Abstract
Human babesiosis is a zoonotic disease caused by protozoan parasites of the Babesia genus, primarily in the Northeastern and Midwest United States due to B. microti, and Western Europe due to B. divergens. Parasites are transmitted by the bite of the ixodid tick when the vector takes a blood meal from the vertebrate host, and the economic importance of bovine babesiosis is well understood. The pathology of human disease is a direct result of the parasite’s ability to invade host’s red blood cells. The current understanding of human babesiosis epidemiology is that many infections remain asymptomatic, especially in younger or immune competent individuals, and the burden of severe pathology resides within older or immunocompromised individuals. However, transfusion-transmitted babesiosis is an emerging threat to public health as asymptomatic carriers donate blood and there are as yet no licensed or regulated tests to screen blood products for this pathogen. Reports of tick-borne cases within new geographical regions such as the Pacific Northwest of the US, through Eastern Europe, and into China are also on the rise. Further, new Babesia spp. have been identified globally as agents of severe human babesiosis, suggesting that the epidemiology of this disease is rapidly changing, and it is clear that human babesiosis is a serious public health concern that requires close monitoring and effective intervention measure.
Keywords: Babesia, malaria, B. microti, B. divergens, zoonosis, Transfusion-transmitted babesiosis
INTRODUCTION
Human babesiosis is a zoonotic infection caused by Babesia parasites transmitted by the bite of ixodid ticks that have distinct geographical distributions based on the presence of their competent natural animal hosts, which include rodents, cattle and deer. The genus Babesia comprises many species of parasites [1], which are transmitted when the ticks takes a blood meal from the vertebrate host [1, 2]. While parasite infection of these natural hosts, such as in cattle, which results in bovine babesiosis causing significant economic cost has long been established [3, 4], the severity of human infection is rapidly becoming apparent, whether the disease has been primarily transmitted from a tick bite or secondarily transmitted via a blood transfusion with infected blood [5-7], or even congenitally during pregnancy [8-11]. The four identified babesia species definitively confirmed that infect humans so far are B. microti [12], B. divergens [13] B. duncani [14, 15], and B. venatorum [16-19]. As sampling has become expansive and techniques have become more sensitive, there is evidence that more B. microti-like and B. divergens-like spp. are able to cause human infection [as detailed in 20]. However, the general life cycle within humans remains the same; Babesia parasites are intracellular obligates that target red blood cells, and the parasite's ability to first recognize and then invade host RBCs is central to the disease pathology.
Vector lifecycle and transmission
The only confirmed vectors of Babesia parasites are members of the Ixodidae family. Not all life-cycles for known parasite-vector-host groups are fully understood yet [20]. The two-year lifecycle of I. scapularis, which is endemic across most of the eastern states of America and Canada [21-23], and its role in B. microti transmission has been well documented [1, 24, 25]. In brief, the larva and nymph stages both need to take a blood meal from their rodent hosts to mature to the next stages, and adults feed primarily on deer as a permanent food source [1, 22, 25]. Reports show that up to 60% of these rodents may be infected with B. microti [26]. Newly hatched larval stages take a blood meal from their vertebrate host at the end of summer, usually August and September, which is when they first acquire parasites if the host is infected. During the winter when they are dormant and molt into nymph stages, the parasites cross the tick gut epithelium, and travel to the salivary glands. It has been shown that these the parasites require some activation from exposure to warm blooded hosts to generate active sporozoites once the ticks feed again [27]. The following summer, the nymph stages are required to feed again in preparation for development into adults later the same year, they now are able to transmit parasites into the vertebrate host. It is at this stage of tick development that zoonotic infection into the human host occurs [1]. The adult stages of I. scapularis feed primarily on the white-tailed deer (Odocoileus virginianus), which are not reservoirs for B. microti but may be a direct contributor to the expansion of ixodes ticks and babesiosis in general. B. divergens is transmitted by the I. ricinus tick, whose life-cycle is 3 years, as the larva, nymph and adults each mature in a consecutive year. Most tick-borne infections are reported between April and October, which coincides not only with the warmer weather when ticks are more active, but also when individuals spend more time within tick-infested areas. Although vaccines are not available, prophylactic antibiotic therapy is not recommended. However, preventative measures, such as suitable clothing, insect repellants containing DEET, and prompt removal of attached ticks when noticed are the best ways of limiting exposure to bites [28].
