Abstract
Background:
Peripheral arterial disease (PAD) is an aftermath of type 2 diabetes posing a significant health problem in developing countries. Its silent progression warrants presymptomatic screening by ankle brachial index (ABI), which cannot be applied to the whole population. We tried to measure the burden of PAD in diabetics of this region correlating various risk factors for it quantitatively and qualitatively.
Materials and Methods:
From various out-patient departments, 110 known under treatment type 2 diabetics were recruited. They underwent thorough assessment for general, symptomatic, medical history and risk factor screening that included 11 well-known risk factors. ABI was measured by Versadop instrument using the standard protocol with ABI <0.9 being considered as abnormal.
Results:
There was a high prevalence of asymptomatism, hypertension, positive family history and age <52 years in the study group. Relative risk was highest for asymptomatism followed by high body mass index, hyperlipidemia, cardiovascular disease and smoking, but less significant for age, gender, fasting sugar level, family history. More adverse ABI profile was noticed with the increase in number of five modifiable risk factors cumulatively.
Conclusion:
There was a high prevalence of low ABI in our region that is an evidence of PAD mainly affected by risk factors many of which were modifiable. Defining those who are at risk to develop PAD in Diabetes, one can use ABI better in early screening and prompt treatment of this complication to stop its further progression and primary prevention can be served as felt the need for health-care effectively.
Keywords: Ankle brachial index, peripheral arterial disease, prevalence, risk factor, type 2 diabetes
Introduction
Type 2 diabetes mellitus is a highly prevailing disease in developing countries of South Asia including India.[1] Peripheral arterial disease (PAD), one of the complications of it,[2] is still remaining under diagnosed and under treated even in western countries[3,4] owing to its silent asymptomatic progression making it difficult to diagnose in early stage. Even in the absence of PAD symptoms, there remains a significant risk for cardiovascular and cerebrovascular morbidity and mortality.[5] Ankle brachial index (ABI) is a symptom-independent reliable screening tool for PAD that is available since long.[6] It is recommended even at primary health-care level,[6,7,8] but yet underused in both communities and hospitals.[9] It can be attributed to its time consuming nature, lack of reimbursement and staff non-availability.[10] However, the use can be optimized by identifying the significant risk factors and those at risk and this primary prevention can be brought out by simple ABI screening. By the present study, we tried to use ABI to quantify the burden of PAD in diabetics in our region and to correlate various risk factors for its relative contribution and to judge how many of them are significant and of them how many are salvageable.
Materials and Methods
Study population
This observational transverse field study was carried out from 15 September 2012 to 10 November 2012 on known diabetic patients taking regular treatment (not insulin) for a minimum of 6 months. After taking approval for the study, sample size was calculated by software Raosoft for population of the city 6 lakhs with 4% national prevalence of diabetes. A size of 110 was sufficient to have 99% confidence level and 5% margin of error. Subjects were chosen randomly from (i) medicine out-patient department (OPD) of Sir Takhtsinhji General Hospital (Tertiary Care Government Hospital) (ii) diabetic OPD of Sir Jaswantsinhji Hospital (Urban Health and Training Center affiliated to Sir T Hospital and Preventive and Social Medicine Department) (iii) diabetic camp at Shree Bajarangdas Bapa Arogyadhaam (Trust Multispecialty Hospital) (iv) private OPD patients. By choosing patients from different set ups belonging to different socio-economic status we tried to have a blend of heterogeneous subjects, which formed a fairly representative sample whose result can be applied to generalized population. Subjects taking irregular treatment, newly diagnosed, having previous vascular intervention, having amputated limb were excluded from the study.
PAD risk factor assessment
All recruited subjects underwent personal interview in the form of pre-designed, pre-validated questionnaires that included general features, demographic characteristics, symptom of PAD, investigations and treatment taken. Specific emphasis was given to identify following 11 risk factors including diabetes itself: (1) Hypertension, (2) hyperlipidemia, (3) smoking, (4) cardiovascular disease (CVD), (5) family history, (6) absence of symptom (asymptomatism), (7) age <52 years, (8) male gender, (9) fasting blood sugar (FBS) >130 mg/dL, (10) body mass index (BMI) >25 kg/m2, (11) diabetes mellitus.
