Abstract
Background:
Preeclampsia and eclampsia (PE) are pregnancy specific syndromes that contribute to maternal and fetal morbidity and mortality. The identification of its predisposing factors in the pre-pregnancy and initial stage of pregnancy will help in reducing the morbidity and mortality.
Aim:
The aim of this study is to determine the risk factors for PE among pregnant women in a tertiary level hospital.
Materials and Methods:
In this study, 122 women who delivered beyond 22 weeks of gestation and diagnosed as preeclampsia or eclampsia were selected. Simultaneously, 122 controls with no diagnosis of preeclampsia or eclampsia were selected from the post natal ward. Cases and controls were administered the same pre-tested questionnaire containing different risk factors.
Results and Conclusion:
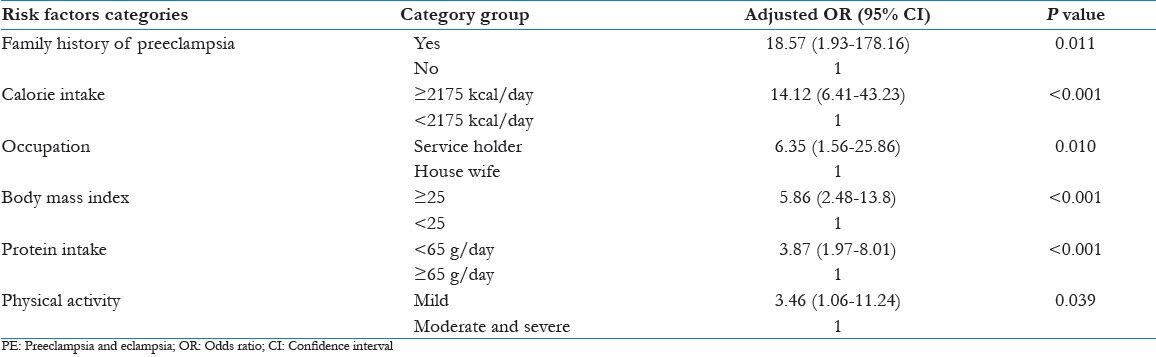
Logistic regression was applied in the statistical analysis. The factors that were found to be significant predictors of risk for development of PE were family history of preeclampsia (adjusted odds ratio [OR] 18.57 [1.93-178.16], P = 0.011), higher calorie intake (adjusted OR 14.12 [6.41-43.23] body mass index (adjusted P < 0.001), employment (adjusted OR 6.35 [1.56-25.82] P = 0.010], less protein intake (adjusted OR 3.87 [1.97-8.01] P < 0.001), increased OR 5.86 [02.48-13.8] P < 0.001), mild physical activities (adjusted OR 3.46 [1.06-11.24] P = 0.039). Past history of hypertension and diabetes mellitus were also associated with development of PE.
Keywords: Case control study, eclampsia, multiple logistic regression, preeclampsia, risk factors
Introduction
Preeclampsia and eclampsia (PE) are major pregnancy specific syndromes that contribute to maternal and fetal morbidity and mortality in India.[1,2] The incidence of PE ranges from 2% to 10%, depending on the population studied and criteria used for diagnosis.[3] PE are gestational hypertensive disorders develop after 22 weeks of pregnancy, in which there is an increase in blood pressure and proteinuria. Preeclampsia causes abortion, prematurity, intra-uterine growth retardation and still birth. It is believed to be of multifactorial origin.
Proper antenatal care remains the important part of prevention. Estimating each woman's individualized risk allow antenatal surveillance to be directed at those women, who are most likely to develop preeclampsia. Such care leads to early diagnosis and intervention, both in terms of maternal or fetal monitoring and timing of delivery. Hence there is a need to develop an integrated model for the estimation of patient specific risk factors for the development of preeclampsia on the basis of maternal demographic, socio-economic, obstetrics, nutritional and anthropometric parameters.[4]
Hence, we conducted this study to determine the risk factors of PE by directly collecting the data using questionnaire schedule from post natal women in the hospital.
Materials and Methods
The study was conducted in Obstetrics and Gynecology ward of a tertiary care hospital in Delhi, India from November 2006 to March 2008. It was designed as a case control study.