Pathogens of human babesiosis
In the Americas
B. microti was first identified in the United States in 1966 [29] and in 2011, babesiosis became a nationally notifiable disease in 18 states, as its emergence and the potential for transfusion-associated cases were recognized [30], but the parasite itself is currently endemic within the Midwest states of Minnesota and Wisconsin and the Northeastern corridor of New Jersey, New York, Connecticut, Massachusetts, and Rhode Island [25], where its main host, the white-footed mouse, (Peromyscus leucopus) is prevalent [20]. The increase in human babesiosis in the northeast corridor is highlighted in the incidence of cases from New Jersey, where babesiosis case reporting began in 1985. During the 1993–2001, only eight of 21 counties reported babesiosis cases [31], but during the following six years from 2006 to 2011, the incidence of reported cases increased 260% with a total of 568 cases reported, and all counties reporting at least 1 case within that time period [32]. Further, a recent incident of B. microti infection in Canada [33] and cases reported further east into Pennsylvania [34] shows that the boundaries of transmission are clearly expanding. On the North-western coast of the United States, there have been limited reported of babesiosis caused by B. duncani or B. duncani–type organisms in healthy individuals [14, 15, 35, 36]. Unlike I. scapularis in the east, I. pacificus is the primary candidate, but this has not been confirmed [37], and the natural host remains unclear [20]. Isolated and severe cases of B. divergens–like infections have been reported in asplenic individuals from Missouri [38], Kentucky [39], and Washington State [40]. In South America, symptomatic human babesiosis infections have been acquired in Brazil [41], Colombia [42], as well as asymptomatic cases of B. bovis in Mexico [36].
In Europe
B. divergens, a natural pathogen of cattle, is the main pathogen of human babesiosis in Europe [41, 43, 44], with the majority of cases being reported in the British Isles and France [45], along with some cases attributed B. microti and B. venatorum, a pathogen of roe deer (Capreolus capreolus), and all are transmitted by the I. ricinus tick [20]. However, a case of B. divergens in Norway [46], a case of B. microti in Germany [47], coupled with the detection of B. microti in two asymptomatic individuals in Poland [48], and B. venatorum infections reported in Germany [18], Austria and Italy [19] shows again that these pathogens are not absolutely segregated geographically, and are becoming increasingly important as pathogens of human disease.
In Africa and Asia
B. divergens–like infections have been reported in on the Canary Islands [22], and other, as-yet uncharacterized babesia species, have been reported in Egypt, Mozambique, South Africa [22, 40, 49]. B. microti–like organisms have been reported in Taiwan [50, 51], Japan [52], South Korea, [53], and a definitive case of B. microti was identified in Australia [54-57]. However it is clear that in recent years, there has be a steady and significant increase of new tick borne infections with the People’s Republic of China, and the incidence rate of these infection is rising with certain regions [58]. I. persulcatus is considered the main vector throughout PR China, and the historical cases of babesiosis have been attributed to either B. microti or B. divergens but have been sporadic [17, 59-61]. Yet two recent reports demonstrate the seriousness of this emerging zoonosis in this region. Firstly, the China–Myanmar border is highly endemic area for malaria. Reports of human babesiosis due to B. microti from this region [58, 61, 62], show that in areas where co-infections with other tick-borne infections and malaria occur, differential diagnoses are essential to determine whether the causative agents of disease are Babesia or Plasmodium spp. This is especially important as the therapeutic regimes for these parasites are different, and Babesia parasites are not known to respond to anti-malarials. If babesiosis infection is misdiagnosed as malaria and treated with standard anti-malarials, it would appear the parasites were drug-resistant. Secondly, a systematic review of 2912 participants from patients attending a hospital in Mudanjiang City, Heilongjiang province, in northeastern China, identified 32 confirmed cases of B. venatorum, presenting with fever, anemia, thrombocytopenia, chills, sweats, headache, myalgia, or arthralgia, and 16 probable, or asymptomatic cases, suggesting an asymptomatic infection rate of ~30% [16]. B. venatorum is phylogenetically related to B. divergens. The disease profile among these 48 individuals significantly differs from those of the European cases in that all European cases were asplenic individuals presenting with severe disease, yet the cohort in the PR China presented a broad range of disease severity, where none of the 48 cases had a splenectomy. This suggests that pathogen virulence may differ greatly between endemic regions and intense, broad range investigations into these emerging pathogens in differing endemic regions are necessary to understand the full scope of disease and implement appropriate treatment regimens at the local level.
