Abstract
The catalytic activity of cytochrome P450 enzymes is known to be affected by presence of organic solvents in in vitro assays. However, these effects tend to be variable and depend on the substrate and CYP450 isoform in question. In the present study, we have investigated effect of ten water miscible organic solvents (methanol, ethanol, propanol, isopropanol, acetone, acetonitrile, dimethylsulphoxide, dimethylformamide, dioxane and PEG400) on water soluble substrates of CYP450, metoprolol and imipramine, at 0, 0.1, 0.25, 0.5, 0.75 and 1% v/v concentration in rat liver microsomes. Organic solvents studied had a concentration dependent inhibitory effect on the metoprolol and imipramine metabolism activity. Metoprolol metabolism was found to be more susceptible to the organic solvents, almost all the ten solvents had more or less inhibitory effect compared to imipramine metabolism. Except acetone, PEG400 and dimethylsulphoxide, all solvents had ~50% inhibition of total metoprolol metabolism activity, while in case of imipramine metabolism activity, only n-propanol, isopropanol and PEG400 had ~50% inhibition at 1% v/v. Interestingly, methanol, dimethylsulphoxide and acetonitrile had negligible effect on the imipramine metabolism (less than 10% inhibition at 1% v/v) while, total metoprolol metabolism activity was substantially inhibited by these solvents (MeOH 52%, DMSO 29% and ACN 47% at 1% v/v). In both cases, dioxane was found to be the most inhibitory solvent (~90% inhibition at 1% v/v).
Keywords: Metoprolol, imipramine, rat liver microsomes, CYP450, organic solvents
Liver is an important organ of metabolism and elimination of the drugs and thus determines the efficacy and toxicity of drugs[1]. The cytochrome P450 (CYP450) enzymes that are predominantly found in liver are a membrane bound superfamily of enzyme that play a critical role in oxidation of drugs[2,3,4]. Various in vitro experiments like microsomal metabolic stability, reaction phenotyping, determination of enzyme kinetic parameters, CYP450 inhibition and induction are performed in various animal liver models, to guide the selection of the lead molecule at discovery stage and to also predict in vivo clearance from in vitro experiments[4,5,6]. The in vitro drug metabolism studies require that the new chemical entities (NCEs) (substrates of CYP450) to be present in dissolved form for enzymatic turnover. However, this a challenging issue due to poor aqueous solubility[7] and hence water miscible organic solvents are generally used at low concentration to improve aqueous solubility of the NCEs[4]. However, these organic solvents have their own disadvantages as they have variable effects on the activity of the CYP450 enzymes at the concentration level they are frequently used and several reports are available of this effect[4,8,9,10,11,12,13,14,15,16]. In most of these reports the investigators had used lipophilic CYP450 probe substrate to study the effects of solvents on their metabolism. Thus, the control incubations to assess effects of solvent were set up by either (a) use of minimal solvent to dissolve substrate (with minimal organic solvent)[9,11,12,13] or (b) in some cases, the substrate was first dissolved in organic solvent, which was evaporated and residue redissolved in microsomes to set up a control (without organic solvent) incubation[10,15,16]. In the first approach a true solvent control was missing while in latter approach complete resolubilization of substrate was assumed.
In the present study, we have studied the effect of ten water miscible organic solvents methanol (MeOH), ethanol (EtOH), n-propanol (nPA), 2-propanol (IPA), acetone (ACE), acetonitrile (ACN), dimethylsulphoxide (DMSO), N,N-dimethylformamide (DMF), dioxane (DIOX) and polyethylene glycol 400 (PEG400) on CYP450 substrates metoprolol and imipramine (having inherently sufficiently high water solubility) metabolism activity in rat liver microsomes (RLM) at 0, 0.1, 0.25, 0.5, 0.75 and 1% v/v concentration. In both these cases, establishment of solvent free control was possible, thus allowing for the true assessment of solvent effects.
MATERIALS AND METHODS
Imipramine (IMI) was obtained from S. D. Fine-Chem Ltd, Mumbai. Metoprolol succinate (MET) was obtained as a gift sample from Ipca Laboratories Ltd, Mumbai. Clomipramine hydrochloride was obtained as a gift sample from Cipla Pvt. Ltd, Mumbai. Pindolol and bovine serum albumin were gift samples obtained from Piramal Life Science Pvt. Ltd., Mumbai. NADPH was obtained from SRL Chemicals Ltd., Mumbai. Tris-HCl was obtained from Sigma Chemicals Co., USA. Sucrose AR, p-nitrophenol, glycerol, ethylenediamine tetraacetic acid AR, dipotassium hydrogen phosphate AR, Triton X-100, sodium dithionite purified, ethanol AR, coomassie brilliant blue G-250, phosphoric acid (85% w/v), conc. HCl, acetone, dioxane, n-propanol and PEG 400 were obtained from S. D. Fine-Chem. Ltd. Mumbai. Calcium chloride AR was obtained from Thomas Baker. Carbon monoxide gas was obtained from Alchemi Gases and Chemicals Pvt. Ltd. Mumbai. ACN, MeOH (HPLC grade), diethyl ether LR was obtained from Qualigens Ltd., Mumbai, DMF and DMSO were obtained from Merck India Ltd. All other chemicals were of analytical grade.
