Abstract
Background: Educational interventions may be a strategy to increase human papillomavirus (HPV) vaccination among female university students, but studies to date have shown mixed results. This study evaluated the effect of MeFirst, an individually tailored, online educational intervention, on HPV vaccine-related knowledge, vaccination intention, and uptake among previously unvaccinated female university students.
Methods: All female students aged 18–26 years who reported being unvaccinated against HPV at a midwestern university were invited via email to enroll. Participants completed an online survey that assessed baseline HPV vaccine-related knowledge, attitudes and vaccination intention. Participants (n = 661) were then randomized to receive either an educational website automatically tailored to their baseline survey responses (MeFirst intervention) or a standard CDC information factsheet on HPV vaccine (control). Vaccine uptake and repeat knowledge and attitude measures were assessed with online surveys 3 months following the intervention and analyzed using logistic regression models.
Results: HPV vaccine uptake was similar in both the MeFirst and control groups at 3 months following the intervention (p = 0.98). Three months after the intervention, the proportion of participants with high knowledge regarding HPV vaccination increased from baseline (32% to 50%; p < 0.0001) but the proportion with favorable intention was unchanged.
Conclusions: We found that an individually tailored, online educational tool had similar effects as a nontailored factsheet on HPV-related knowledge, intention to HPV undergo vaccination, and HPV vaccine uptake among previously unvaccinated female university students.
Introduction
Genital human papillomavirus (HPV) is the most common sexually transmitted infection in the United States, with adolescents and young adults at highest risk.1,2 Although most infections clear spontaneously, HPV causes genital warts and cervical cancer and is associated with other anogenital cancers and precancers.3 Since 2007, the CDC's Advisory Committee on Immunization Practices (ACIP) has recommended routine HPV vaccination of girls ages 11–12 and “catch up” vaccination for females aged 13–26 years who have not been previously vaccinated.3
Although the introduction of HPV vaccines has been associated with a dramatic reduction in vaccine-type HPV prevalence (types 6, 11, 16, and 18) among females aged 14–19 years, no similar change has been demonstrated among females aged 20–24 years.4 National prevalence of at least one vaccine-type HPV infection remained at 19%–20% in this age group during the first four years after vaccine introduction.4 Low levels of catch-up vaccination likely contributed to the lack of observed effect. In 2011, only 29.5% of females aged 19–26 had received any doses of HPV vaccine.5 Thus, young adult women remain an important target group for HPV vaccination.
Female college students are one group of young adult women for whom interventions could theoretically be delivered with low cost and effort through existing university structures such as university health centers or campus email. Interventions to encourage HPV vaccination in this population have had mixed results.6–10 One intervention, based on sharing common HPV-vaccine decision stories in a narrative format nearly doubled vaccine uptake at 2 months,6 and two other interventions increased participant intention to be vaccinated.7,8 However, two additional studies found no effect of educational interventions on vaccine uptake.9,10
Tailored health communications are modified based on patient-specific characteristics to increase relevance to the recipient;11 this approach has already shown promise among HPV unvaccinated female college students.8 Since only 65% of American women aged 18–24 years had a routine checkup in 2009,12 and only 55% of women aged 15–24 accessed sexual and reproductive health services between 2006 and 2010,13 relying on clinician recommendation is an extremely limited strategy to increase HPV vaccination in this age group. A recent meta-analysis found that tailored computer-based interventions with decision, behavior, and/or emotional support increased sexual health knowledge and self-efficacy of participants.14 In college populations, tailored interventions delivered via online platforms have been effective at improving health knowledge and behavior in other research areas.15,16 Because an online intervention could potentially reach many women who otherwise may not receive health messaging related to HPV vaccination, in 2010 our team began developing a novel, online, highly tailored educational intervention for unvaccinated female college students.
The primary objective of this study was to determine the impact of an individually tailored online educational intervention (“MeFirst”) on HPV vaccine uptake among previously unvaccinated female university students, compared with an untailored intervention. The secondary objective was to quantify the effect of MeFirst on proposed mediators of uptake, namely HPV vaccine-related knowledge, risk perception, and intention to be vaccinated.
Materials and Methods
Subject pool and recruitment
A university Institutional Review Board approved all methods (No. HUM00069032) and this study was registered with clinicaltrials.gov (N0. NCT01769560) prior to study start. Potential subjects were recruited among the female student population at a large public midwestern university. All female students aged 18–26 were sent three recruitment emails by the Office of the Registrar in January 2013. The recruitment emails specified the study was regarding HPV vaccination and that female university students aged 18–26 who had never been vaccinated against HPV were eligible to participate in a voluntary research study. Additional inclusion criteria included either full or part-time student status at the university. Females with prior receipt of any doses of HPV vaccine were ineligible for the study. Eligible students were invited to participate in the study and gave their informed consent by enrolling in the study. Raffle entries were offered as incentives. Interested students took a brief online eligibility survey prior to enrollment.