Sample size
The sample size was calculated with the following assumptions, Type 1 error 5%, power of study 80%, prevalence of nulliparity (a risk factor) among control 20%, ratio of the case and control 1:1 and estimated odds ratio (OR) for nulliparity was 2.35.[5] Estimated sample size was 122 cases for uncorrected Chi-square test and equal numbers of controls.[6]
Cases (exposure)
A case was defined with the following definitions.[7]
Preeclampsia was diagnosed with minimum criteria of blood pressure ≥140/90 mm Hg and Proteinuria ≥0.3 g/24 h (≥1 + dipstick) after 22 weeks of gestation.[7]
Eclampsia was diagnosed as the presence of convulsions that cannot be attributed to other causes in a woman with preeclampsia.
The study was approved by Institutional Ethics Committee and informed consent was received from all the participants. From the postnatal ward case sheets of PE were collected. The women were interviewed using a pre-tested questionnaire. In case, an eclamptic patient was comatose after delivery, the history was taken when she regained consciousness. If consciousness could not be gained by her, the history was taken from relatives who accompanied her throughout the pregnancy period.
Controls
Women who delivered after 22 weeks of gestation and admitted to the post natal wards with no history of preeclampsia or eclampsia were recruited as controls. Controls were selected randomly in the same time of cases selection. The controls were administered the same questionnaire. Matching among cases and controls was not performed because most of the socio-demographic parameters are established risk factors for PE.
Data collection
Data was gathered using a pre-tested questionnaire. The questionnaire was administered to both cases and controls by the first author. The questionnaire included demographic and socio-economic information. The history of gravidity, parity, abortion, bad obstetrics history, past history of eclampsia, diabetes mellitus and hypertension were elicited. Both cases and controls were asked about their food intake patterns during pregnancy (cereals, pulses, egg, meat, fish, sugar, milk and dairy products and fruits) by using food frequency questionnaire. Physical activity level during pregnancy was asked and categorized as mild, moderate and severe.[8] Height was measured for both cases and controls and pre-pregnancy weight was noted.
The data was entered in SPSS for window version 12. SPSS Inc., Chicago, IL 60606-6412, USA Crude OR was calculated for all possible risk factors for PE by bivariate analysis using Chi-square test. Adjusted OR was also computed using multiple logistic regression analysis to control for influence of all the risk factors.
Results
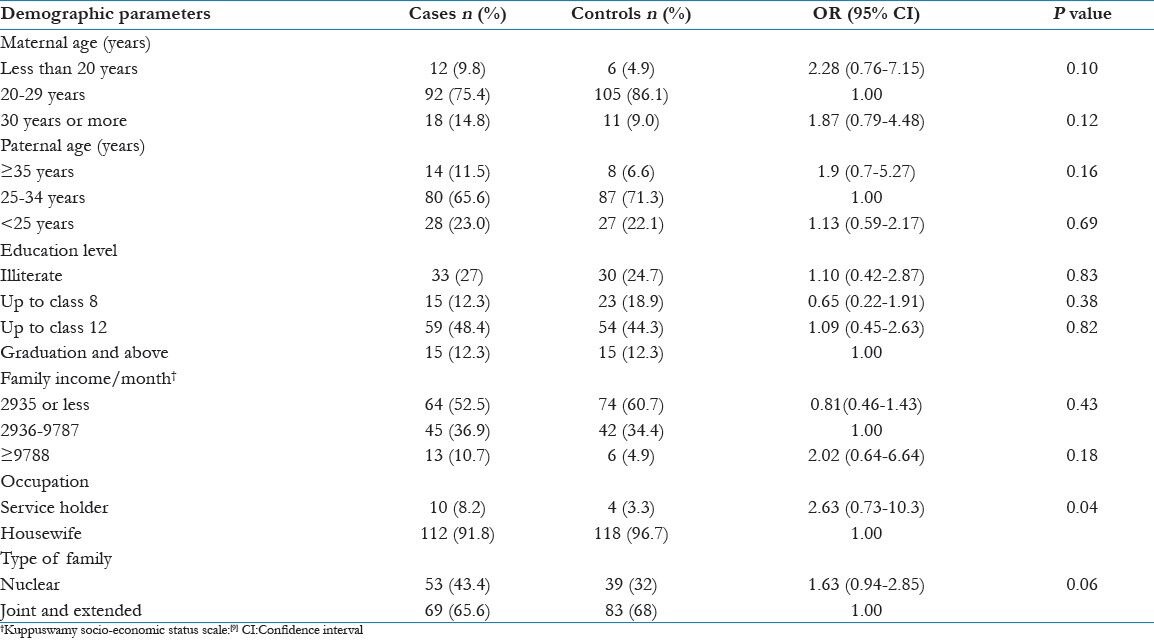
In this study, the mean age of cases and controls were 24.4 ± 4.2 years and 23.9 ± 3.6 years respectively. Socio-economic risk factors such as maternal age, paternal age, education level, family income, occupation, type of family were not significantly associated with development of PE [Table 1].