Isolation and characterization of rat liver microsomes:
Livers used in this study were obtained from rats sacrificed as a part of other experiments approved by the Institutional Animal Ethics Committee of the Bombay College of Pharmacy, Kalina. RLM were prepared in-house by calcium chloride aggregation method[17]. The entire microsomal isolation protocol was carried out at 0-4°, in an ice bath. Briefly, finely chopped pooled liver from upto six rats (10 g) were mixed with four volumes (40 ml) of ice cold 10 mM Tris-HCl buffer, containing 0.25 M sucrose, pH 7.4, and then homogenized in a potter glass homogenizer equipped with Teflon pestle. The homogenate was centrifuged at 13 000×g for 10 min at 4°, and the precipitate was discarded. To the supernatant, CaCl2 was added to yield a final concentration of 10 mM. The solution was stirred for 15-20 min and then centrifuged at 25 000×g for 10 min at 4°. The firmly packed pellets of microsomes were resuspended by homogenization in 100 mM Tris-HCl buffer containing 20% w/v glycerol and 10 mM EDTA, pH 7.4. The microsomes were stored at -70° until use. Further, RLM were characterized for spectral CYP450 content by the method of Omura and Sato and for protein content by Bradford method.
Effect of water-miscible organic solvents on imipramine and metoprolol metabolism:
The effect of ten different water-miscible organic solvents, MeOH, EtOH, nPA, IPA, ACE, ACN, DMF, DMSO, DIOX and PEG400 on imipramine metabolism and metoprolol metabolism activities were studied at varying concentration (0.1, 0.25, 0.5, 0.75 and 1% v/v).
Metoprolol metabolism activity:
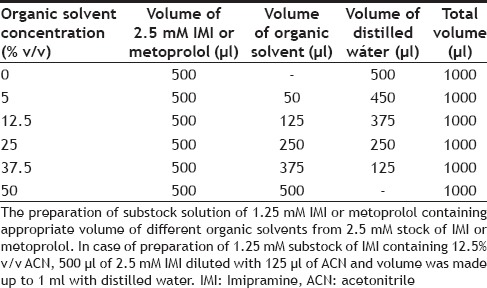
A stock solution of 2.5 mM MET was prepared in distilled water, and 500 μl of this substock was further diluted appropriately with respective organic solvent-distilled water mixture to get 1.25 mM of MET and 0, 5, 12.5, 25, 37.5 and 50% v/v of organic content as shown in Table 1. Further, 20 μl of 1.25 mM MET substocks containing 0, 5, 12.5, 25, 37.5 and 50% v/v of respective organic solvents were incubated with 30 μl of RLM. The total volume of reaction (1000 μl) was adjusted with phosphate buffer to obtain 0, 0.1, 0.25, 0.5, 0.75 and 1% v/v final concentration of organic solvents, and a final concentration of 25 μM MET, respectively. The reaction was initiated by addition of 100 μl of 6 mM NADPH and maintained at 37° on water bath shaker for 30 min. The reaction was then terminated by addition of 200 μl perchloric acid (6% v/v). Then, 100 μl of 0.4 mg/ml pindolol, as an internal standard was added to each tube and stored at -70° until analysis. For the HPLC analysis, 500 μl of HPLC mobile phase was added to each reaction tube, and then samples were vortexed and centrifuged at 7000×g for 10 min. A Jasco HPLC instrument equipped with PU2080 plus HPLC pump, manual Rheodyne injector with a fluorescence detector (FP2080 plus) and chromatograms were analyzed by Borwin 1.50 software. Stationary phase used was a reverse phase Thermo Hypersil BDS, C18 (250×4.6 mm, 5 μM). Mobile phase containing 1% v/v triethylamine pH 3.0 adjusted with orthophosphoric acid and ACN was used in the ratio of 90:10. The analytes were eluted at flow rate of 1 ml/min and detected at excitation and emission wavelength of 228 nm and 310 nm, respectively. MET substock prepared in distilled water (0% v/v organic solvent) was considered as control. Each experiment was conducted in triplicate.
TABLE 1.