Pathogen lifecycle in humans
When Babesia spp. sporozoites are first injected into the human host, they target the host RBCs immediately, unlike Plasmodium spp. which are required to undergo an exo-erythrocytic phase in hepatic cells. Further, infected RBCs remain circulating in peripheral blood stream, including regularly passing through the hosts’ spleen, and do not sequester to the fine capillaries of the bone marrow or organs. It is the parasite's ability to first recognize and then invade host RBCs that is central to human babesiosis and the parasites invade RBCs using multiple complex interactions between parasite proteins and the host cell surface, which are not fully elucidated yet [63-70]. Once inside the RBC, the parasite begins a cycle of maturation and growth. The early stages of the cycle are morphologically indistinguishable from Plasmodium spp., with both appearing as ring-like parasites. Replication occurs by budding, where one ring forms divides into two, often referred to as “figure eight” form. Budding may occur again, giving ride to the tetrad form know as a “Maltese Cross” [65]. Both these morphological forms are unique to Babesia spp. and are the basis of definitive diagnosis by microscopy, especially if Plasmodium spp. are also suspected. Once the parasites have concluded division, the resulting merozoites egress from the RBCs, destroying it in the process, and seek new, uninfected RBCs to invade, perpetuating the intracellular cycle of infection.
Clinical disease
Infections vary greatly in their presentation, and are dependent on a multiple of factors, such as parasite species, the age and immune competency of the host. Although individuals of any age can harbor parasites, the burden of disease pathology is associated with age, with severe symptoms presenting in neonates, usually due to congenitally-transmitted infection [8-11], or to older adults, possibly due to depressed cellular immunity inhibiting the ability to prevent mild infections developing into severe disease [45, 71, 72]. Further, individuals of any age that are immunocompromised, particularly those which are asplenic, are greater risk from presenting with severe, acute disease, compared to healthy immune competent individuals.
B. microti infections in healthy individuals are usually silent and asymptomatic, with very low or undetectable levels of parasites [45, 73], especially in younger age groups, as shown by sero-prevalence data from endemic regions, ranging from about 6% [74] to 16 % [75], and the numbers of actual reported cases, and as such the disease prevalence is probably underestimated. However, in 2011, babesiosis became a reportable disease in 18 of the United States and in that year alone, 1124 cases were reported [30]. Although the long-term effects of circulating parasites are not well understood, the greatest risk of asymptomatic infection is the ability to donate blood and contribute to transfusion-transmitted babesiosis. Mild disease caused by B. microti usually presents with intermittent fever, general malaise and weakness, often accompanied with chills, sweats, headache, anorexia, myalgia, and upon examination, patients often have splenomegaly and hepatomegaly. In patients that were hospitalized with severe B. microti infection, death occurred in about 10% of cases [76, 77]. But if patients are splenectomized or are immune-compromised in other ways, such as with HIV infection, or are receiving immunosuppressive therapy, or are elderly, then the severe symptoms, usually high fever (40-41°C), chills, night sweats, myalgia, hemolytic anemia, and hemoglobinuria are more likely to develop.
Although comparative animal studies have indicated B. duncani may be more virulent than B. microti [78], it remains unclear from the clinical data what the true virulence of B. duncani truly is as the few reported cases range in severity from asymptomatic to fatal, the same disease spectrum as B. microti [15, 44]. The few B. venatorum cases in Europe [19, 32] would indicate this parasite causes a moderate to severe infection, however the larger studies of this species performed in China show that approximately a third of the confirmed B. venatorum cases were asymptomatic, nearly half of which were children, and the median age of individuals infected was 45 [40], a prevalence profile very similar to B. microti infection.
B. divergens infections are much rarer than B. microti, with less than 50 cases having been reported so far throughout Europe [4, 20, 25, 43, 45], but cases present with much greater severity pathology, usually with hemoglobinuria as the presenting symptom, but jaundice due to hemolysis, vomiting and diarrhea are often present, and the toxins and anoxia, resulting from the hemolysis and the host immunological response, may cause respiratory, cardiac, renal, or hepatic failure [4, 41]. As such, they require immediate treatment and are treated as medical emergencies.