Table 1.
Distribution of socio-economic risk factors among cases and controls
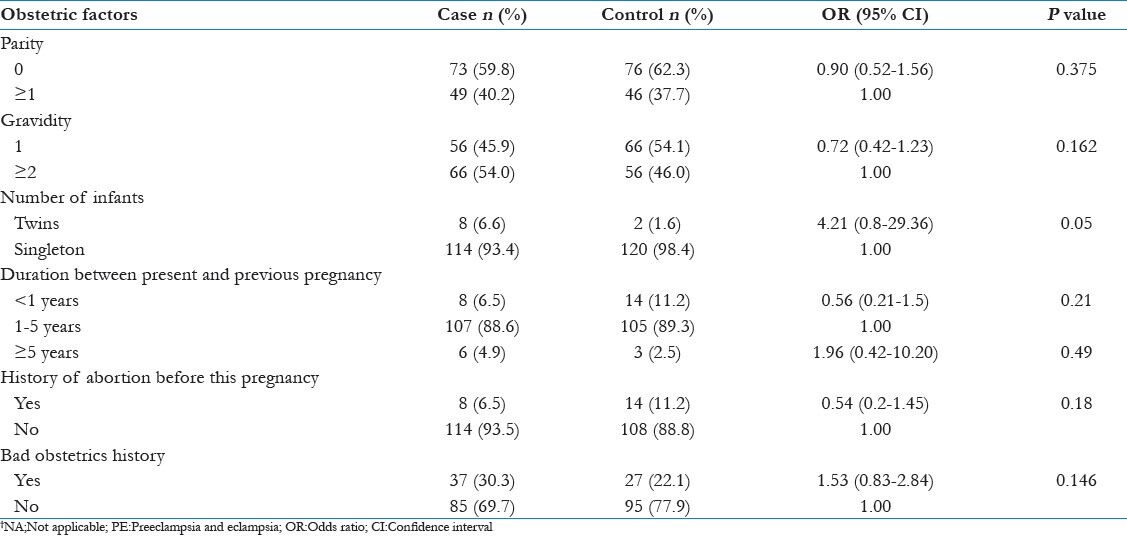
In bivariate analysis nulliparity, primigravida, twin pregnancy, bad obstetrics history and history of abortion were not significantly associated with development of PE [Table 2].
Table 2.
Bivariate analysis showing obstetric risk factors for PE
Past history of PE, family history of PE and past history of hypertension and diabetes mellitus were associated with development of PE [Table 3].
Table 3.
Bivariate analysis showing past and family history risk factors for PE

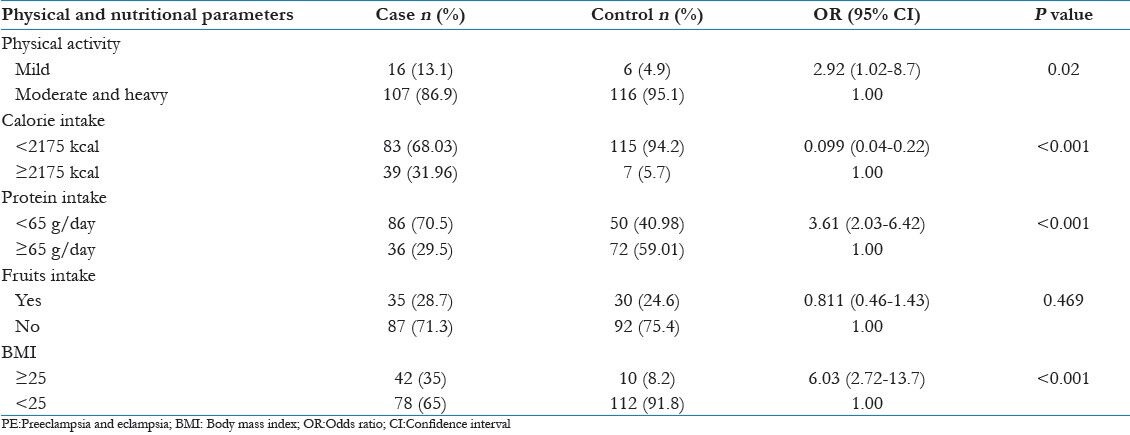
Proportion of cases (31.96%) taking higher calorie (≥2175 kcal/day) during pregnancy were 14.12 times more at risk for development of PE in comparison with controls (5.7%) [adjusted OR = 14.12 [6.41-43.23] P < 0.001]. Low protein intake during pregnancy (<65 g/day) was more in cases (70.5%) compared with controls (40.98%) and cases were 3.61 times at risk for development of PE, [OR = 3.61 [2.05-6.45] P < 0.001]. Fruits intake during pregnancy was found to be similar in both groups.