PROTOCOL FOR PREPARATION OF 1.25 mM IMI OR METOPROLOL CONTAINING 0, 5, 12.5, 25, 37.5 AND 50% V/V RESPECTIVE ORGANIC SOLVENTS
Imipramine metabolism activity:
A stock solution 2.5 mM IMI was prepared in distilled water. Then, 500 μl of this substock was further diluted appropriately with respective organic solvent-distilled water mixture to get 1.25 mM of IMI and 0, 5, 12.5, 25, 37.5 and 50% v/v of organic content as shown in Table 1. Further, 10 μl of 1.25 mM IMI substocks containing 0, 5, 12.5, 25, 37.5 and 50% v/v of respective organic solvents were incubated with 10 μl of RLM. The total volume of reaction (500 μl) was adjusted with phosphate buffer to obtain 0, 0.1, 0.25, 0.5, 0.75 and 1% v/v final concentration of organic solvents, and a final concentration of 25 μM IMI, respectively. The reaction was initiated by addition of 50 μl of 6 mM NADPH and maintained at 37° on water bath shaker for 10 min. The reaction was then terminated by addition of 2000 μl ammonia solution (12.5% v/v). Then, 30 μl of 1 mM clomipramine as an internal standard was added to each tube and stored at -70° until analysis. For the HPLC analysis, samples were extracted using diethyl ether (5 ml). The two layers (i.e. aqueous and ether) were allowed to separate. All the samples were placed in the dry ice-acetone bath for 20 s. After the freezing of the bottom aqueous layer, the upper ether layer was decanted and collected in separate glass centrifuge tubes. The ether layer was evaporated on a water bath at 40°. The residue was reconstituted in 400 ml of mobile phase for HPLC analysis. A Thermo HPLC instrument equipped with pump (Spectra System P2000), Auto-sampler (Spectra System AS3000) and a variable wavelength detector (Spectra System UV1000) was used and chromatograms were analyzed by Chromquest 4.1 software. Stationary phase used was a reverse phase Thermo Hypersil BDS, C18 (250×4.6 mm, 5 μM). Mobile phase containing 0.5% v/v triethylamine pH 3.0 adjusted with orthophosphoric acid and ACN was used in the ratio of 35:65. The analytes were eluted at flow rate of 1 ml/min and detected at 252 nm. IMI substock prepared in distilled water (0% v/v organic solvent) was considered as control. Each experiment was conducted in triplicate.
Data analysis:
The peak area ratio of metabolite to internal standard was calculated for each incubation sample and then the extent of inhibition or enhancement in the activity was determined by comparing the ratio of peak areas of metabolite to IS in the test incubations (with organic solvent) over the control incubation (no organic solvent), expressed as a percentage. Results are represented as a mean of triplicate incubation.
RESULTS
MET incubation with RLM resulted in the formation of two metabolites while incubation of imipramine with RLM resulted in one metabolite formation as shown in typical chromatograms of incubation in figs. 1 and 2, respectively. Since MET metabolism in RLM gave two metabolite peaks in HPLC analysis, the effect of organic solvents on individual metabolite formation i.e. MET1 and MET2 formation was studied and expressed as % activity remaining as shown in figs. 3 and 4. All the solvents studied had a concentration dependent inhibitory effect of MET1 and MET2 formation. Most solvents showed little or no effect at 0.1% v/v concentration (except nPA for MET1 and acetonitrile for MET2). Only DMSO, ACN, PEG 400 and ACE showed less than 50% inhibition at 1% v/v. The organic solvents like DMSO, MeOH, ACN, DMF and nPA appeared to have more inhibitory effect on the MET1 formation compared to MET2 formation as shown in figs. 3 and 4. However, organic solvents like ACE, PEG400, EtOH, IPA and DIOX showed similar extent of inhibitory effect on MET1 and 2 formation as shown in figs. 3 and 4. DIOX was found to be the most inhibitory solvent for the MET1 (92% inhibition at 1% v/v) and MET2 formation (94% inhibition at 1% v/v). DMSO showed no inhibition of MET2 formation (<10% inhibition at 1% v/v) however, MET1 formation was inhibited by 38% at 1% v/v concentration. Acetonitrile at lower concentration had activation effect (33% activation at 0.1% v/v) but at higher concentration had inhibitory effect (38% inhibition at 1% v/v) on MET2 formation. Also, the trend of effect of organic solvent observed on these two metabolite formation were similar, thus total peak area of the MET1 and MET2 was also considered to evaluate effect of these organic solvents on overall metoprolol metabolism activity. Initially, total metabolite peak area to IS peak area ratio was calculated for each sample and then compared with that of control (no organic solvent) to calculate percent activity remaining of metoprolol metabolism. Over all, ACE showed least inhibitory effect on total metoprolol metabolism (27% inhibition at 1% v/v) and DIOX was found to be the most inhibitory solvent of metoprolol metabolism (92% inhibition at 1% v/v) as shown in fig. 5.
Fig. 1.
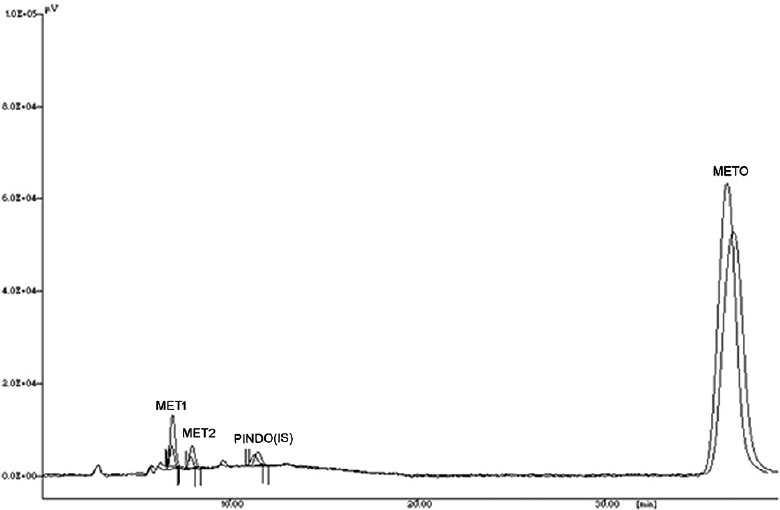
A representative overlain chromatogram of metoprolol metabolism.