Transfusion Transmitted Babesiosis
Almost 5 million individuals receive a blood transfusion each year in the United States [7]. The current blood-banking safeguards to prevent transmission of babesia through the blood supply relies on a blood donor questionnaire to self-identify any previous history of babesiosis [5]. Although individuals that answer affirmatively to such queries are barred immediately and indefinitely from donating, the effectiveness of self-identified screening measures are limiting due the fact that individuals may be asymptomatic for disease, and thus remain parasitemic and infectious carriers. Further, as asymptomatic individuals can harbor parasites for extended periods of time, they are able to contribute to the donor pool at any time, and not during the seasons associated with tick-borne infections, TTB cases occur year round. And as recipients of blood products are immunocompromised to some degree in the very nature of requiring a donated unit, they are at greater risk of developing severe disease. In areas of highest prevalence, studies suggest there is a transmission risk of 1 per 601 blood units [79]. Since 1980, there have been approximately 162 reported cases of babesiosis, which included 12 fatalities from 2005-2008, making it the most frequent transfusion-transmitted infection [5, 7, 80], and the Food and Drug Administration (FDA) reporting that 3.6% of all transfusion related fatalities from 2005 to 2010 were due to TTB [81]. Unlike many other blood-borne pathogens such as HIV and hepatitis, there are no licensed screening technologies available in order to detect Babesia spp. in the blood supply, and studies have shown that B. divergens parasites can survive the routine cold-storage all donated blood is subjected to for up to 31 days and still yield high end-point parasitemia [82]. Although there are no current pathogen reduction and/or inactivation technologies that are commercially available to use against Babesia, promise has been made in this area. The greatest hurdle to overcome is inactivating the parasite inside the red cell whilst ensuring the absolute competency of the cells themselves. These technologies, such as the Mirasol system utilizing riboflavin and ultraviolet light to inactivate B. microti [83, 84], and the S-303 inactivation system [85] have become significantly more reliable in maintaining red cell integrity whilst making headway in preventing blood-borne pathogens from being transmitted through donated blood. The significant advantage of these technologies is they are being designed as ‘in bag’ treatments that can be ubiquitously applied to all units, without significantly increasing the processing steps between donor and recipient, thus keeping processing costs to a minimum whilst maximizing the safety of the donor pool.
DIAGNOSIS AND TREATMENT OF BABESIOSIS
Direct detection
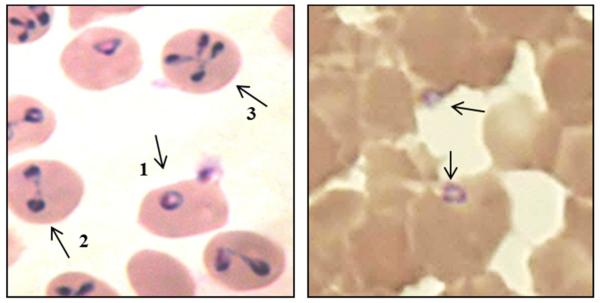
The observation of parasites within Giemsa- or Wright-stained blood films remains the classic method of diagnosis, but it can be difficult to directly observe parasites less than about 0.1% to 0.5 % parasitemia, thus lacks the sensitivity required by some asymptomatic and chronic cases [20]. However, parasitemias greater than 5% are rarely observed in mild cases so serial smears are recommended in mild cases, though have been known to reach as high as 85% in asplenic individuals [86]. The intracellular trophozoites, and the extracellular merozoites which can sometimes be observed, especially in vitro conditions, are usually ring or oval shaped, with red or pink chromatin and blue/purple cytoplasm (see Figure 1). Despite the range in size of various Babesia spp., from ~1 µm to ~5 µm, it is generally impossible to distinguish Babesia spp. morphologically from each other [87], resulting in the need to develop more sensitive detection assays based on PCR. The early ring stages of development can be indistinguishable from some blood-stages of Plasmodium spp., and the differential diagnosis is the observation of the tretrad or ‘Maltese Cross” forms (left panel, Figure 1). As the symptoms of human babesiosis and malaria are often similar, this differential diagnosis is essential, especially in regions where these pathogens are known to coexist, especially as the trophozoites, schizonts, and particularly gametocyte stages of Plasmodium spp. can be more easily recognized if present in peripheral blood smear, meaning coinfections can be easily overlooked.
Figure 1.
Left panel: Standard asynchronous in vitro culture of B. diergens in human RBCs with classic morphological ring (1), "Figure eight" (2) and Maltese Cross (3) forms. Right panel: B. microti parsites from a symptomatic individiual.
The polymerase chain reaction (PCR), particularly real-time or quantitative (qPCR) based on the 18s rRNA gene is much more sensitive than microscopy and are becoming common for the detection of B. microti, where the limits of pathogen detection are about~100 gene copies, equivalent to about 5-10 parasites/µL [88, 89], and PCR technology lends itself to high throughput screening more readily than microscopy. One drawback of the qPCR method is the need for a standard curve for each assay, and as yet, no multiplex exists to detect multiple species per sample. Adaptations to the qPCR method include the droplet digital PCR (ddPCR), where the amplification reaction containing the DNA sample, fluorescently-labeled probe, is divided into many microscopic reaction droplets, each containing one or less copies of the target DNA. This technology has recently been applied to the detection of B. microti and B. duncani, and were shown to discriminate B. microti from B. duncani at a limit of limits of detection of ~10 gene copies [90].
