Abstract
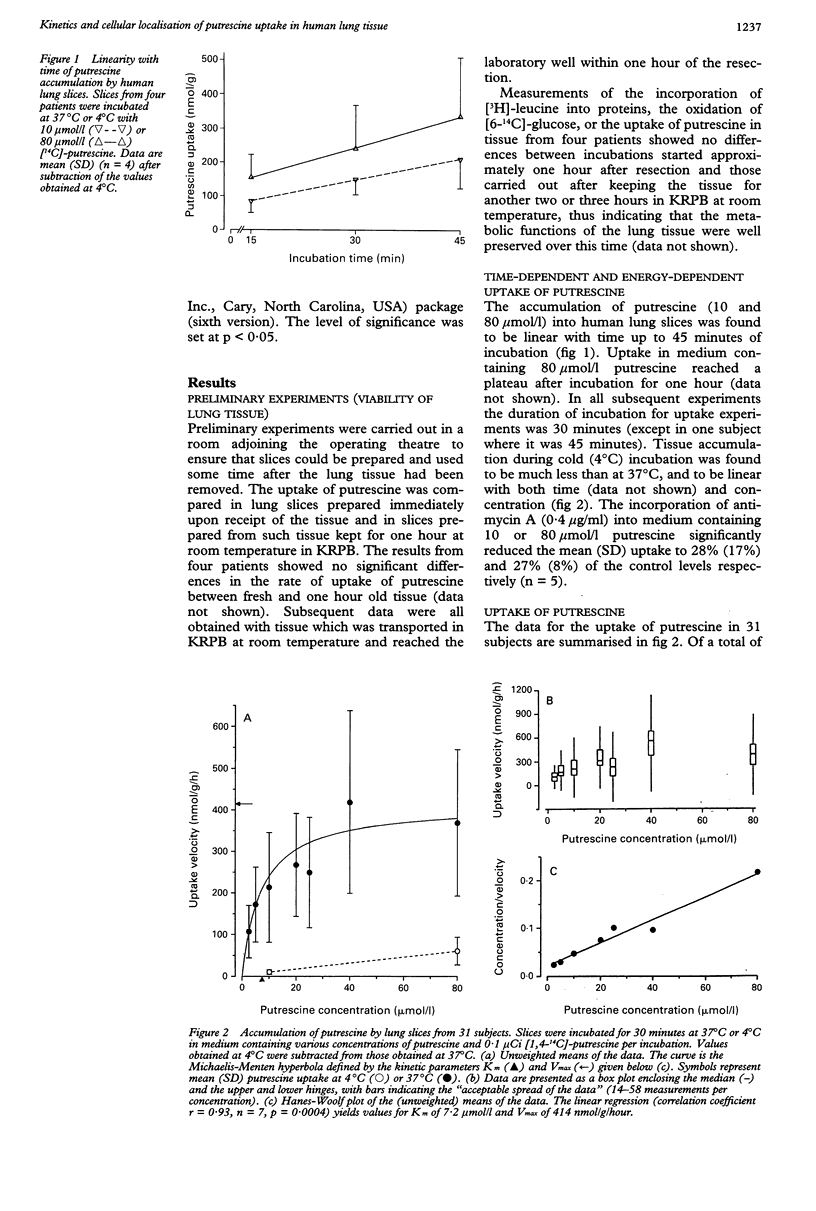
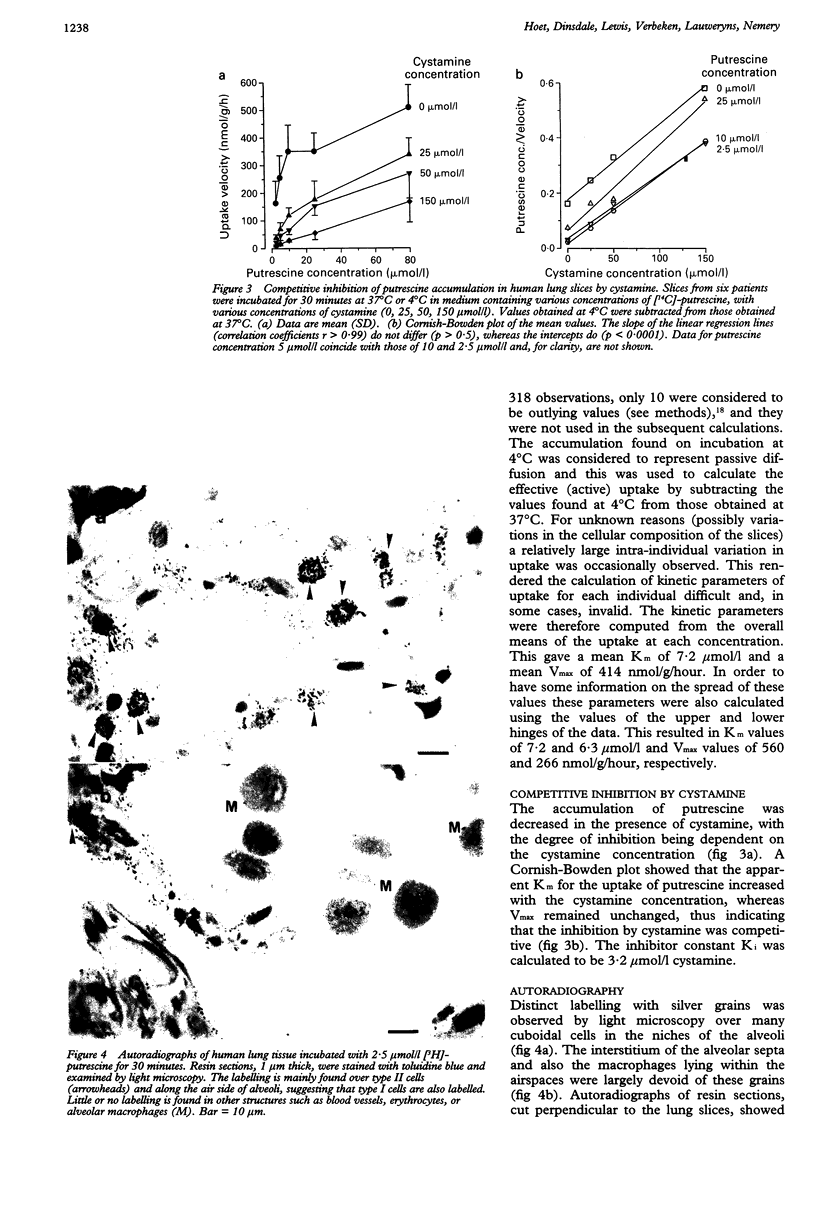
BACKGROUND--The polyamines (putrescine, spermidine, and spermine) are involved in cellular growth, proliferation, and differentiation. In the lungs of various species, polyamines are accumulated by an active uptake system which also mediates the uptake of cystamine and paraquat. In the rat lung putrescine uptake has been shown to be cell-specific, occurring predominantly in the alveolar epithelium. The aim of this study was to characterise the uptake of putrescine in human lung. METHODS--Lung tissue was obtained from 31 patients undergoing surgery for lung cancer. Slices (0.7 mm thick) from non-tumour containing lung parenchyma were incubated for 15-60 minutes in Krebs-Ringer phosphate buffer with various concentrations of putrescine (2.5 to 80 mumol/l) containing 0.1 microCi [1,4-14C]-putrescine. Uptake was assessed from tissue radioactivity. For autoradiographic imaging, slices were incubated for 30 minutes with 2.5 mumol/l putrescine containing 2.5 mCi [1,4n-3H]-putrescine. RESULTS--The accumulation of [14C]-putrescine into slices was time-dependent and energy-dependent, and obeyed saturation kinetics, with mean calculated values for Vmax (maximal rate of uptake) of 414 nmol/g/hour and for Km (medium concentration at which the rate of uptake is half Vmax) of 7.2 mumol/l, with a large interindividual variation. Competitive inhibition was observed on incubation with cystamine, which appears to have a high affinity for the uptake system since its calculated Ki (concentration of inhibitor at which the Km is doubled) was 3.2 mumol/l. Ultrastructural autoradiography showed labelling over both type I and type II cells of the alveolar epithelium, but not over the endothelium or any cells of the interstitium. Alveolar macrophages were also devoid of label. CONCLUSIONS--These results show that the human lung possesses an active uptake system for putrescine, and probably also cystamine, which is located in both cell types of the alveolar epithelium. These findings may be used to develop tests for the assessment of the alveolar epithelium.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooke-Taylor S., Smith L. L., Cohen G. M. The accumulation of polyamines and paraquat by human peripheral lung. Biochem Pharmacol. 1983 Feb 15;32(4):717–720. doi: 10.1016/0006-2952(83)90500-2. [DOI] [PubMed] [Google Scholar]