The overlain chromatogram of metoprolol metabolism indicates decrease in metabolite formation with respect to control. Incubation sample contains dimethylformamide (0.75% v/v) as compared to solvent free control incubation.
Fig. 2.
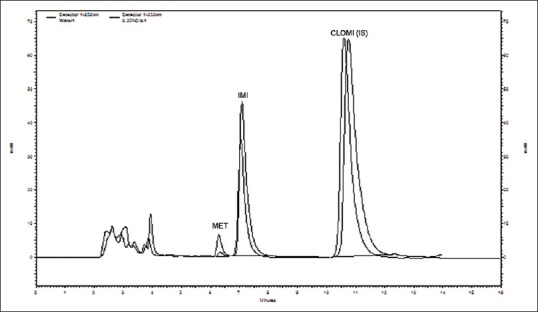
A representative overlain chromatogram of imipramine metabolism.
The overlain chromatogram of imipramine metabolism indicated decrease in metabolites formation with respect to control. Incubation sample contains dioxane (0.25% v/v) as compared to a solvent free control incubation.
Fig. 3.
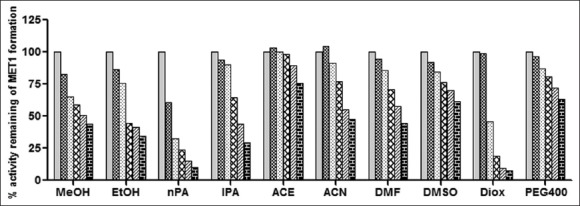
The effect of ten organic solvents on the metoprolol metabolism in RLM.
Organic solvent effect on metoprolol metabolism was determined by co-incubation of each solvent at (0, 0.1, 0.25, 0.5, 0.75 and 1% v/v) concentration with rat liver microsomes, 0.6 mM NADPH and MET (25 μM) represents effect on MET1 formation. Each bar represents mean of triplicate incubation expressed as a percent activity remaining.  (0% v/v),
(0% v/v),  (0.1% v/v),
(0.1% v/v),  (0.25% v/v),
(0.25% v/v),  (0.5% v/v),
(0.5% v/v),  (0.75% v/v),
(0.75% v/v),  (1% v/v).
(1% v/v).
Fig. 4.
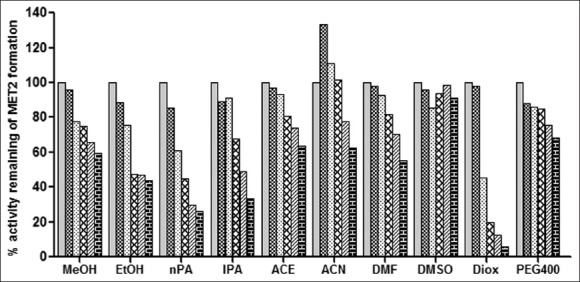
The effect of ten organic solvents on the metoprolol metabolism in RLM.
Organic solvent effect on metoprolol metabolism activity was determined by co-incubation of each solvent at (0, 0.1, 0.25, 0.5, 0.75 and 1% v/v) concentration with rat liver microsomes, 0.6 mM NADPH and MET (25 μM) represents effect on MET2 formation. Each bar represents mean of triplicate incubation expressed as a percent activity remaining.  (0% v/v),
(0% v/v),  (0.1% v/v),
(0.1% v/v),  (0.25% v/v),
(0.25% v/v),  (0.5% v/v),
(0.5% v/v),  (1% v/v),
(1% v/v),  (0.75% v/v).
(0.75% v/v).
Fig. 5.
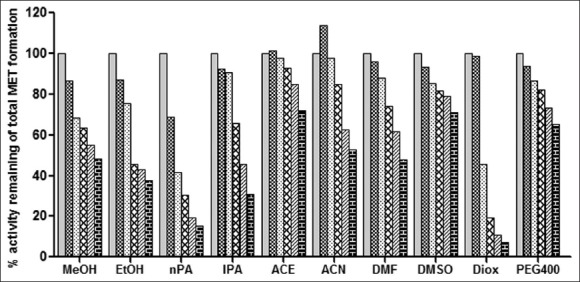
The effect of ten organic solvents on the metoprolol metabolism in RLM.
Organic solvent effect on metoprolol metabolism activity was determined by co-incubation of each solvent at (0, 0.1, 0.25, 0.5, 0.75 and 1% v/v) concentration with rat liver microsomes, 0.6 mM NADPH and METO (25 μM) represents effect on total metabolite formation of metoprolol metabolism activity. Each bar represents mean of triplicate incubation expressed as a percent activity remaining.  (0% v/v),
(0% v/v),  (0.1% v/v),
(0.1% v/v),  (0.25% v/v),
(0.25% v/v),  (0.5% v/v),
(0.5% v/v),  (1% v/v),
(1% v/v), (0.75% v/v).