Indirect detection
As chronic infections do not always present with observable levels of parasites, serological testing, usually in the form of immuno-fluoresence assay, can be used to support clinical diagnostics of human babesiosis. Nearly all asymptomatic chronic B. microti infections seroconvert in immunocompetent individuals, but the presence of anti-B. microti antibodies merely suggest an infection at some point in time, as B. microti-specific IgG titers of 1:64 have been detected up 12 months after the parasite infection has cleared. However, IgG titers exceeding 1:1024 have been shown to correlate well with acute B. microti infection, and is an accurate indicator of recent infection, as well as the presence of Babesia-specific IgM [91, 92]. In severe cases, such as B. divergens infected or immunocompromised individuals, serology is rarely performed as the sudden onset of disease pathology and need for treatment prevents the host from generating anti-Babesia antibodies, and direct detection methods are usually suitable for diagnosis. Although sera from individuals infected with B. microti and B. duncani do not cross react with antigens of other Babesia spp., it has been shown that sera from B. venatorum and B. divergens-like infections does cross react with B. divergens antigens, meaning, a sero-positive tests for B. divergens would also need further investigation to determine which of these three species was the causative agent [25, 45].
Treatment
Treatment for mild babesiosis is clindamycin for 7–10 days in B. divergens and B. venatorum [22, 45], with the addition of quinidine or quinine for B. duncani. As the drug-related toxicity for quinine can be significant [93], intravenously administered quinidine is a recommended alternative [44, 45]. In mild B. microti infections, a 7-10 day course atovoquone plus azithromycin is the combination of choice after it was shown this is was just as effective but with fewer side effects compared with the clindamycin/quinine combination [94]. Indeed atovoquone plus azithromycin was successfully used applied used to treat a B. venatorum infection in Germany [18], suggesting that investigation into the most effective and most tolerable treatments are necessary. It is also suggested that chronic B. microti parasite-positive but asymptomatic infection lasting more than 3 months are also treated with this same combination [95]. However, in immunocompromised individuals or any severe case with symptoms of severe hemolysis, renal or hepatic distress, compromised respiratory function, or a parasitemia greater than 10%, regardless of species, a 10 day course of intravenous clindamycin plus quinine coupled with exchange transfusion is the recommended course of treatment [41, 44, 96]. The additive measure of exchange transfusion swiftly removes the parasitized RBCs from the host circulation, resolving any pathologies arising from anemia, such as low hematocrit and circulating toxic metabolites which released into the host’s circulatory system from the cyclical destruction of the RBCs.
Drug resistance
Although the two main treatment regimens of clindamycin/quinine or atovoquone plus azithromycin appear to remain effective in general, problems with speed of response to therapy and parasite persistence have been reported both in Europe [18] and in the United States [97], though parasite drug resistance itself is not suspected in these cases, and highlights the need to monitor patient parasitemia throughout treatment. However, three incidents of drug resistance to atovoquone/azithromycin, as defined by parasite relapse after more than 28 days of anti-babesial treatment, in immunocompromised patients with B. microti has been reported [95]. The authors note that they used more stringent definitions of resistance than is commonly used for other parasites such as malaria. The results highlight the need to robustly and systematically assess anti-babesial drugs and compounds where possible. Recent in vitro studies with artemether and lumefantrine showed a positive synergistic interaction of these compounds against B. gibsoni [98], and compounds that directly target the pathways of parasite DNA and RNA synthesis of B. gibsoni and B. bovis, such as mycophenolate mofetil, mizoribine, ribavirin, and 7-nitroindole, directly inhibit in the in vitro propagations of these parasites.
