at the initiation of the study (baseline) revealed a statistically significant increase in the laser group 6 months after the treatment. This can be the result of many factors, such as individual host response and confounding factors in the healing mechanism of the peri-implant alveolar bone. However, a negative impact related to the use of DL may also be involved. It was reported that an uncontrolled increase of temperature in the DL-applied area might jeopardize healing conditions because of thermal damage.18,37 Nevertheless, the power output of the recently used DL was below that of the previous studies, and a similar methodology was used in the clinical application. Because the sample size of the present clinical study cannot be used for a generalized conclusion, clinicians should be aware of such dangers in the DL application in the nonsurgical treatment of peri-implantitis.
Conclusions
Based on the results of this study, it was concluded that in the nonsurgical treatment of peri-implantitis, adjunctive use of a DL, did not yield any additional positive influence on the peri-implant microbiota compared with conventional scaling alone. Further studies are required to explore the reasons for MBL around diseased implants treated by DL after conventional scaling.
Acknowledgments
We thank Dr. Sevda Özel of the Department of Biostatistics and Medical Informatics, Istanbul University, Faculty of Medicine for her support with the statistical analysis of the study data. This study was supported by a grant from Istanbul University Research Fund (No: 22148).
Author Disclosure Statement
No competing financial interests exist. All authors are full-time academic staff (Selehattin Volkan Arıcı is a PhD student at the Institute of Health Sciences at Istanbul University, Istanbul, Turkey, and Zihni Cuneyt Karabuda, Volkan Arısan, Nursen Topçuoğlu, and Güven Külekçi are full time professors employed by Istanbul University, Istanbul, Turkey).
References
- 1.Albrektsson T, Isidor F. Consensus Report of Session IV. In: Proceedings of the First European Workshop on Periodontology Lang N, Karring T. (eds.). London: Quintessence, 1994, pp. 382–384 [Google Scholar]
- 2.Roos-Jansaker AM, Renvert S, Egelberg J. Treatment of peri-implant infections: a literature review. J Clin Periodontol 2003;30:467–485 [DOI] [PubMed] [Google Scholar]
- 3.Zitzmann NU, Berglundh T. Definition and prevalence of peri-implant diseases. J Clin Periodontol 2008;35:286–291 [DOI] [PubMed] [Google Scholar]
- 4.Klinge B. Peri-implant marginal bone loss: an academic controversy or a clinical challenge? Eur J Oral Implantol 2012;5 Suppl:S13–S19 [PubMed] [Google Scholar]
- 5.Subramani K, Wismeijer D. Decontamination of titanium implant surface and re-osseointegration to treat peri-implantitis: a literature review. Int J Oral Maxillofac Implants 2012;27:1043–1054 [PubMed] [Google Scholar]
- 6.Gosau M, Hahnel S, Schwarz F, Gerlach T, Reichert TE, Burgers R. Effect of six different peri-implantitis disinfection methods on in vivo human oral biofilm. Clin Oral Implants Res 2010;21:866–872 [DOI] [PubMed] [Google Scholar]
- 7.Tastepe CS, van Waas R, Liu Y, Wismeijer D. Air powder abrasive treatment as an implant surface cleaning method: a literature review. Int J Oral Maxillofac Implants 2012;27:1461–1473 [PubMed] [Google Scholar]
- 8.Salmeron S, Rezende ML, Consolaro A, et al. Laser therapy as an effective method for implant surface decontamination: a histomorphometric study in rats. J Periodontol 2013;84:641–649 [DOI] [PubMed] [Google Scholar]
- 9.Pick RM, Pecaro BC, Silberman CJ. The laser gingivectomy. The use of the CO2 laser for the removal of phenytoin hyperplasia. J Periodontol 1985;56:492–496 [DOI] [PubMed] [Google Scholar]
- 10.Moritz A, Schoop U, Goharkhay K, et al. Treatment of periodontal pockets with a diode laser. Lasers Surg Med 1998;22:302–311 [DOI] [PubMed] [Google Scholar]
- 11.Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000 1998;17:63–76 [DOI] [PubMed] [Google Scholar]
- 12.Patz J, Gulzow HJ. Epidemiology of dental caries [in German]. MMW Munch Med Wochenschr 1977;119:381–386 [PubMed] [Google Scholar]
- 13.Loe H. The gingival index, the plaque index and the retention index systems. J Periodontol 1967;38(Suppl):610–616 [DOI] [PubMed] [Google Scholar]
- 14.Arisan V, Bolukbasi N, Ersanli S, Ozdemir T. Evaluation of 316 narrow diameter implants followed for 5–10 years: a clinical and radiographic retrospective study. Clin Oral Implants Res 2010;21:296–307 [DOI] [PubMed] [Google Scholar]
- 15.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr. Microbial complexes in subgingival plaque. J Clin Periodontol 1998;25:134–144 [DOI] [PubMed] [Google Scholar]