Baseline data collection
Upon enrollment, all participants were given a 144 question baseline survey that included knowledge about HPV-related disease and the HPV vaccine, opinions about the HPV vaccine including perceptions of susceptibility, intentions to receive the HPV vaccine, most recent medical visit, history of Papanicolaou (Pap) smear, sexual history, and demographic information. Knowledge about HPV and the HPV vaccine was measured using 10 true/ false/don't know statements. Perceived risk of HPV infection and intention to be vaccinated were measured by agreement with the statements “I am likely to get an HPV infection in my lifetime,” and “I intend to get vaccinated against HPV,” respectively, using five-point scales of strongly disagree/ disagree/ neutral/ agree/ strongly agree. Participants who did not complete the baseline survey within 7 days of enrollment were sent up to two automated reminder emails.
Randomization and interventions
Participants who completed the baseline survey were randomized via an automated algorithm to either a MeFirst tailored intervention website or a control website; their intervention site was immediately available after survey completion with one mouse click. The randomization was performed stratified by age group (<21 or ≥21 years) to balance age of participants in each arm. The control was a webpage of text from the CDC Vaccine Information Statement on the quadrivalent HPV vaccine.17 The MeFirst intervention website was a unique, tailored website automatically configured for the individual participant based on their baseline survey responses. It consisted of seven tailored topic webpages that the participant could toggle through using a navigation bar. The topic pages had factual information on HPV and the HPV vaccine, including statistics on the incidence of HPV infection and cervical cancer, risks associated with HPV infection, costs of vaccination, safety and efficacy of the HPV vaccine, and suggestions for how to talk to a doctor about the vaccine. Previous qualitative work by this research group guided MeFirst development. Tailoring was multifaceted but especially focused on barriers to vaccination the participant had previously endorsed, including perceived susceptibility (a list of tailoring elements are available by request). Website tailoring also included addressing the participant by their first name, using their doctor's name, and displaying background photos of young women of the same self-identified racial background as the participant. In total, up to 160 items could be tailored to the participant on their individual MeFirst website. Participants who received a MeFirst website were not exposed to a control website.
Follow-up
Three months following randomization, participants were emailed a request to log-in at their website to provide feedback on their decision of whether to get vaccinated against HPV. After returning to the site, they were given a 37-question survey that included the same knowledge, risk perception, and intention items as the baseline survey and additionally assessed HPV vaccine uptake. Participants who did not complete the 3-month follow-up survey within 15 days were sent up to two automated reminder emails.
Primary outcome
HPV vaccine use was measured with the statement, “I have received at least one dose of the HPV vaccine” with possible responses of yes, no, or don't know.
Secondary outcomes
Knowledge was scored as the number of correct responses out of 10 to yield a binary measure of a high level of knowledge (≥9 correct answers) versus a low level of knowledge (<9 correct answers). Nine was chosen as the cutoff because it included the 75th percentile of baseline scores. Barrier contraception use was analyzed as binary (high, always or mostly, vs. low, occasionally or never). Intention and risk perception were analyzed both as binary variables (positive, strongly agree, agree, or neutral, vs. negative, disagree or strongly disagree) and ordinal variables; results were similar, so only binary findings are presented for clarity. Vaccine uptake was treated as a binary variable (yes vs. no or don't know).
Statistical analysis
We conducted all analyses using SAS® statistical software, Version 9.3, of the SAS system for Windows (Copyright © 2002–2010 SAS Institute Inc.; SAS and all other SAS Institute Inc. product or service names are registered trademarks or trademarks of SAS Institute Inc., Cary, NC). Data was checked for internal consistency and logic prior to further analysis. An intention-to-treat analysis was performed; participants who were randomized were analyzed regardless of whether they actually viewed the intervention or not. Descriptive statistics of all variables were calculated, including means and standard deviations or frequencies as appropriate. Randomization and potential bias in enrollment and follow-up were each assessed by comparing characteristics using a Wilcoxon Rank Sum, chi-squared, or Fisher Exact test as appropriate.