(0.75% v/v).
In case of imipramine metabolism activity, among the ten solvents evaluated, except for MeOH and DMSO, all solvents had concentration dependent inhibition of imipramine metabolism activity as shown in fig. 6. DIOX showed maximum inhibition (93% at 1% v/v), followed by PEG400, nPA and IPA (45-58% at 1% v/v). Solvents like EtOH, ACE and DMF showed less than 50% inhibition at 1% v/v concentration. Interestingly, DMSO one of the most-inhibitory solvent (>90% inhibition of p-nitrophenol hydroxylation activity, (data not shown) and 40% inhibition of MET1 formation at 1% v/v concentration) has negligible effect on the IMI metabolism and MET2 formation of MET metabolism activity.
Fig. 6.
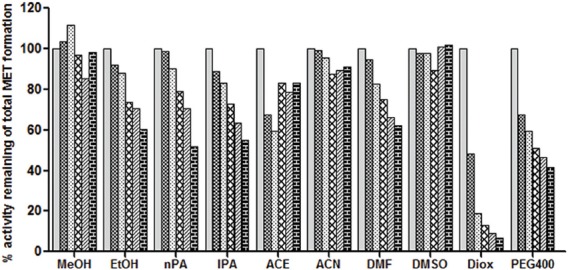
The effect of ten organic solvents on the imipramine metabolism activity in RLM.
Organic solvent effect on imipramine metabolism activity was determined by co-incubation of each solvent at (0, 0.1, 0.25, 0.5, 0.75 and 1% v/v) concentration with rat liver microsomes, 0.6 mM NADPH and IMI (25 μM). Each bar represents mean of triplicate incubation expressed as a percent activity remaining.  (0% v/v),
(0% v/v), (0.1% v/v),
(0.1% v/v),  (0.25% v/v),
(0.25% v/v),  (0.5% v/v),
(0.5% v/v),  (1% v/v),
(1% v/v),  (0.75% v/v).
(0.75% v/v).
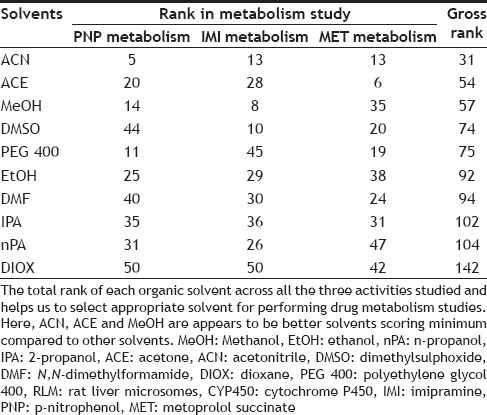
For evaluating the best solvent out of the ten solvents studied to assay total MET metabolism and IMI metabolism activity, the inhibition shown by solvents were rank ordered at each concentration level, for example, solvent showing least inhibition at 0.1% v/v concentration was ranked as 1 and solvent showing maximum inhibition at 0.1% v/v concentration was ranked as 10. Thus, the best solvent would score rank 5 (rank of 1 at each of the five concentration levels studies) while worst solvent would score rank 50 (rank of 10 at each of the five concentration levels studies) as given in Tables 2 and 3. Finally, the total score of each solvent for each study (i.e. IMI, MET and p-nitrophenol metabolism study, data not shown) was obtained by addition of individual score to a overall score out of 150 indicating the gross effect of each water miscible solvent on CYP450 mediated metabolism as shown in Table 4. Thus, the solvent with least score can be projected to be the most preferable solvent to perform CYP450 mediated metabolism studies in RLM. Among the ten solvents studied, ACN appeared to be best solvent scoring least (31 out of 150) while DIOX appeared to be worst solvent (142 out of 150).
TABLE 2.
RANK ORDER FOR EACH SOLVENT BASED ON INHIBITION ON TOTAL METOPROLOL METABOLISM ACTIVITY IN RLM AT DIFFERENT CONCENTRATION
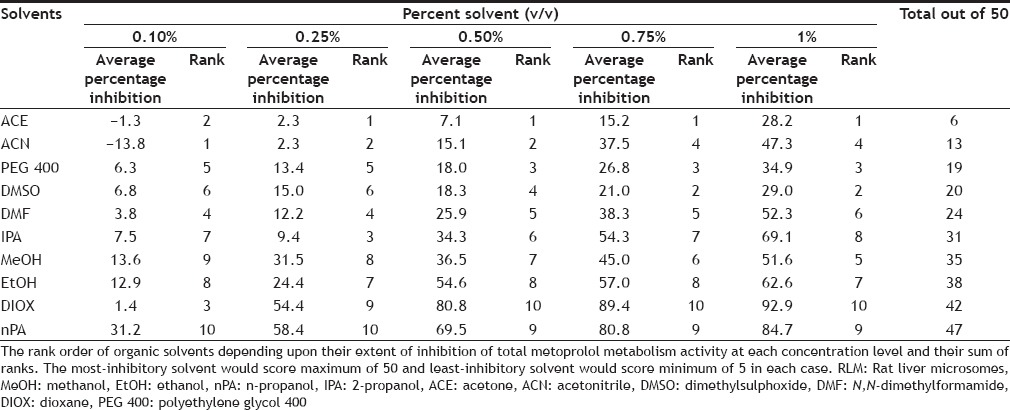
TABLE 3.