- Cardenas A., Nemery B. Effects of pneumotoxic trialkylphosphorothioates on the pentose phosphate pathway in rat lung slices. Toxicol Lett. 1991 May;56(3):339–348. doi: 10.1016/0378-4274(91)90162-y. [DOI] [PubMed] [Google Scholar]
- Clayton F. Bronchioloalveolar carcinomas. Cell types, patterns of growth, and prognostic correlates. Cancer. 1986 Apr 15;57(8):1555–1564. doi: 10.1002/1097-0142(19860415)57:8<1555::aid-cncr2820570820>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Davies D. S. Paraquat poisoning: the rationale for current treatment regimes. Hum Toxicol. 1987 Jan;6(1):37–40. doi: 10.1177/096032718700600106. [DOI] [PubMed] [Google Scholar]
- Dinsdale D., Preston S. G., Nemery B. Effects of injury on [3H]putrescine uptake by types I and II cells in rat lung slices. Exp Mol Pathol. 1991 Jun;54(3):218–229. doi: 10.1016/0014-4800(91)90032-s. [DOI] [PubMed] [Google Scholar]
- Eisenthal R., Cornish-Bowden A. The direct linear plot. A new graphical procedure for estimating enzyme kinetic parameters. Biochem J. 1974 Jun;139(3):715–720. doi: 10.1042/bj1390715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. B., Reicherter J. Pentose pathway of glucose metabolism in isolated granular pneumocytes. Metabolic regulation and stimulation by paraquat. Biochem Pharmacol. 1984 Apr 15;33(8):1349–1353. doi: 10.1016/0006-2952(84)90191-6. [DOI] [PubMed] [Google Scholar]
- Jänne J., Alhonen L., Leinonen P. Polyamines: from molecular biology to clinical applications. Ann Med. 1991 Aug;23(3):241–259. doi: 10.3109/07853899109148056. [DOI] [PubMed] [Google Scholar]
- Lewis C. P., Demedts M., Nemery B. Indices of oxidative stress in hamster lung following exposure to cobalt(II) ions: in vivo and in vitro studies. Am J Respir Cell Mol Biol. 1991 Aug;5(2):163–169. doi: 10.1165/ajrcmb/5.2.163. [DOI] [PubMed] [Google Scholar]
- Lewis C. P., Haschek W. M., Wyatt I., Cohen G. M., Smith L. L. The accumulation of cystamine and its metabolism to taurine in rat lung slices. Biochem Pharmacol. 1989 Feb 1;38(3):481–488. doi: 10.1016/0006-2952(89)90388-2. [DOI] [PubMed] [Google Scholar]
- Nemery B., Dinsdale D., Verschoyle R. D. Detecting and evaluating chemical-induced lung damage in experimental animals. Bull Eur Physiopathol Respir. 1987 Sep-Oct;23(5):501–528. [PubMed] [Google Scholar]