In our study, mild physical activity during pregnancy was a significant association (2.92 times) with development of PE (P = 0.02). Women with higher body mass index (BMI) (≥25) were 6 times at risk for development of PE than women with BMI <25 (P < 0.001) [Table 4].
Table 4.
Bivariate analysis showing physical activity level and nutritional intake risk factors for PE
Forward step wise logistic regression was applied to the data. Cut-off point was taken at P = 0.05. The variables, which were found to be a significant contributor in development of PE by bivariate analysis entered into logistic regression equation starting from highly significant to least significant variable. The variables that were found to be significant predictors of PE were: family history of preeclampsia, higher calorie intake, employment, low protein intake, less physical activity during pregnancy and increased BMI [Table 5].
Table 5.
Logistic regression analysis for risk factors of PE
Discussion
Preeclampsia, a syndrome unique to human pregnancy, is associated with maternal and fetal complications. The risk factors identified that influence the development of preeclampsia included extremes of maternal age, race, socio-economic factors, change of paternity, twin pregnancy, nulliparity, increased birth interval, increased BMI, increased systolic and diastolic blood pressure early in pregnancy, increased rate of weight gain during pregnancy and the presence of gestational diabetes.[3,10,11,12,13,14]
In this study, 11.5% and 3.3% cases had a past history of hypertension and diabetes mellitus respectively, which is greater than controls (1.6% and 0% respectively). Ros et al. quoted that in diabetic women, high levels of plasma triglycerides cause endothelial cells to accumulate triglycerides leading to endothelial cell dysfunction that predisposes to develop high blood pressure.[10]
Past history of preeclampsia was associated with development of preeclampsia.[3,15] Similarly our study observed 4.1% of cases experienced same sign and symptom in present pregnancy as in a previous pregnancy. However, none of the controls reported past history of preeclampsia. There may be chances of recall bias because the cases had a good recall that they experience the same sign and symptoms than controls.
In the present study, 9% of PE cases had a family history of PE as compared with 0.8% of controls. Family history of preeclampsia reported in four mothers, four sisters, one grandmother and two mothers-in-law of cases, while only in one mother-in-law of controls. The family history of convulsions, i.e., eclampsia could be elicited easily while history of raised blood pressure was difficult to recall by patients. Severe preeclampsia is of familial origin, as has been shown by many investigators.[16] There is primary evidence of the genetic component in the determination of disease and implies that it would be good practice to enquire routinely the family history of severe preeclampsia to obtain early warning of possible “high risk” cases.
Similar to other studies in our study the women doing mild physical activities were nearly 3 times risk for development of PE than women doing moderate and heavy activities. Saftlas et al. described in their study that physical activity has a protective effect against development of preeclampsia.[17]
The proportion of women with calorie intake ≥2175 kcal/day was a risk for development of PE. This may be explained that women with higher calorie intake may have higher pre-pregnancy maternal weight that predisposes to increase the incidence of PE.
The increased BMI in the pre-pregnancy period is found to be a risk factor for PE in many studies. Our study also correlated the same.
Being employed outside the home is a risk factor for development of PE as compared with a housewife. As depicted in the previous studies working status of women increases the risk.[10] Working women are more prone to develop PE as they are more stressed (in terms of travelling during pregnancy and job responsibility) than housewives.
Nulliparity was not significantly associated with development of PE. This could be due to reason that nulliparous women are more likely to have institutional delivery and increased numbers of antenatal checkup as also shown in the recent National Family Health Survey-3.[18] That may be the reason for early detection and treatment of preeclampsia.
The limitations of this study were first it was a hospital based study hence can’t be generalized to the whole population. Second chances of recall bias were more among both controls and cases.
Conclusion
To conclude higher calorie intake, less protein intake, mild physical activities and employment during pregnancy; pre-pregnancy high BMI, family history of preeclampsia, past history of diabetes and hypertension are the major risk factors for PE. A format containing all the risk factors is to be asked routinely in antenatal checkup to pregnant women to prevent the development of PE.