Proportions of participants with high knowledge score, positive risk perception, or positive vaccine intention were compared between MeFirst and control groups using chi squared tests at baseline and repeated at 3 months. For each of these measures, change in proportion from baseline to 3 months was tested within intervention groups using McNemar's tests. Unadjusted odds of vaccine uptake for MeFirst versus control participants were calculated using a logistic regression model. Unadjusted logistic regression models were also used to examine bivariate associations between each baseline characteristic and HPV vaccine uptake. Multivariable logistic regression models were then used to determine the independent effect of the intervention, adjusting for factors found to be statistically significant at the α = 0.10 level in the bivariate analyses. Results of logistic regression models were expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). All final statistical tests were two sided with α = 0.05.
Results
Characteristics of the study sample at baseline
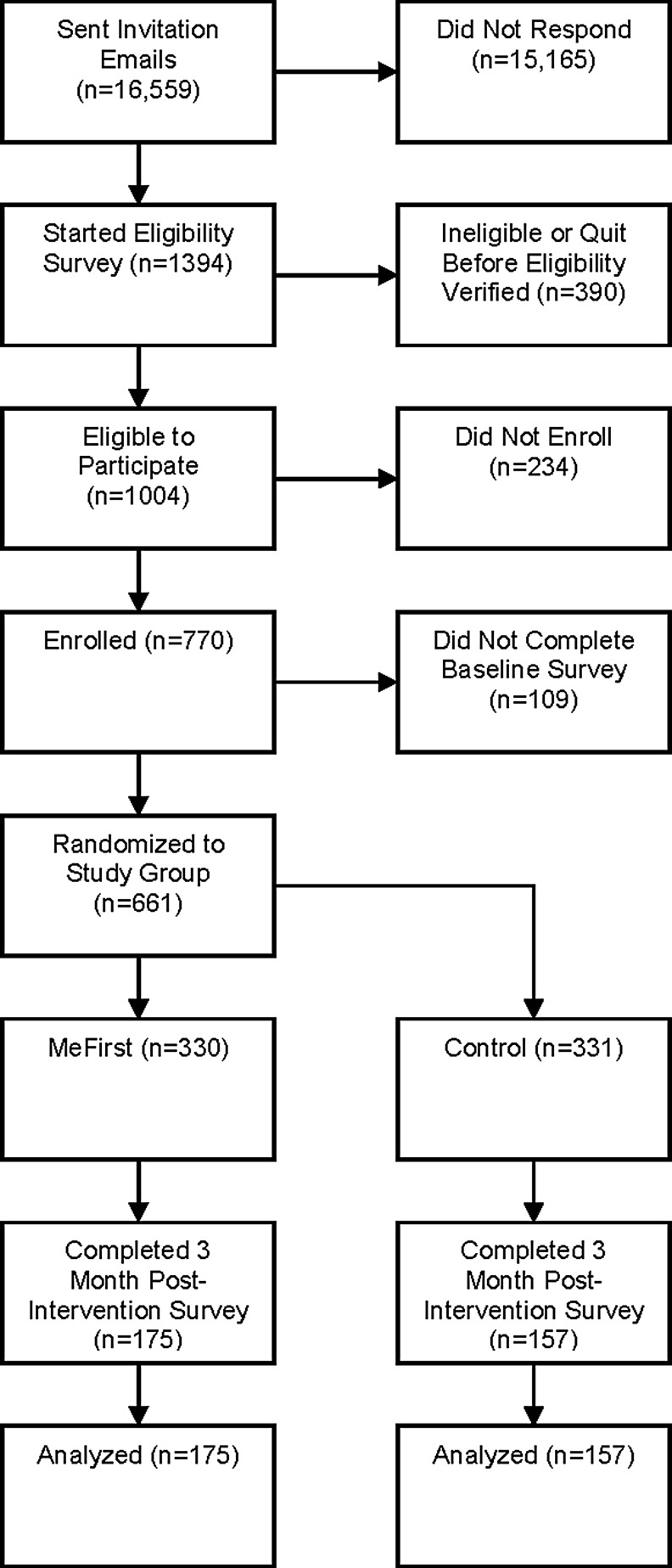
Selection of the sample population is presented in Figure 1. Of enrolled participants, 85.8% completed the baseline survey and thus were randomized to one of two study groups. Participants who completed the baseline survey did not differ significantly on age, student enrollment status, or student type from those who were eligible but did not enroll or complete the baseline survey (data not shown).
FIG. 1.
Sample population.
There were no substantial differences between randomization groups in baseline demographic characteristics (see Table 1). Most participants were single full-time students with health insurance coverage. Relevant sexual and medical history characteristics were also similar between randomization groups with the exception that more women randomized to MeFirst had engaged in anal sex (p = 0.02). Among the 70% of participants who reported a history of sexual activity, the majority of women had only one partner in the past 6 months (66%) and more than three partners in their lifetime (61%); 32.7% of all randomized participants intended to be vaccinated at baseline, 26.4% were neutral, and 40.9% did not intend to be vaccinated.