CONCLUSIONS
The clinical epidemiology of human babesiosis appears to be changing and this increased awareness will improve the management of local incidence of disease. The pathology of babesiosis, is a direct consequence of the cyclical replication of parasites in RBC’s. Like malaria, the parasite's ability to first recognize and then invade host RBCs is central to the disease process, and thus the invasion step provide a vulnerable point in the parasite’s lifecycle. The recent publication of the B. divergens [99] and B. microti [100] genomes will greatly assist the discovery of therapeutic agents that target this stage in the parasite lifecycle. Utilizing these tools to mine for agents that might prove effective against Babesia parasites is especially important for two diverse reasons. Firstly, the potential that human pathogens have develop and then swiftly spread resistance, rendering therapeutic agents ineffective has been shown many times before. Secondly, B. microti cannot currently be cultured in vitro and relies upon rodent models for testing the effectiveness of potential anti-B. microti agents, often making such studies difficult to perform or translate into the human system. The current treatment options for Babesia infections are limited to clindamycin plus quinidine or quinine or atovoquone plus azithromycin, yet there is evidence that drug resistance to atovoquone plus azithromycin may already have been observed in immunocompromised individuals. Advances in the treatment of donated blood products are a significant step forward in protecting the blood supply and limiting recipients to the risk of transfusion-transmitted babesiosis, yet more assessment needs to be done to ensure the parasites are completely inactivated without compromising the integrity of the blood components. However, it is clear that as clinical information becomes more readily available, there are significant gaps in our understanding of the basic biology of these parasites which warrants immediate and extensive investigation.
Acknowledgments
The authors state that this work has been completed in the authors’ laboratory is supported by grants from the NIH to CAL: HL105694 and HL129215 and a grant from the George Link Jr. Foundation
Contributor Information
Rosalynn Louise Ord, Department of Blood-Borne Parasites, Lindsley Kimball Research Institute, New York Blood Center, New York, NY, 10065, USA.
Cheryl A. Lobo, Department of Blood-Borne Parasites, Lindsley Kimball Research Institute, New York Blood Center, New York, NY, 10065, USA
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Spielman A, Wilson ML, Levine JF, Piesman J. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol. 1985;30:439–60. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- 2.Lantos PM, Krause PJ. Babesiosis: similar to malaria but different. Pediatr Ann. 2002;31(3):192–7. doi: 10.3928/0090-4481-20020301-10. [DOI] [PubMed] [Google Scholar]
- 3.Schnittger L, Rodriguez AE, Florin-Christensen M, Morrison DA. Babesia: a world emerging. Infect Genet Evol. 2012;12(8):1788–809. doi: 10.1016/j.meegid.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Zintl A, Mulcahy G, Skerrett HE, Taylor SM, Gray JS. Babesia divergens, a bovine blood parasite of veterinary and zoonotic importance. Clin Microbiol Rev. 2003;16(4):622–36. doi: 10.1128/CMR.16.4.622-636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leiby DA. Transfusion-associated babesiosis: shouldn't we be ticked off? Ann Intern Med. 2011;155(8):556–7. doi: 10.7326/0003-4819-155-8-201110180-00363. [DOI] [PubMed] [Google Scholar]
- 6.Leiby DA. Transfusion-transmitted Babesia spp. bull's-eye on Babesia microti. Clin Microbiol Rev. 2011;24(1):14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubernot DM, Nakhasi HL, Mied PA, Asher DM, Epstein JS, Kumar S. Transfusion-transmitted babesiosis in the United States: summary of a workshop. Transfusion. 2009;49(12):2759–71. doi: 10.1111/j.1537-2995.2009.02429.x. [DOI] [PubMed] [Google Scholar]
- 8.Yager PH, Luginbuhl LM, Dekker JP. Case records of the Massachusetts General Hospital. Case 6-2014. A 35-day-old boy with fever, vomiting, mottled skin, and severe anemia. N Engl J Med. 2014;370(8):753–62. doi: 10.1056/NEJMcpc1208155. [DOI] [PubMed] [Google Scholar]