- 16.Zitzmann NU, Berglundh T, Marinello CP, Lindhe J. Experimental peri-implant mucositis in man. J Clin Periodontol 2001;28:517–523 [DOI] [PubMed] [Google Scholar]
- 17.Block CM, Mayo JA, Evans GH. Effects of the Nd:YAG dental laser on plasma-sprayed and hydroxyapatite-coated titanium dental implants: surface alteration and attempted sterilization. Int J Oral Maxillofac Implants 1992;7:441–449 [PubMed] [Google Scholar]
- 18.Castro GL, Gallas M, Nunez IR, Borrajo JL, Alvarez JC, Varela LG. Scanning electron microscopic analysis of diode laser-treated titanium implant surfaces. Photomed Laser Surg 2007;25:124–128 [DOI] [PubMed] [Google Scholar]
- 19.Goncalves F, Zanetti AL, Zanetti RV, et al. Effectiveness of 980-mm diode and 1064-nm extra-long-pulse neodymium-doped yttrium aluminum garnet lasers in implant disinfection. Photomed Laser Surg 2010;28:273–280 [DOI] [PubMed] [Google Scholar]
- 20.Klinge B, Meyle J. Peri-implant tissue destruction. The Third EAO Consensus Conference 2012. Clin Oral Implants Res 2012;23 Suppl 6:108–110 [DOI] [PubMed] [Google Scholar]
- 21.Jemt T, Lekholm U, Adell R. Osseointegrated implants in the treatment of partially edentulous patients: a preliminary study on 876 consecutively placed fixtures. Int J Oral Maxillofac Implants 1989;4:211–217 [PubMed] [Google Scholar]
- 22.Lekholm U, Ericsson I, Adell R, Slots J. The condition of the soft tissues at tooth and fixture abutments supporting fixed bridges. A microbiological and histological study. J Clin Periodontol 1986;13:558–562 [DOI] [PubMed] [Google Scholar]
- 23.Mombelli A, Schmid B, Rutar A, Lang NP. Local antibiotic therapy guided by microbiological diagnosis. J Clin Periodontol 2002;29:743–749 [DOI] [PubMed] [Google Scholar]
- 24.Fontana CR, Kurachi C, Mendonca CR, Bagnato VS. Microbial reduction in periodontal pockets under exposition of a medium power diode laser: an experimental study in rats. Lasers Surg Med 2004;35:263–268 [DOI] [PubMed] [Google Scholar]
- 25.Moritz A, Gutknecht N, Doertbudak O, et al. Bacterial reduction in periodontal pockets through irradiation with a diode laser: a pilot study. J Clin Laser Med Surg 1997;15:33–37 [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Kim J, Lim W, et al. In vitro bactericidal effects of 625, 525, and 425 nm wavelength (red, green, and blue) light-emitting diode irradiation. Photomed Laser Surg 2013;31:554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson M, Dobson J, Sarkar S. Sensitization of periodontopathogenic bacteria to killing by light from a low-power laser. Oral Microbiol Immunol 1993;8:182–187 [DOI] [PubMed] [Google Scholar]
- 28.Bassir SH, Moslemi N, Jamali R, et al. Photoactivated disinfection using light-emitting diode as an adjunct in the management of chronic periodontitis: a pilot double-blind split-mouth randomized clinical trial. J Clin Periodontol 2013;40:65–72 [DOI] [PubMed] [Google Scholar]
- 29.Kreisler M, Meyer C, Stender E, Daublander M, Willershausen–Zonnchen B, d'Hoedt B. Effect of diode laser irradiation on the attachment rate of periodontal ligament cells: an in vitro study. J Periodontol 2001;72:1312–1317 [DOI] [PubMed] [Google Scholar]
- 30.Dostalova T, Jelinkova H. Lasers in dentistry: overview and perspectives. Photomed Laser Surg 2013;31:147–149 [DOI] [PubMed] [Google Scholar]
- 31.Lindhe J, Meyle J. Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 2008;35:282–285 [DOI] [PubMed] [Google Scholar]
- 32.Schwarz F, Bieling K, Nuesry E, Sculean A, Becker J. Clinical and histological healing pattern of peri-implantitis lesions following non-surgical treatment with an Er:YAG laser. Lasers Surg Med 2006;38:663–671 [DOI] [PubMed] [Google Scholar]
- 33.Schwarz F, Herten M, Sager M, Bieling K, Sculean A, Becker J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res 2007;18:161–170 [DOI] [PubMed] [Google Scholar]
- 34.Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: a randomized controlled clinical study. J Clin Periodontol 2011;38:276–284 [DOI] [PubMed] [Google Scholar]
- 35.Schwarz F, Sculean A, Rothamel D, Schwenzer K, Georg T, Becker J. Clinical evaluation of an Er:YAG laser for nonsurgical treatment of peri-implantitis: a pilot study. Clin Oral Implants Res 2005;16:44–52 [DOI] [PubMed] [Google Scholar]
- 36.Esposito M, Klinge B, Meyle J, et al. Working Group on the Treatment Options for the Maintenance of Marginal Bone Around Endosseous Oral Implants, Stockholm, Sweden, 8 and 9 September 2011. Consensus statements. Eur J Oral Implantol 2012;5(Suppl):S105–S106 [PubMed] [Google Scholar]
- 37.Fontana CR, Kurachi C, Mendonca CR, Bagnato VS. Temperature variation at soft periodontal and rat bone tissues during a medium-power diode laser exposure. Photomed Laser Surg 2004;22:519–522 [DOI] [PubMed] [Google Scholar]