ABI assessment
ABI was measured in the supine position by investigators themselves after taking consent using the principle of Doppler effect by portable instrument Versadop (table top vascular Doppler with 8 MHz of Diabetik Foot Care India Limited, Chennai, India) having 12 cm occluding cuff. Ankle brachial pressure index was derived by dividing the higher reading of the ankle pressure at dorsalis pedis artery by brachial pressure of the same side.[11] ABI ≥0.9 was considered as normal and ABI <0.9 was defined as PAD.[12]
Statistical analysis
The data was transferred on Excel spreadsheet and descriptive analysis was expressed as mean ± standard deviation. All calculations were accomplished by using Graph Pad in Stat 3 software. We evaluated the strength of association of individual risk factor for PAD by finding the relative risk (RR) keeping confidence interval 95% with ABI <0.9 as a positive outcome and ABI ≥0.9 as a negative outcome. Five out 11 were modifiable risk factors whose cumulative RR was calculated with aforementioned criteria and difference in mean distribution was calculated by Student t-test. Difference was considered as statistically significant with P < 0.05.
Results
Table 1 shows general information for the study group highlighting more females than males, high mean BMI high prevalence of PAD symptom, high mean FBS level, average duration of diabetes 6.28 years and average of risk factors out of 11 being 5.6.
Table 1.
Baseline data of study group
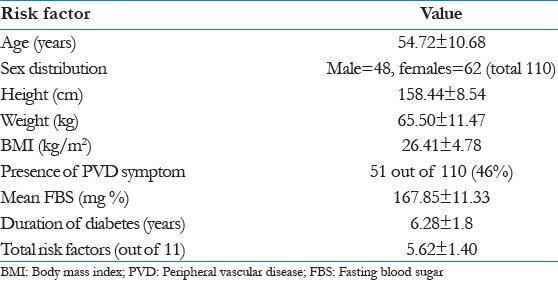
Table 2 shows the prevalence of all risk factors for PAD in diabetes with distribution of subjects with regard to low or normal ABI. RR for individual risk factor is calculated with Z and P value for each. It clearly indicates (a) strong positive and significant correlation with hyperlipidemia, asymptomatism for PAD, age <52 years, BMI >25 kg/m2; (b) weak and insignificant correlation with smoking, CVD, FBS >130 mg/dL; (c) no correlation with male gender and positive family history and (d) negative correlation with hypertension and age >52 years.
Table 2.
Effect of individual risk factor for PAD on ABI results with prevalence and relative risk
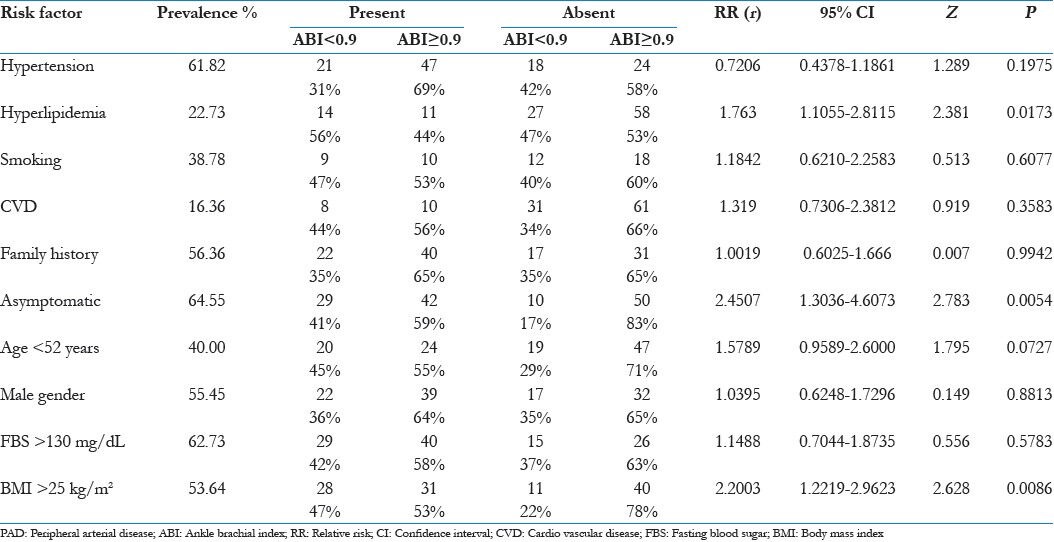
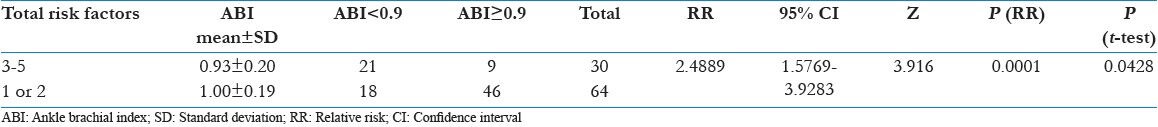
Table 3 shows the cumulative effect of modifiable risk factor five in total in group based on the total number of these risk factors. It shows linear and significant increase in RR for PAD with the increase in their total number in diabetic subject.