- 9.Aderinboye O, Syed SS. Congenital babesiosis in a four-week-old female infant. Pediatr Infect Dis J. 2010;29(2):188. doi: 10.1097/INF.0b013e3181c3c971. [DOI] [PubMed] [Google Scholar]
- 10.New DL, Quinn JB, Qureshi MZ, Sigler SJ. Vertically transmitted babesiosis. J Pediatr. 1997;131:163–4. doi: 10.1016/s0022-3476(97)70143-4. 1 Pt 1. [DOI] [PubMed] [Google Scholar]
- 11.Sethi S, Alcid D, Kesarwala H, Tolan RW., Jr. Probable congenital babesiosis in infant, new jersey, USA. Emerg Infect Dis. 2009;15(5):788–91. doi: 10.3201/eid1505.070808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Western KA, Benson GD, Gleason NN, Healy GR, Schultz MG. Babesiosis in a Massachusetts resident. N Engl J Med. 1970;283(16):854–6. doi: 10.1056/NEJM197010152831607. [DOI] [PubMed] [Google Scholar]
- 13.Skrabalo Z, Deanovic Z. Piroplasmosis in man; report of a case. Doc Med Geogr Trop. 1957;9(1):11–6. [PubMed] [Google Scholar]
- 14.Bloch EM, Herwaldt BL, Leiby DA, Shaieb A, Herron RM, Chervenak M, et al. The third described case of transfusion-transmitted Babesia duncani. Transfusion. 2012;52(7):1517–22. doi: 10.1111/j.1537-2995.2011.03467.x. [DOI] [PubMed] [Google Scholar]
- 15.Conrad PA, Kjemtrup AM, Carreno RA, Thomford J, Wainwright K, Eberhard M, et al. Description of Babesia duncani n.sp. (Apicomplexa: Babesiidae) from humans and its differentiation from other piroplasms. Int J Parasitol. 2006;36(7):779–89. doi: 10.1016/j.ijpara.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 16.Jiang JF, Zheng YC, Jiang RR, Li H, Huo QB, Jiang BG, et al. Epidemiological, clinical, and laboratory characteristics of 48 cases of "Babesia venatorum" infection in China: a descriptive study. Lancet Infect Dis. 2015;15(2):196–203. doi: 10.1016/S1473-3099(14)71046-1. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y, Li SG, Jiang JF, Wang X, Zhang Y, Wang H, et al. Babesia venatorum Infection in Child, China. Emerg Infect Dis. 2014;20(5):896–7. doi: 10.3201/eid2005.121034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haselbarth K, Tenter AM, Brade V, Krieger G, Hunfeld KP. First case of human babesiosis in Germany - Clinical presentation and molecular characterisation of the pathogen. Int J Med Microbiol. 2007;297(3):197–204. doi: 10.1016/j.ijmm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Herwaldt BL, Caccio S, Gherlinzoni F, Aspock H, Slemenda SB, Piccaluga P, et al. Molecular characterization of a non-Babesia divergens organism causing zoonotic babesiosis in Europe. Emerg Infect Dis. 2003;9(8):942–8. doi: 10.3201/eid0908.020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabsley MJ, Shock BC. Natural history of Zoonotic Babesia: Role of wildlife reservoirs. Int J Parasitol Parasites Wildl. 2013;2:18–31. doi: 10.1016/j.ijppaw.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spielman A. Human babesiosis on Nantucket Island: transmission by nymphal Ixodes ticks. Am J Trop Med Hyg. 1976;25(6):784–7. doi: 10.4269/ajtmh.1976.25.784. [DOI] [PubMed] [Google Scholar]
- 22.Vannier E, Krause PJ. Human babesiosis. N Engl J Med. 2012;366(25):2397–407. doi: 10.1056/NEJMra1202018. [DOI] [PubMed] [Google Scholar]
- 23.Hersh MH, Tibbetts M, Strauss M, Ostfeld RS, Keesing F. Reservoir competence of wildlife host species for Babesia microti. Emerg Infect Dis. 2012;18(12):1951–7. doi: 10.3201/eid1812.111392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaschitz M, Narodoslavsky-Gfoller M, Kanzler M, Stanek G, Walochnik J. Babesia species occurring in Austrian Ixodes ricinus ticks. Appl Environ Microbiol. 2008;74(15):4841–6. doi: 10.1128/AEM.00035-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am. 2015;29(2):357–70. doi: 10.1016/j.idc.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Etkind P, Piesman J, Ruebush TK, 2nd, Spielman A, Juranek DD. Methods for detecting Babesia microti infection in wild rodents. J Parasitol. 1980;66(1):107–10. [PubMed] [Google Scholar]
- 27.Piesman J, Mather TN, Dammin GJ, Telford SR, 3rd, Lastavica CC, Spielman A. Seasonal variation of transmission risk of Lyme disease and human babesiosis. Am J Epidemiol. 1987;126(6):1187–9. doi: 10.1093/oxfordjournals.aje.a114757. [DOI] [PubMed] [Google Scholar]