Acknowledgment
We acknowledge all pregnant women, nursing staffs and paramedical staffs for their cooperation and help during the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Sawhney H, Aggarwal N, Biswas R, Vasishta K, Gopalan S. Maternal mortality associated with eclampsia and severe preeclampsia of pregnancy. J Obstet Gynaecol Res. 2000;26:351–6. doi: 10.1111/j.1447-0756.2000.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 2.Singhal SR, Deepika, Anshu, Nanda S. Maternal and perinatal outcome in severe pre eclampsia and eclampsia. J South Asian Fed Obstet Gynecol. 2009;1:25–8. [Google Scholar]
- 3.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The calcium for preeclampsia prevention (CPEP) study group. Am J Obstet Gynecol. 1997;177:1003–10. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 4.Yu CK, Smith GC, Papageorghiou AT, Cacho AM, Nicolaides KH Fetal Medicine Foundation Second Trimester Screening Group. An integrated model for the prediction of preeclampsia using maternal factors and uterine artery Doppler velocimetry in unselected low-risk women. Am J Obstet Gynecol. 2005;193:429–36. doi: 10.1016/j.ajog.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 5.Duckitt K, Harrington D. Risk factors for pre-eclampsia at antenatal booking: Systematic review of controlled studies. BMJ. 2005;330:565. doi: 10.1136/bmj.38380.674340.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dupout WD, Plummer WD. PS power and sample size program available for free on the internet. Control Clin Trials. 1997;18:274. [Google Scholar]
- 7.Cunningham FG, Lenovo KJ, Bloom SL, Hauth JC, Gilstrap LC, Wenstrrom KD. Williams Obstetrics. 22nd ed. New York: McGraw Hill; 2005. Hypertensive disorders in pregnancy; pp. 761–808. [Google Scholar]
- 8.Gopalan C, Sastri RB, Balasubramanian SC, Rao BS, Deosthale YG, Pant KC. Hyderabad: National Institute of Nutrition, ICMR; 2011. Nutritive Value of Indian Foods. [Google Scholar]
- 9.Kumar N, Shekhar C, Kumar P, Kundu AS. Kuppuswamy's socioeconomic status scale-updating for 2007. Indian J Pediatr. 2007;74:1131–2. [PubMed] [Google Scholar]
- 10.Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol. 1998;147:1062–70. doi: 10.1093/oxfordjournals.aje.a009400. [DOI] [PubMed] [Google Scholar]
- 11.Eskenazi B, Fenster L, Sidney S. A multivariate analysis of risk factors for preeclampsia. JAMA. 1991;266:237–41. [PubMed] [Google Scholar]
- 12.Coonrod DV, Hickok DE, Zhu K, Easterling TR, Daling JR. Risk factors for preeclampsia in twin pregnancies: A population-based cohort study. Am J Obstet Gynecol. 1995;85:645–50. doi: 10.1016/0029-7844(95)00049-w. [DOI] [PubMed] [Google Scholar]
- 13.Dekker GA, Sibai BM. Etiology and pathogenesis of preeclampsia: Current concepts. Am J Obstet Gynecol. 1998;179:1359–75. doi: 10.1016/s0002-9378(98)70160-7. [DOI] [PubMed] [Google Scholar]
- 14.Skjaerven R, Wilcox AJ, Lie RT. The interval between pregnancies and the risk of preeclampsia. N Engl J Med. 2002;346:33–8. doi: 10.1056/NEJMoa011379. [DOI] [PubMed] [Google Scholar]
- 15.Sibai BM, Lindheimer M, Hauth J, Caritis S, VanDorsten P, Klebanoff M, et al. Risk factors for preeclampsia, abruptio placentae, and adverse neonatal outcomes among women with chronic hypertension. National institute of child health and human development network of maternal-fetal medicine units. N Engl J Med. 1998;339:667–71. doi: 10.1056/NEJM199809033391004. [DOI] [PubMed] [Google Scholar]
- 16.Cincotta RB, Brennecke SP. Family history of pre-eclampsia as a predictor for pre-eclampsia in primigravidas. Int J Gynaecol Obstet. 1998;60:23–7. doi: 10.1016/s0020-7292(97)00241-5. [DOI] [PubMed] [Google Scholar]
- 17.Saftlas AF, Logsden-Sackett N, Wang W, Woolson R, Bracken MB. Work, leisure-time physical activity, and risk of preeclampsia and gestational hypertension. Am J Epidemiol. 2004;160:758–65. doi: 10.1093/aje/kwh277. [DOI] [PubMed] [Google Scholar]
- 18.Chapter 8, 2005-2006. Vol. 1. Mumbai, India: International Institute for Population Sciences (IIPS); Maternal Health, National Family Health Survey (NFHS-3) Report; pp. 191–222. [Google Scholar]