Table 1.
Demographic, Medical, and Sexual Characteristics of Study Participants Who Completed the Baseline Survey
| Characteristic | Total (N = 661) | MeFirst (N = 330) | Control (N = 331) | p-value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, median years (SE) | 21.0 (0.1) | 21.0 (0.13) | 21.0 (0.13) | 0.88 |
| Student enrollment, n (%) | 0.33 | |||
| Full-time | 634 (95.9) | 319 (96.7) | 315 (95.2) | |
| Part-time | 27 (4.1) | 11 (3.3) | 16 (4.8) | |
| Student standing, n (%) | 0.31 | |||
| Undergraduate | 445 (67.3) | 218 (66.1) | 227 (68.6) | |
| Graduate | 182 (27.5) | 98 (29.7) | 84 (25.4) | |
| Professional | 34 (5.1) | 14 (4.2) | 20 (6.0) | |
| Race, n (%) | 0.25 | |||
| Hispanic | 30 (4.5) | 17 (5.2) | 13 (3.9) | |
| Asian | 98 (14.8) | 56 (17.0) | 42 (12.7) | |
| Non-Hispanic black | 36 (5.5) | 21 (6.4) | 15 (4.5) | |
| Non-Hispanic white | 445 (67.3) | 21 (6.4) | 15 (4.5) | |
| Other/ multiracial | 52 (7.9) | 22 (6.7) | 30 (9.1) | |
| Health insurance, n (%) | 0.48 | |||
| UM SHIP or GradCare | 106 (16.0) | 58 (8.8) | 48 (7.3) | |
| Parent's private | 438 (66.3) | 212 (32.1) | 226 (34.2) | |
| Medicaid | 10(1.5) | 5 (0.8) | 5 (0.8) | |
| Other insurance | 41 (6.2) | 17 (2.6) | 24 (3.6) | |
| None | 43 (6.5) | 26 (3.9) | 17 (2.6) | |
| Don't know/missing | 23 (3.5) | 12 (1.8) | 11 (1.7) | |
| Employment, n (%) | 0.14 | |||
| Full-time | 55 (8.3) | 35 (10.6) | 20 (6.0) | |
| Part-time | 323 (48.9) | 159 (48.2) | 164 (49.6) | |
| Unemployed | 280 (42.4) | 134 (40.6) | 146 (44.1) | |
| Missing | 3 (0.5) | 2 (0.6) | 1 (0.3) | |
| Marital status, n (%) | 0.65 | |||
| Single | 545 (82.5) | 268 (81.2) | 277 (83.7) | |
| Married | 32 (4.8) | 16 (4.9) | 16 (4.8) | |
| Separated | 1 (0.2) | 0 | 1(0.3) | |
| In unmarried couple | 82 (12.4) | 45 (13.6) | 37 (11.2) | |
| Missing | 1 (0.2) | 1(0.3) | 0 | |
| Medical history | ||||
| Gets regular checkups, n (%) | 374 (56.6) | 181 (54.9) | 193 (58.3) | 0.11 |
| Health care visit in past 12 months, n (%) | 540 (81.7) | 258 (78.2) | 282 (85.2) | 0.07 |
| Ever had a Papanicolaou (Pap) test, n (%) | 292 (44.2) | 144 (43.6) | 148 (44.7) | 0.81 |
| Pap in past 12 months, n (%) | 205 (31.0) | 107 (32.4) | 98 (29.6) | 0.53 |
| Ever had an abnormal Pap test, n (%) | 39 (5.9) | 24 (7.3) | 15 (4.5) | 0.27 |
| Ever had HPV, n (%) | 16 (2.4) | 9 (2.7) | 7 (2.1) | 0.77 |
| Ever had STI (non-HPV), n (%) | 23 (3.5) | 12 (3.6) | 11 (3.3) | 0.94 |
| Smoked in past 7 days, n (%) | 34 (5.1) | 14 (4.2) | 20 (6.0) | 0.38 |
| Sexual history | ||||
| Ever had sexual intercourse, n (%) | 463 (70.1) | 224 (67.8) | 239 (72.2) | 0.32 |
| Age at sexual debut, median years (SE) | 18.0 (0.1) | 18.0 (0.2) | 18.0 (0.1) | 0.41 |
| Frequency of barrier method use, n (%) | 0.78 | |||
| Never | 104 (22.3) | 53 (23.4) | 51 (21.3) | |
| Occasionally | 71 (45.2) | 31 (13.7) | 40 (16.7) | |
| Mostly | 133 (28.5) | 66 (29.1) | 67 (27.9) | |
| Always | 153 (32.8) | 73 (32.2) | 80 (33.3) | |
| Missing | 6 (1.3) | 4 (1.8) | 2 (0.8) | |
HPV, human papilloma virus; SE, standard error; STI, sexually transmitted infection; UM SHIP, University of Michigan Student Health Insurance Plan.