- Nemery B., Smith L. L., Aldridge W. N. Putrescine and 5-hydroxytryptamine accumulation in rat lung slices: cellular localization and responses to cell-specific lung injury. Toxicol Appl Pharmacol. 1987 Oct;91(1):107–120. doi: 10.1016/0041-008x(87)90198-0. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Rannels D. E., Pegg A. E., Clark R. S., Addison J. L. Interaction of paraquat and amine uptake by rat lungs perfused in situ. Am J Physiol. 1985 Nov;249(5 Pt 1):E506–E513. doi: 10.1152/ajpendo.1985.249.5.E506. [DOI] [PubMed] [Google Scholar]
- Rao S. B., Mehendale H. M. Passive sequestration of putrescine, spermidine and spermine by rat lungs. Biochim Biophys Acta. 1988 Jul 14;966(1):22–29. doi: 10.1016/0304-4165(88)90124-9. [DOI] [PubMed] [Google Scholar]
- Rose M. S., Smith L. L., Wyatt I. Evidence for energy-dependent accumulation of paraquat into rat lung. Nature. 1974 Nov 22;252(5481):314–315. doi: 10.1038/252314b0. [DOI] [PubMed] [Google Scholar]
- Smith L. L., Lewis C. P., Wyatt I., Cohen G. M. The importance of epithelial uptake systems in lung toxicity. Environ Health Perspect. 1990 Apr;85:25–30. doi: 10.1289/ehp.85-1568342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. L. Mechanism of paraquat toxicity in lung and its relevance to treatment. Hum Toxicol. 1987 Jan;6(1):31–36. doi: 10.1177/096032718700600105. [DOI] [PubMed] [Google Scholar]
- Smith L. L., Wyatt I., Cohen G. M. The accumulation of diamines and polyamines into rat lung slices. Biochem Pharmacol. 1982 Oct 1;31(19):3029–3033. doi: 10.1016/0006-2952(82)90075-2. [DOI] [PubMed] [Google Scholar]
- Smith L. L., Wyatt I. The accumulation of putrescine into slices of rat lung and brain and its relationship to the accumulation of paraquat. Biochem Pharmacol. 1981 May 15;30(10):1053–1058. doi: 10.1016/0006-2952(81)90441-x. [DOI] [PubMed] [Google Scholar]
- Smith L. L. Young Scientists Award lecture 1981: The identification of an accumulation system for diamines and polyamines into the lung and its relevance to paraquat toxicity. Arch Toxicol Suppl. 1982;5:1–14. doi: 10.1007/978-3-642-68511-8_1. [DOI] [PubMed] [Google Scholar]
- Williamson D. F., Parker R. A., Kendrick J. S. The box plot: a simple visual method to interpret data. Ann Intern Med. 1989 Jun 1;110(11):916–921. doi: 10.7326/0003-4819-110-11-916. [DOI] [PubMed] [Google Scholar]
- Wyatt I., Soames A. R., Clay M. F., Smith L. L. The accumulation and localisation of putrescine, spermidine, spermine and paraquat in the rat lung. In vitro and in vivo studies. Biochem Pharmacol. 1988 May 15;37(10):1909–1918. doi: 10.1016/0006-2952(88)90536-9. [DOI] [PubMed] [Google Scholar]