Table 3.
Cumulative effects of modifiable risk factors on ABI results
Discussion
By present community based study, we tried to find the prevalence of PAD in diabetics as indicted by low ABI and to associate various risk factors for it in a sample of urban population of this region. Low ABI prevailed in 35% of subjects having type 2 diabetes mellitus reflecting the possible burden of this problem on the health-care system to be faced in the future. This is higher than few other similar studies done in India.[13,14] It can be attributed to high BMI, high mean age, high mean FBS and female preponderance in our study group and first three can be generalized to overall diabetic population of our region. Indians are at higher risk to develop PAD and Diabetes as compared to other ethnic groups and that is proven true even if they are residing outside India.[15,16,17] This fact points toward some genetic susceptibility that remains unaffected by environmental factors.
In our study group, hypertension, high FBS, high BMI, asymptomatism, positive family history proved to be highly prevalent out of which first three are modifiable. CVD and hyperlipidemia though modifiable prevailed very low.
We observed highest RR with asymptomatism, which can be understood by iceberg nature of the disease. Asymptomatic cases receive fewer medications than symptomatic ones as in line with others.[18] Inclusions of asymptomatics with few other risk factors can help in diagnosing presymptomatic atherosclerosis[19] and one can shift secondary prevention to primary prevention that target rigorous treatment. So, one can identify disease early and prevent adverse outcomes like major amputation[20] or mortality due to cardiovascular or cerebrovascular diseases.
Hyperlipidemia and high BMI pose RR of 1.763 and 2.2 respectively in our study. It is supported by a western study with RR being 1.68.[21] It can be explained by their effects in the form of oxidized low-density lipoprotein damage, Insulin resistance, plaque formation and thrombus formation.[22] Dyslipidemia can be treated by statins,[23] but their cost-effectiveness is in question. However, life-style modification such as stoppage of tobacco consumption, exercise[24] and diet therapy[25] are better alternatives perhaps for us, which have been tested successfully, but not implemented successfully. High BMI was given as a cause for high prevalence of PAD in Indians among various South Asian countries.[26] Smoking was single most important risk factor for PAD in India mainly for males whose cessation can stop disease progression.[27] Continuous tobacco increases the risk and reduces the success of vascular intervention.
CVD is established risk factor for PAD as proven by few studies.[28] In ellipse study[29] diabetes did not prove a statistically significant risk factor and they explained it by the fact that the study group like ours was having low CVD risk and fewer comorbid conditions. Low prevalence of hyperlipidemia and CVD in our sample raises further importance of BMI and hypertension that prevailed in nearly 60% of subjects.
In India, about 50% of diabetics have hypertension.[30] Tight glycemic control decreases only the risk of microvascular complications such as retinopathy, nephropathy and neuropathy without affecting macrovascular complications like PAD.[31] Blood pressure regulation gives instantaneous result than glycemic control and is more cost-effective for both patients as well as primary physicians. In the present study, we contrastingly found that hypertensive diabetics have fewer PADs than normotensive diabetics. This can be explained by the fact that more than 90% of hypertensives were taking angiotensin-converting enzyme inhibitors drugs like enalapril or ramipril regularly as a first line of treatment. This drug keeps in check angiotensin II and prevents various vascular complications of diabetes as documented by previous studies.[32,33]
We did not find any significant correlation of PAD with a positive family history or gender in line with others,[34,35] who found them inconsistent and insignificant at times. These factors cannot be modified so their beneficial preventive role does not exist. With ageing there is atherosclerosis leading to stiffness in arteries that tend to raise ABI value, which exactly oppose the decline in ABI value by PAD due to diabetes.[36] So, one cannot ascertain exactly age as a risk factor for this disease and it is definitely impossible to modify it even if it is proven so.