Characteristics of respondents to three-month survey
Half of participants (332/661, 50.2%) responded to the 3-month follow-up survey and reported their HPV vaccine status; the other half was lost to follow-up. Respondents to the 3-month survey were significantly racially different from nonrespondents, particularly among MeFirst participants (chi-squared = 11.61, p = 0.02). Proportionally fewer Asian women took the 3-month survey, and proportionally more Hispanic and white women did take it. Participants who smoked in the week before intervention were less likely to follow-up 3 months later (chi-squared = 9.16, p = 0.01). Among participants who had the MeFirst intervention, but not among those who had the control intervention, participants with lower baseline intentions were more likely to respond to the three months survey (Wilcoxon = 27,203, p = 0.03) than those with higher baseline intention.
Outcomes at three months following the intervention
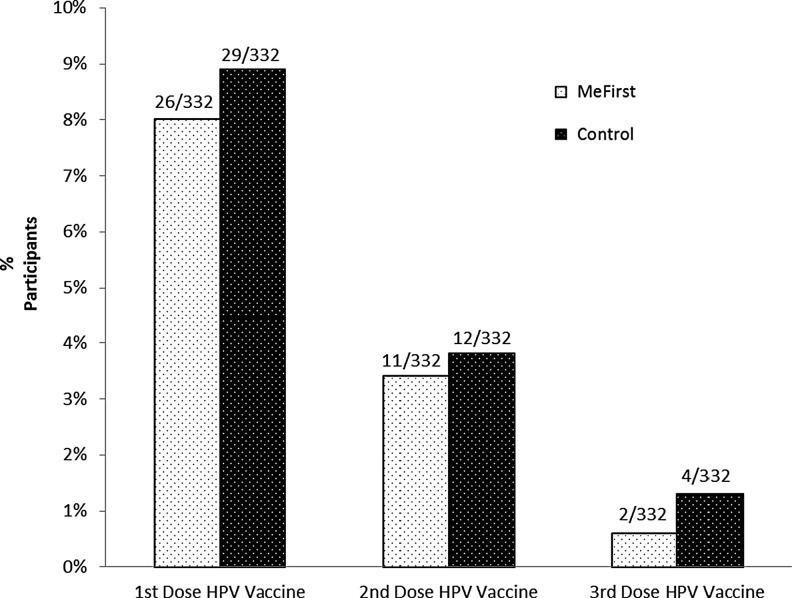
Three months after intervention, there was no difference in HPV vaccine uptake among participants randomized to the MeFirst intervention compared with controls (chi-squared = 0.09, p = 0.76); 8.4% of all participants initiated the vaccine series (see Figure 2). Of those who received the first dose of the HPV vaccine series, 42.9% (6/14) also received a second dose in each study group. The proportion of all participants with a high level of knowledge increased from 32% at baseline to 50% at three months (McNemar's statistic = 33.4; p < 0.0001). This increase in knowledge is not associated with intervention assignment. Risk perception and intention to be vaccinated did not change significantly from baseline to 3 months in either group. Of participants who intended to undergo HPV vaccination at baseline, 82.4% remained unvaccinated 3 months later.
FIG. 2.
HPV vaccine uptake at 3 months, MeFirst versus control.
Correlates of HPV vaccine uptake at three months following intervention
Knowledge change was not significantly associated with HPV vaccine uptake at 3 months on bivariate analysis (OR 0.92; 95% CI 0.78–1.09); the variables that were significantly associated with HPV vaccine uptake were nonemployment (OR 3.28; 95% CI 1.44–7.50), low sexual barrier use (OR 4.71; 95% CI 1.65–13.47), and high baseline intention to be vaccinated (OR 5.10; 95% CI 2.22–11.70). In the final logistic regression model, which included the MeFirst intervention and these significant covariates, nonemployment (OR = 3.60; 95% CI 1.27–10.20), low frequency use of sexual barriers (OR = 7.09; 95% CI 2.27–22.14), and high baseline intention to be vaccinated (OR 5.84, 95% CI 2.04–16.77) all remained significant. However, the effect of the MeFirst intervention on HPV vaccine uptake at 3 months remained nonsignificant (OR 0.92; 95% CI 0.34–2.54).