RANK ORDER FOR EACH SOLVENT BASED ON INHIBITION ON IMIPRAMINE METABOLISM ACTIVITY IN RLM AT DIFFERENT CONCENTRATION
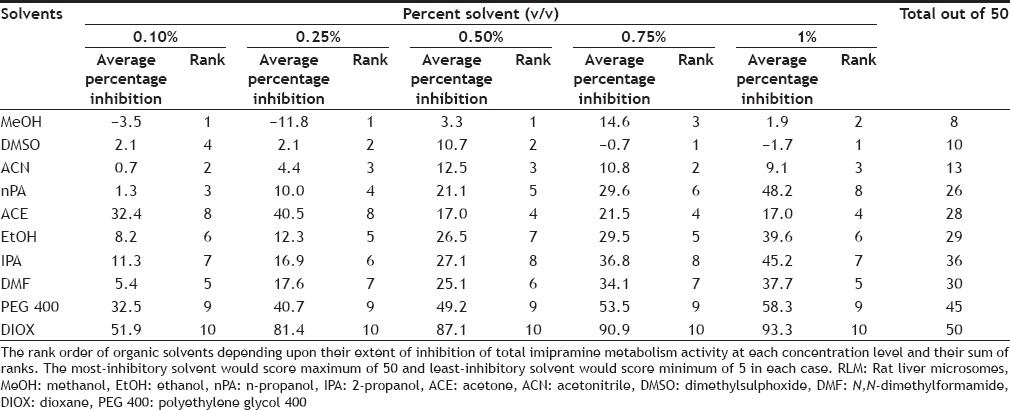
TABLE 4.
GROSS EFFECT OF WATER MISCIBLE SOLVENT ON CYP450 MEDIATED METABOLISM IN RLM
DISCUSSION
In the present study, MET metabolism and IMI metabolism activity in RLM were selected to study the effects of water miscible organic solvents. The incubation of metoprolol with RLM gave two major metabolites possibly O-desmethyl MET and α-hydroxy MET[18,19] while IMI incubation possibly resulted in N-demethylated metabolite of IMI (desipramine) as a major metabolite[20,21,22]. However, due to unavailability of standard metabolites they were not identified and tentatively called as MET1 or MET2 and metabolite in case of MET and IMI metabolism activity, respectively. All water miscible organic solvents that could be potentially used for dissolving NCEs/substrates while performing in vitro incubations were selected. These included some alcohols (MeOH, EtOH, nPA and IPA), ketone (ACE), ether (DIOX), nitrile (ACN), amide (DMF), sulphoxide (DMSO) and PEG400.
CYP450 are membrane bound enzymes found on the endoplasmic reticulum and require cytochrome P450 reductase (CPR) for their activity. Thus, the organic solvents can interact with substrate (solvation effect), lipid membrane, CYP450 (altering substrate binding and/or catalysis) and CPR (altering interaction between CYP450 and CPR). Thus, the CYP450 activity can be potentially affected by interaction of organic solvents at one or more of these factors.
If the organic solvents had an effect at only the substrate level (solvation of substrate) then in case of metoprolol metabolism activity, each organic solvent should have had a similar effect on both the metabolite formation. Although the trend of effect of organic solvents, except DMSO and ACN, on MET1 and MET2 formation was similar, MET1 formation was found to be more susceptible to organic solvent effect (DMSO, MeOH, ACN, DMF and nPA appeared to have more inhibitory effect on the MET1 formation compared to MET2). Further, DMSO did not affect MET2 formation while MET1 formation was inhibited (38% at 1% v/v), ACN showed activation of MET2 formation (33% activation at 0.1% v/v). This evidence indicates that in case of metoprolol metabolism activity, inhibitory effect of organic solvents was not solely due to the solvation of substrate, which enables the binding of substrate to active site at altered energy cost[23].
Further, almost all the organic solvents studied have influenced MET and IMI metabolism activities to varying extent, indicating that the organic solvents might have effect either on the phospholipid membrane and affecting structural stability of CYP450 or have affected the CPR, altering the reduction of CYP450, an initial step in the oxygen activation cycle. However, varying effect of the organic solvents was observed, MeOH, DMSO and ACN had negligible effect on the imipramine metabolism (less than 10% inhibition at 1% v/v) while, total MET metabolism was substantially inhibited by these solvents (MeOH 52%, DMSO 29% and ACN 47% at 1% v/v). Further, in case of MET metabolism, the activation of MET2 formation activity (33% activation at 0.1%v/v) and in case of p-nitrophenol hydroxylation activity (23% activation at 1% v/v, data not shown) was observed when ACN was used as an organic solvent. Similarly, ACN was previously known to show activation of tolbutamide hydroxylase CYP2C8/2C9 activity (139% of control) at 5% v/v concentration[10] in human liver microsomes. In another report, activation of caffeine N3-demethylation (CYP1A2) activity in human liver microsomes was observed in presence of ACE, ACN and IPA[13]. These evidences (activation and selective inhibition) indicate that in addition to effects on lipid bilayer and/or CPR, solvents may interact with CYP450 enzymes, either by (a) affecting the binding of substrate with the active site, (b) through noncompetitive effects, thus reducing the amount of enzyme available for catalysis or (c) through inactivation of enzyme.