In the present study, glycemic control was measured by FBS and not by hemoglobin A1c as later one is costly and not practically possible in all set ups in a developing country like India. A recent study has shown former as a more reliable test than later to correlate glycemic control with ABI results.[37] We found controlled sugar level in just one-third of the subjects and it is must to have FBS within normal upper limit. Still for PAD ABI is surely a better tool to correlate than mere blood sugar level, which does not require any specific laboratory and reagent and can be practiced even at primary health-care level.
It was seen that more the number of modifiable risk factors in totality more adverse was the ABI profile owing to the cumulative effect of them and this observation is in line with previous such attempt. Out of 110 subjects only 16 were having no modifiable risk factors out of five and one can easily see the benefit of targeting risk modification after proper risk stratification in all diabetics and timely intervention.
As India is heading to be the hub of diabetes by 2025 one can predict necessity of primary preventive measures for PAD that is offered by ABI. However, this test cannot be generalized for the whole diabetic population and it is better to be offered to those who are at risk with certain benefit from screening and intervention. It is always better preventing at primary level rather than treating at secondary and tertiary care level. General Practitioners who are the backbone of health-care systems world-wide more so in the context of India can be sensitized about the burden of this modern epidemic and they can play a significant role in risk stratification for vasculopathy in type 2 diabetes as many of patients with the disease are coming for a regular visit at primary health facilitators. More emphasis should be put on modifying the modifiable risk factors by life-style modifications, drug therapy and other interventions that come well within the domain of family medicine and primary care.
Conclusion
ABI measurement in diabetics revealed a high prevalence of PAD in our population with the presence of more than one risk factor in most. We found a high prevalence and positive correlation of modifiable risk factors such as high BMI, uncontrolled blood sugar level, smoking, hypertension and hyperlipidemia. Asymptomatic nature of disease progression can be nullified by optimum use of ABI as a screening tool preceded by risk factor stratification of diabetic subjects. It can be implemented as a primary preventive measure even at primary health-care level to fight against this modern epidemic of diabetics and its aftermath PAD.
Acknowledgments
I am thankful to Department of Physiology Government Medical College Bhavnagar for providing me an opportunity to conduct this study. I am also thankful to UHTC, Bhavnagar, Medicine Department Government Medical College Bhavnagar, Shree Bajarangdas Bapa Arogyadhaam, Dr. Krunal Chandarana and other private practitioners for allowing me to recruit the subjects for the study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Kiechl S, Willeit J. The natural course of atherosclerosis. Part I: Incidence and progression. Arterioscler Thromb Vasc Biol. 1999;19:1484–90. doi: 10.1161/01.atv.19.6.1484. [DOI] [PubMed] [Google Scholar]
- 3.Welten GM, Schouten O, Hoeks SE, Chonchol M, Vidakovic R, van Domburg RT, et al. Long-term prognosis of patients with peripheral arterial disease: A comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51:1588–96. doi: 10.1016/j.jacc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 4.Khan S, Flather M, Mister R, Delahunty N, Fowkes G, Bradbury A, et al. Characteristics and treatments of patients with peripheral arterial disease referred to UK vascular clinics: Results of a prospective registry. Eur J Vasc Endovasc Surg. 2007;33:442–50. doi: 10.1016/j.ejvs.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Shemanski L, Manolio TA, Cushman M, Mittelmark M, Polak JF, et al. Ankle-arm index as a predictor of cardiovascular disease and mortality in the cardiovascular health study. The cardiovascular health study Group. Arterioscler Thromb Vasc Biol. 1999;19:538–45. doi: 10.1161/01.atv.19.3.538. [DOI] [PubMed] [Google Scholar]