Discussion
We found no difference in HPV vaccine uptake between intervention arms, suggesting this individually tailored intervention had no greater effect than the CDC Vaccine Information Statement on HPV vaccine initiation among our study sample of unvaccinated female university students. The incidence of HPV vaccine initiation in our sample, 8% over a 3-month period, is consistent with rates among control participants in previously reported unvaccinated student cohorts of 6–12% with 2–10 months of follow-up.6,9,10 While this study showed no difference in effect of individually tailored and nontailored educational materials on vaccine uptake rates, we also found overall low uptake rates in both groups. It remains unclear whether individually tailored and/or standard HPV education can improve vaccine uptake enough to have a population impact.
Our secondary outcomes and multivariate model may provide clues about why this intervention was not associated with vaccine uptake. First, both intervention groups showed significant improvement in knowledge at 3 months following the intervention, but knowledge change was unassociated with uptake; this would suggest, as others have,18 that increasing knowledge by itself is insufficient to increase vaccine uptake (i.e., behavior change) in the absence of additional measures such as explicit provider endorsement of vaccination or systems improvement to provide immediate vaccination once readiness to vaccinate is identified. Second, intention was significantly associated with uptake; MeFirst may have failed to affect uptake because it failed to impact intention. The adult population in the study may have already been firm in their intentions about vaccination, highlighting the need for continued work increasing uptake among the pediatric population.
Comparison with previous interventions
The value of educational interventions to increase HPV vaccination among female university students is unclear, possibly because knowledge is not a strong predictor of vaccine behavior. Although initial cross-sectional studies after vaccine approval showed limited positive associations between HPV-related knowledge and intention to be vaccinated against HPV in young female adults,19–21 more recent investigations have shown that knowledge is not associated with interest in vaccination or vaccine uptake after adjustment for other factors.18,22 Factually driven educational interventions targeting participant knowledge have shown to have some impact on intention to be vaccinated7 but have not been associated with change in actual vaccine uptake.9,10 Taken together, these studies suggest that a knowledge-focused approach may impact immediate vaccine intent, but does not appear to affect behavior.
Educational interventions that have specifically targeted the female university population in some way have been more promising. Gerend et al. reported that additional educational information tailored to the individual participant's perceived barriers increased intention to be vaccinated versus standard CDC-based information.8 Hopfer reported that vaccine uptake doubled among participants randomized to a combined peer–expert vaccine decision narrative video compared with control.6 This intervention was based on culture-centric narrative theory6 as described by Larkey and Hecht23 and focused on HPV susceptibility, self-efficacy, vaccine safety, and vaccine use regardless of sexual experience.6 These personally or culturally tailored approaches may work by providing information that participants find particularly relevant.
MeFirst was similar to these successful interventions in several ways despite our null findings. Our intervention was substantially more tailored than that of Gerend et al.;8 we tailored on demographics, attitudes, and behavioral variable in addition to perceived barriers. We do not know if MeFirst increased immediate intention to be vaccinated, as Gerend's tailored intervention did,8 but any effects it had immediately did not translate to the more important outcome of vaccine uptake after 3 months. MeFirst provided information on the same four themes Hopfer addressed, but presented this information in an educational question and answer style without personal stories or any particular narrator; ours may have been less efficacious because of this difference in approach. However, only one of three of Hopfer's narrative interventions was effective,6 indicating that a better understanding of the mechanisms of HPV vaccine decision making is still necessary to develop useful interventions.
Strengths and limitations of the study
The strengths of this study include the randomized, controlled design and the use of a novel, individually tailored, online intervention that could be easily adapted to other settings. If this study had shown an important increase in HPV vaccination using MeFirst, other universities could have easily used it as a cost-effective measure to increase vaccine uptake among their students. Several limitations of this study should be considered. First, our study may have been biased by participant self-selection. Although a significant proportion of invited students were likely vaccinated and thus ineligible, a large proportion (92%) of students who were invited to participate did not respond, even after three email invitations. If proportionally more women who were already set in their intention to undergo HPV vaccination enrolled than in the general population, our study would have been biased towards the null. However, it was somewhat reassuring that participants who completed the baseline survey were similar on key demographic characteristics as those who were eligible but did not enroll or complete the baseline survey. Second, we had substantial loss to follow-up. Our pre-intervention survey was long, which may have fatigued participants and contributed to attrition before the 3-month survey. All of these factors limit our ability to generalize our findings on the effectiveness of an individually tailored intervention for HPV vaccine uptake to broader populations.