The altered effect of same organic solvents in case of MET metabolism on each metabolite formation, activation of MET2 formation by ACN (33% activation at 0.1% v/v) and negligible effect on the MET1 formation while DMSO inhibited MET1 formation (40% at 1% v/v) and negligible effect on MET2 formation, indicates that these solvents alter catalysis by affecting the binding of metoprolol with the active sites or through noncompetitive effects on the amount of enzyme available for catalysis. This is also evident from the previous findings of Kumar et al., who have shown that the binding of nelfinavir to CYP3A4 enzyme improves in presence of EtOH. Kumar et al. reported that the spectral dissociation constant of nelfinavir decreases from 0.227 to 0.041 μM in presence of 20 mM EtOH[24]. Similarly, Backes et al. found that binding of ethylbenzene to the rat liver microsomes varies with the organic solvents (apparent binding constant of ethylbenzene was found to be 28, 23, 16, and 25 mM in presence of MeOH, EtOH, ACE and nPA, respectively)[25] thus, indicating that solvents seem to affect the first step of catalysis i.e. binding of the substrate to the active site of CYP450. To establish that this may occur in our case warrants detailed study of enzyme kinetics (Km and Vmax) of the metoprolol metabolism activity.
Overall, our studies suggest that the effect of organic solvents on CYP450 activity probably represents a combined effect on the phospholipid bilayer containing the CYP450 system, the binding of substrate with the active site of enzyme and enzyme inactivation, substrate/enzyme pair. However, the exact mechanism and the relative contribution of above stated factors on the activity of the CYP450 enzyme are difficult to deconvolute.
It is evident that pure aqueous incubations always be preferred for CYP450s activity studies. Further, since the effects of solvents on the activity of CYP450s are complicated, one must be very cautious about the choice of solvents and its concentration while performing such studies. Our studies however do indicate that, if one has to use an organic solvent then the concentration should be kept below 0.25% v/v and, since ACN and MeOH appear to have the least influence compared to the other solvents studied on the CYP450 activity, they appear to be the solvents of choice for CYP450 activity studies.
Financial support and sponsorship:
PP and TS are recipients of AICTE fellowship and SK is a BMS Research Fellow.
Conflicts of interest:
There are no conflicts of interest.
Acknowledgements:
PP and TS are recipients of AICTE fellowship and SK is a BMS Research Fellow, and PP, TS, SK & KI thank BMS and AICTE for their financial support.
Footnotes
Shah, et al.: Effects of Solvents on Metoprolol and Imipramine Metabolism
REFERENCES
- 1.Murry DJ, Crom WR, Reddick WE, Bhargava R, Evans WE. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metab Dispos. 1995;23:1110–6. [PubMed] [Google Scholar]
- 2.Lin JH, Lu AY. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35:361–90. doi: 10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- 3.Danielson PB. The cytochrome P450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr Drug Metab. 2002;3:561–97. doi: 10.2174/1389200023337054. [DOI] [PubMed] [Google Scholar]
- 4.Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat Rev Drug Discov. 2005;4:825–33. doi: 10.1038/nrd1851. [DOI] [PubMed] [Google Scholar]
- 5.Riley RJ, McGinnity DF, Austin RP. A unified model for predicting human hepatic, metabolic clearance from in vitro intrinsic clearance data in hepatocytes and microsomes. Drug Metab Dispos. 2005;33:1304–11. doi: 10.1124/dmd.105.004259. [DOI] [PubMed] [Google Scholar]
- 6.Obach RS. Prediction of human clearance of twenty-nine drugs from hepatic microsomal intrinsic clearance data: An examination of in vitro half-life approach and nonspecific binding to microsomes. Drug Metab Dispos. 1999;27:1350–9. [PubMed] [Google Scholar]
- 7.Lewis DF, Jacobs MN, Dickins M. Compound lipophilicity for substrate binding to human P450s in drug metabolism. Drug Discov Today. 2004;9:530–7. doi: 10.1016/S1359-6446(04)03115-0. [DOI] [PubMed] [Google Scholar]
- 8.Aasa J, Hu Y, Eklund G, Lindgren A, Baranczewski P, Malmquist J, et al. Effect of solvents on the time-dependent inhibition of CYP3A4 and the biotransformation of AZD3839 in human liver microsomes and hepatocytes. Drug Metab Dispos. 2013;41:159–69. doi: 10.1124/dmd.112.047597. [DOI] [PubMed] [Google Scholar]