- 6.Hirsch AT, Haskal ZJ, Hertzer NR, Bakal CW, Creager MA, Halperin JL, et al. ACC/AHA 2005 Practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American association for vascular surgery/society for vascular surgery, society for cardiovascular angiography and interventions, society for vascular medicine and biology, society of interventional radiology, and the ACC/AHA task force on practice guidelines (Writing Committee to develop guidelines for the management of patients with peripheral arterial disease): Endorsed by the American association of cardiovascular and pulmonary rehabilitation; national heart, lung, and blood institute; society for vascular nursing; transatlantic inter-society consensus; and vascular disease foundation. Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 7.Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, et al. Inter-Society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(Suppl 1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–41. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 9.Mohler ER, 3rd, Treat-Jacobson D, Reilly MP, Cunningham KE, Miani M, Criqui MH, et al. Utility and barriers to performance of the ankle-brachial index in primary care practice. Vasc Med. 2004;9:253–60. doi: 10.1191/1358863x04vm559oa. [DOI] [PubMed] [Google Scholar]
- 10.Schröder F, Diehm N, Kareem S, Ames M, Pira A, Zwettler U, et al. A modified calculation of ankle-brachial pressure index is far more sensitive in the detection of peripheral arterial disease. J Vasc Surg. 2006;44:531–6. doi: 10.1016/j.jvs.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 11.Mohler ER., 3rd Peripheral arterial disease: Identification and implications. Arch Intern Med. 2003;163:2306–14. doi: 10.1001/archinte.163.19.2306. [DOI] [PubMed] [Google Scholar]
- 12.Murabito JM, Evans JC, Larson MG, Nieto K, Levy D, Wilson PW, et al. The ankle-brachial index in the elderly and risk of stroke, coronary disease, and death: The Framingham study. Arch Intern Med. 2003;163:1939–42. doi: 10.1001/archinte.163.16.1939. [DOI] [PubMed] [Google Scholar]
- 13.Shana PK, Sengupta N, Chowdhury S. High prevalence of neuropathy and peripheral arterial disease in type 2 diabetes in a tertiary care centre in eastern India. Internet J Endocrinol. 2011:6. [Google Scholar]
- 14.Doza B, Kaur M, Chopra S, Kapoor R. Cardiovascular risk factors and distributions of the ankle-brachial index among type 2 diabetes mellitus patients. Int J Hypertens 2012. 2012 doi: 10.1155/2012/485812. 485812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnick HE, Lindsay RS, McDermott MM, Devereux RB, Jones KL, Fabsitz RR, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: The strong heart study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 16.Rabia K, Khoo EM. Prevalence of peripheral arterial disease in patients with diabetes mellitus in a primary care setting. Med J Malaysia. 2007;62:130–3. [PubMed] [Google Scholar]
- 17.Chao A, Koh W. Prevalence of peripheral arterial disease in Asians. Apopulation screening study. ANZ J Surg. 2003;73(Supp1):A120. [Google Scholar]
- 18.Ramos R, Quesada M, Solanas P, Subirana I, Sala J, Vila J, et al. Prevalence of symptomatic and asymptomatic peripheral arterial disease and the value of the ankle-brachial index to stratify cardiovascular risk. Eur J Vasc Endovasc Surg. 2009;38:305–11. doi: 10.1016/j.ejvs.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Meijer WT, Grobbee DE, Hunink MG, Hofman A, Hoes AW. Determinants of peripheral arterial disease in the elderly: The Rotterdam study. Arch Intern Med. 2000;160:2934–8. doi: 10.1001/archinte.160.19.2934. [DOI] [PubMed] [Google Scholar]
- 20.Jude EB, Oyibo SO, Chalmers N, Boulton AJ. Peripheral arterial disease in diabetic and nondiabetic patients: A comparison of severity and outcome. Diabetes Care. 2001;24:1433–7. doi: 10.2337/diacare.24.8.1433. [DOI] [PubMed] [Google Scholar]