Implications for future research
Increasing vaccine uptake among young adults likely requires a multifaceted approach. In this study, the adjusted odds of initiating the HPV vaccine series were nearly six times higher for those who intended to get the vaccine at baseline versus those who were not sure or did not intend to get it, yet over 80% of participants who intended to get vaccinated remained unvaccinated 3 months later. Other factors, such as practical barriers, must be important. Since the Affordable Care Act now mandates no-cost sharing on the HPV vaccine for eligible children and adults for those with non-grandfathered private insurance,24 there is an ongoing natural experiment of the impact of decreasing the practical barrier of cost. Future research efforts could consider interventions to increase convenience of vaccine administration, like on-site vaccination at community events, such as freshman move-in day for university communities.
Interventions to increase catch-up HPV vaccination must move beyond female university students because other young adults, including nonstudents and men, may be at higher risk for nonvaccination. Large college surveys have reported female student vaccination rates of 57% in 200922 and 55% in 201025 compared with 21% of all females aged 19–26 years in 2010.26 Although CDC data is not yet available for 2012, the available information suggests that vaccine uptake has been low for men since the ACIP recommended male routine vaccination.27 Future intervention studies in young adults should include a broader range of participants to increase utility and generalizability of the research.
Implications for practice
Cumulatively, the current evidence suggests that the resources invested in developing highly tailored interventions to improve acceptance of HPV vaccination may be better reassigned to horizontal integration of care delivery with routine identification of those in need of vaccination at multiple points of entry into the university health system, offering HPV vaccination to those in need at any point of entry and facilitating vaccine service delivery within the same visit, if possible, those who are ready to be vaccinated. For those who are in need, but not ready to be vaccinated, rigorously developed educational material such as a standard CDC information factsheet on HPV vaccine may be sufficient.
Conclusions
In this randomized controlled trial, an individually tailored online intervention was not associated with a difference in uptake of HPV vaccine among female university students 3 months post-intervention compared with the standard CDC HPV Vaccine Information Statement. Both intervention groups showed increased knowledge from baseline, but this change in knowledge did not mediate uptake. We found no evidence that HPV vaccine uptake among unvaccinated female university students was affected by use of this individually tailored online intervention. Future interventions should target both sexes and consider decreasing practical barriers to uptake. More research could help determine whether investing significant time and money on tailored educational interventions is valuable for increasing HPV vaccine uptake among female university students.
Acknowledgments
We gratefully acknowledge the substantive contributions of Kelli Hall, PhD, Viji Ramaswami, and Mike Nowak for their work on the design and conduct of this study; all women who participated in this trial; and our sources of funding:
• Center for Healthcare Research and Transformation: Teachable Moments to Enhance Human Papillomavirus (HPV) Vaccination in Young Women Ages 18–26 (PI, R.C. Carlos);
• National Institutes of Health National Cancer Institute No. 1 R21 CA133333: Using Maternal Cancer Screening Visits to Improve Adolescent HPV Vaccinations (PI, R.C. Carlos);
• National Center for Advancing Translational Sciences of the National Institutes of Health: Research reported in this publication was supported under award number 2TL1TR000435. (Trainee, A.T. Bennett); and
• University of Michigan Department of Obstetrics and Gynecology Milton Goldrath Resident Research Support Award (Trainee, A.M. Chi).
The first manuscript draft was written by Alaina Town Bennett.
The content reported here is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
Carlos receives salary support from the American College of Radiology as Deputy Editor of the Journal of the America College of Radiology and is funded in part by Eastern Cooperative Oncology Group (ECOG)-American College of Radiology Imaging Network (ACRIN). V.K. Dalton has been paid to serve on an advisory committee by McNeil. V.K. Dalton is also an expert witness for Bayer. The remaining authors and contributors have no competing financial interests. No study sponsors were involved in the design, conduct, writing, or submission of this manuscript.