- 9.Busby WF, Jr, Ackermann JM, Crespi CL. Effect of methanol, ethanol, dimethyl sulfoxide, and acetonitrile on in vitro activities of cDNA-expressed human cytochromes P-450. Drug Metab Dispos. 1999;27:246–9. [PubMed] [Google Scholar]
- 10.Chauret N, Gauthier A, Nicoll-Griffith DA. Effect of common organic solvents on in vitro cytochrome P450-mediated metabolic activities in human liver microsomes. Drug Metab Dispos. 1998;26:1–4. [PubMed] [Google Scholar]
- 11.Easterbrook J, Lu C, Sakai Y, Li AP. Effects of organic solvents on the activities of cytochrome P450 isoforms, UDP-dependent glucuronyl transferase, and phenol sulfotransferase in human hepatocytes. Drug Metab Dispos. 2001;29:141–4. [PubMed] [Google Scholar]
- 12.González-Pérez V, Connolly EA, Bridges AS, Wienkers LC, Paine MF. Impact of organic solvents on cytochrome P450 probe reactions: Filling the gap with (S)-Warfarin and midazolam hydroxylation. Drug Metab Dispos. 2012;40:2136–42. doi: 10.1124/dmd.112.047134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hickman D, Wang JP, Wang Y, Unadkat JD. Evaluation of the selectivity ofIn vitro probes and suitability of organic solvents for the measurement of human cytochrome P450 monooxygenase activities. Drug Metab Dispos. 1998;26:207–15. [PubMed] [Google Scholar]
- 14.Li D, Han Y, Meng X, Sun X, Yu Q, Li Y, et al. Effect of regular organic solvents on cytochrome P450-mediated metabolic activities in rat liver microsomes. Drug Metab Dispos. 2010;38:1922–5. doi: 10.1124/dmd.110.033894. [DOI] [PubMed] [Google Scholar]
- 15.Tang C, Shou M, Rodrigues AD. Substrate-dependent effect of acetonitrile on human liver microsomal cytochrome P450 2C9 (CYP2C9) activity. Drug Metab Dispos. 2000;28:567–72. [PubMed] [Google Scholar]
- 16.Palamanda J, Feng WW, Lin CC, Nomeir AA. Stimulation of tolbutamide hydroxylation by acetone and acetonitrile in human liver microsomes and in a cytochrome P-450 2C9-reconstituted system. Drug Metab Dispos. 2000;28:38–43. [PubMed] [Google Scholar]
- 17.Walawalkar PS, Serai PS, Iyer K. Isolation and catalytic competence of different animal liver microsomal fractions prepared by calcium-aggregation method. Indian J Pharm Sci. 2006;68:262–5. [Google Scholar]
- 18.Lennard M. Quantitative analysis of metoprolol and three of its metabolites in urine and liver microsomes by high-performance liquid chromatography. J Chromatogr Biomed. 1985;342:199–205. doi: 10.1016/s0378-4347(00)84504-1. [DOI] [PubMed] [Google Scholar]
- 19.Lennard MS, Crewe HK, Tucker GT, Woods HF. Metoprolol oxidation by rat liver microsomes. Inhibition by debrisoquine and other drugs. Biochem Pharmacol. 1986;35:2757–61. doi: 10.1016/0006-2952(86)90186-3. [DOI] [PubMed] [Google Scholar]
- 20.Bull S, Catalani P, Garle M, Coecke S, Clothier R. Imipramine for cytochrome P450 activity determination: A multiple-species metabolic probe. ToxicolIn Vitro. 1999;13:537–41. doi: 10.1016/s0887-2333(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 21.Chiba M, Fujita S, Suzuki T. Kinetic properties of the metabolism of imipramine and desipramine in isolated rat hepatocytes. Biochem Pharmacol. 1990;39:367–72. doi: 10.1016/0006-2952(90)90036-k. [DOI] [PubMed] [Google Scholar]
- 22.Lemoine A, Gautier JC, Azoulay D, Kiffel L, Belloc C, Guengerich FP, et al. Major pathway of imipramine metabolism is catalyzed by cytochromes P-450 1A2 and P-450 3A4 in human liver. Mol Pharmacol. 1993;43:827–32. [PubMed] [Google Scholar]
- 23.Rokitta D, Pfeiffer K, Streich C, Gerwin H, Fuhr U. The effect of organic solvents on enzyme kinetic parameters of human CYP3A4 and CYP1A2 in vitro. Toxicol Mech Methods. 2013;23:576–83. doi: 10.3109/15376516.2013.806622. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Earla R, Jin M, Mitra AK, Kumar A. Effect of ethanol on spectral binding, inhibition, and activity of CYP3A4 with an antiretroviral drug nelfinavir. Biochem Biophys Res Commun. 2010;402:163–7. doi: 10.1016/j.bbrc.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 25.Backes WL, Canady WJ. The interaction of hepatic cytochrome P-450 with organic solvents. The effect of organic solvents on apparent spectral binding constants for hydrocarbon substrates. J Biol Chem. 1981;256:7213–27. [PubMed] [Google Scholar]