- 21.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: Results from the national health and nutrition examination survey, 1999-2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 22.Uemura S, Matsushita H, Li W, Glassford AJ, Asagami T, Lee KH, et al. Diabetes mellitus enhances vascular matrix metalloproteinase activity: Role of oxidative stress. Circ Res. 2001;88:1291–8. doi: 10.1161/hh1201.092042. [DOI] [PubMed] [Google Scholar]
- 23.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): Multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 24.Tsai JC, Chan P, Wang CH, Jeng C, Hsieh MH, Kao PF, et al. The effects of exercise training on walking function and perception of health status in elderly patients with peripheral arterial occlusive disease. J Intern Med. 2002;252:448–55. doi: 10.1046/j.1365-2796.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- 25.Afaghi A, Ziaee A, Afaghi M. Effect of low-glycemic load diet on changes in cardiovascular risk factors in poorly controlled diabetic patients. Indian J Endocrinol Metab. 2012;16:991–5. doi: 10.4103/2230-8210.103010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnett AH, Dixon AN, Bellary S, Hanif MW, O’hare JP, Raymond NT, et al. Type 2 diabetes and cardiovascular risk in the UK South Asian community. Diabetologia. 2006;49:2234–46. doi: 10.1007/s00125-006-0325-1. [DOI] [PubMed] [Google Scholar]
- 27.Basit A, Hydrie MZ, Ahmed K, Hakeem R. Prevalence of diabetes, impaired fasting glucose and associated risk factors in a rural area of Baluchistan province according to new ADA criteria. J Pak Med Assoc. 2002;52:357–60. [PubMed] [Google Scholar]
- 28.Cacoub PP, Abola MT, Baumgartner I, Bhatt DL, Creager MA, Liau CS, et al. Cardiovascular risk factor control and outcomes in peripheral artery disease patients in the reduction of Atherothrombosis for continued health (REACH) registry. Atherosclerosis. 2009;204:e86–92. doi: 10.1016/j.atherosclerosis.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Mourad JJ, Cacoub P, Collet JP, Becker F, Pinel JF, Huet D, et al. Screening of unrecognized peripheral arterial disease (PAD) using ankle-brachial index in high cardiovascular risk patients free from symptomatic PAD. J Vasc Surg. 2009;50:572–80. doi: 10.1016/j.jvs.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 30.Doobay AV, Anand SS. Sensitivity and specificity of the ankle-brachial index to predict future cardiovascular outcomes: A systematic review. Arterioscler Thromb Vasc Biol. 2005;25:1463–9. doi: 10.1161/01.ATV.0000168911.78624.b7. [DOI] [PubMed] [Google Scholar]
- 31.Singh RB, Beegom R, Rastogi V, Rastogi SS, Madhu V. Clinical characteristics and hypertension among known patients of non-insulin dependent diabetes mellitus in North and South Indians. J Diabetes Assoc India. 1996;36:45–50. [Google Scholar]
- 32.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The diabetes control and complications trial/epidemiology of diabetes interventions and complications research group. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arauz-Pacheco C, Parrott MA, Raskin P. American diabetes association. Treatment of hypertension in adults with diabetes. Diabetes Care. 2003;26(Suppl 1):S80–2. doi: 10.2337/diacare.26.2007.s80. [DOI] [PubMed] [Google Scholar]
- 34.Jayawardena R, Ranasinghe P, Byrne NM, Soares MJ, Katulanda P, Hills AP. Prevalence and trends of the diabetes epidemic in South Asia: A systematic review and meta-analysis. BMC Public Health. 2012;12:380. doi: 10.1186/1471-2458-12-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alzamora MT, Forés R, Baena-Díez JM, Pera G, Toran P, Sorribes M, et al. The peripheral arterial disease study (PERART/ARTPER): Prevalence and risk factors in the general population. BMC Public Health. 2010;10:38. [Google Scholar]
- 36.Norman PE, Eikelboom JW, Hankey GJ. Peripheral arterial disease: Prognostic significance and prevention of atherothrombotic complications. Med J Aust. 2004;181:150–4. doi: 10.5694/j.1326-5377.2004.tb06206.x. [DOI] [PubMed] [Google Scholar]
- 37.Dziemidok P, Szczes´niak G, Kostrzewa-Zabl´ocka E, Paprzycki P, Korzon-Burakowska A. Is the advancement of diabetic angiopathy evaluated as ankle-brachial index directly associated with current glycaemic control? Ann Agric Environ Med. 2012;19:563–6. [PubMed] [Google Scholar]