References
- 1.Forhan SE, Gottlieb SL, Sternberg MR, et al. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics 2009;124:1505–1512 [DOI] [PubMed] [Google Scholar]
- 2.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis 2011;204:566–573 [DOI] [PubMed] [Google Scholar]
- 3.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent Human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007;56:1–24 [PubMed] [Google Scholar]
- 4.Markowitz LE, Hariri S, Lin C, et al. Reduction in human papillomavirus (HPV) prevalence among young women following HPV vaccine introduction in the United States, National Health and Nutrition Examination Surveys, 2003–2010. J Infect Dis 2013;208:385–393 [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Noninfluenza vaccination coverage among adults – United States, 2011. MMWR Morb Mortal Wkly Rep 2013;62:66–72 [PMC free article] [PubMed] [Google Scholar]
- 6.Hopfer S. Effects of a narrative HPV vaccination intervention aimed at reaching college women: A randomized controlled trial. Prev Sci 2012;13:173–182 [DOI] [PubMed] [Google Scholar]
- 7.Krawczyk A, Lau E, Perez S, et al. How to inform: Comparing written and video education interventions to increase human papillomavirus knowledge and vaccination intentions in young adults. J Am Coll Health 2012;60:316–322 [DOI] [PubMed] [Google Scholar]
- 8.Gerend MA, Shepherd MA, Lustria ML. Increasing human papillomavirus vaccine acceptability by tailoring messages to young adult women's perceived barriers. Sex Transm Dis 2013;40:401–405 [DOI] [PubMed] [Google Scholar]
- 9.Patel DA, Zochowski M, Peterman S, et al. Human papillomavirus vaccine intent and uptake among female college students. J Am Coll Health 2012;60:151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerend MA, Shepherd JE. Predicting human papillomavirus vaccine uptake in young adult women: Comparing the health belief model and theory of planned behavior. Ann Behav Med 2012;44:171–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kreuter MW, Wray RJ. Tailored and targeted health communication: Strategies for enhancing information relevance. Am J Health Behav 2003;27 Suppl 3:S227–232 [DOI] [PubMed] [Google Scholar]
- 12.Willet MN, Hayes DK, Zaha RL, et al. Social-emotional support, life satisfaction, and mental health on reproductive age women's health utilization, U.S., 2009. Matern Child Health J 2012;16 Suppl 2:203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hall KS, Moreau C, Trussell J. Continuing social disparities despite upward trends in sexual and reproductive health service use among young women in the United States. Contraception 2012;86:681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey JV, Murray E, Rait G, et al. Interactive computer-based interventions for sexual health promotion. Cochrane Database Syst Rev 2010;:CD006483. [DOI] [PubMed] [Google Scholar]
- 15.Bingham CR, Barretto AI, Walton MA, et al. Efficacy of a web-based, tailored, alcohol prevention/intervention program for college students: Initial findings. J Am Coll Health 2010;58:349–356 [DOI] [PubMed] [Google Scholar]
- 16.Milan JE, White AA. Impact of a stage-tailored, web-based intervention on folic acid-containing multivitamin use by college women. Am J Health Promot 2010;24:388–395 [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. Gardasil vaccine information statement. 2012. www.cdc.gov/vaccines/pubs/vis/vis-rtf-files.htm Accessed November2, 2012
- 18.Ratanasiripong NT, Cheng AL, Enriquez M. What college women know, think, and do about human papillomavirus (HPV) and HPV vaccine. Vaccine 2013;31:1370–1376 [DOI] [PubMed] [Google Scholar]
- 19.Kahn JA, Rosenthal SL, Jin Y, et al. Rates of human papillomavirus vaccination, attitudes about vaccination, and human papillomavirus prevalence in young women. Obstet Gynecol 2008;111:1103–1110 [DOI] [PubMed] [Google Scholar]
- 20.Almeida CM, Tiro JA, Rodriguez MA, et al. Evaluating associations between sources of information, knowledge of the human papillomavirus, and human papillomavirus vaccine uptake for adult women in California. Vaccine 2012;30:3003–3008 [DOI] [PubMed] [Google Scholar]
- 21.Allen JD, Mohllajee AP, Shelton RC, et al. Stage of adoption of the human papillomavirus vaccine among college women. Prev Med 2009;48:420–425 [DOI] [PubMed] [Google Scholar]
- 22.Roberts ME, Gerrard M, Reimer R, et al. Mother-daughter communication and human papillomavirus vaccine uptake by college students. Pediatrics 2010;125:982–989 [DOI] [PubMed] [Google Scholar]
- 23.Larkey LK, Hecht M. A model of effects of narrative as culture-centric health promotion. J Health Commun 2010;15:114–135 [DOI] [PubMed] [Google Scholar]
- 24.Simmons A, Skopec L. 47 million women will have guaranteed access to women's preventive services with zero cost-sharing under the Affordable Care Act. Washington, DC: U.S. Department of Health and Human Services, 2012 [Google Scholar]
- 25.Patel DA, Grunzweig KA, Zochowski MK, et al. Human papillomavirus vaccine stages of change among male and female university students: Ready or not? J Am Coll Health 2013;61:336–346 [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Adult vaccination coverage–United States, 2010. MMWR Morb Mortal Wkly Rep 2012;61:66–72 [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011;60:1705–1708 [PubMed] [Google Scholar]