- 28.Buckingham SC. Tick-borne diseases of the USA: Ten things clinicians should know. J Infect. 2015;71(Suppl 1):S88–96. doi: 10.1016/j.jinf.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Scholtens RG, Braff EH, Healey GA, Gleason N. A case of babesiosis in man in the United States. Am J Trop Med Hyg. 1968;17(6):810–3. doi: 10.4269/ajtmh.1968.17.810. [DOI] [PubMed] [Google Scholar]
- 30.Herwaldt BL, Montgomery S, Woodhall D, Bosserman E. Babesiosis surveillance - 18 States, 2011. MMWR Morb Mortal Wkly Rep. 2012;61(27):505–9. [PubMed] [Google Scholar]
- 31.Herwaldt BL, McGovern PC, Gerwel MP, Easton RM, MacGregor RR. Endemic babesiosis in another eastern state: New Jersey. Emerg Infect Dis. 2003;9(2):184–8. doi: 10.3201/eid0902.020271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apostolou A, Sorhage F, Tan C. Babesiosis surveillance, new jersey, USA, 2006-2011. Emerg Infect Dis. 2014;20(8):1407–9. doi: 10.3201/eid2008.131591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullard JM, Ahsanuddin AN, Perry AM, Lindsay LR, Iranpour M, Dibernardo A, et al. The first case of locally acquired tick-borne Babesia microti infection in Canada. Can J Infect Dis Med Microbiol. 2014;25(6):e87–9. doi: 10.1155/2014/209521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Acosta ME, Ender PT, Smith EM, Jahre JA. Babesia microti infection, eastern Pennsylvania, USA. Emerg Infect Dis. 2013;19(7):1105–7. doi: 10.3201/eid1907.121593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Persing DH, Herwaldt BL, Glaser C, Lane RS, Thomford JW, Mathiesen D, et al. Infection with a babesia-like organism in northern California. N Engl J Med. 1995;332(5):298–303. doi: 10.1056/NEJM199502023320504. [DOI] [PubMed] [Google Scholar]
- 36.Kjemtrup AM, Conrad PA. Human babesiosis: an emerging tick-borne disease. Int J Parasitol. 2000;30(12-13):1323–37. doi: 10.1016/s0020-7519(00)00137-5. [DOI] [PubMed] [Google Scholar]
- 37.Kjemtrup AM, Wainwright K, Miller M, Penzhorn BL, Carreno RA. Babesia conradae, sp. Nov., a small canine Babesia identified in California. Vet Parasitol. 2006;138(1-2):103–11. doi: 10.1016/j.vetpar.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 38.Herwaldt B, Persing DH, Precigout EA, Goff WL, Mathiesen DA, Taylor PW, et al. A fatal case of babesiosis in Missouri: identification of another piroplasm that infects humans. Ann Intern Med. 1996;124(7):643–50. doi: 10.7326/0003-4819-124-7-199604010-00004. [DOI] [PubMed] [Google Scholar]
- 39.Beattie JF, Michelson ML, Holman PJ. Acute babesiosis caused by Babesia divergens in a resident of Kentucky. N Engl J Med. 2002;347(9):697–8. doi: 10.1056/NEJM200208293470921. [DOI] [PubMed] [Google Scholar]
- 40.Herwaldt BL, de Bruyn G, Pieniazek NJ, Homer M, Lofy KH, Slemenda SB, et al. Babesia divergens-like infection, Washington State. Emerg Infect Dis. 2004;10(4):622–9. doi: 10.3201/eid1004.030377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunfeld KP, Hildebrandt A, Gray JS. Babesiosis: recent insights into an ancient disease. Int J Parasitol. 2008;38(11):1219–37. doi: 10.1016/j.ijpara.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Rios L, Alvarez G, Blair S. Serological and parasitological study and report of the first case of human babesiosis in Colombia. Rev Soc Bras Med Trop. 2003;36(4):493–8. doi: 10.1590/s0037-86822003000400010. [DOI] [PubMed] [Google Scholar]
- 43.Gorenflot A, Moubri K, Precigout E, Carcy B, Schetters TP. Human babesiosis. Ann Trop Med Parasitol. 1998;92(4):489–501. doi: 10.1080/00034989859465. [DOI] [PubMed] [Google Scholar]
- 44.Gray J, Zintl A, Hildebrandt A, Hunfeld KP, Weiss L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis. 2010;1(1):3–10. doi: 10.1016/j.ttbdis.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Hildebrandt A, Gray JS, Hunfeld KP. Human babesiosis in Europe: what clinicians need to know. Infection. 2013;41(6):1057–72. doi: 10.1007/s15010-013-0526-8. [DOI] [PubMed] [Google Scholar]
- 46.Morch K, Holmaas G, Frolander PS, Kristoffersen EK. Severe human Babesia divergens infection in Norway. Int J Infect Dis. 2015;33:37–8. doi: 10.1016/j.ijid.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Hildebrandt A, Hunfeld KP, Baier M, Krumbholz A, Sachse S, Lorenzen T, et al. First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis. 2007;26(8):595–601. doi: 10.1007/s10096-007-0333-1. [DOI] [PubMed] [Google Scholar]
- 48.Welc-Faleciak R, Pawelczyk A, Radkowski M, Pancewicz SA, Zajkowska J, Sinski E. First report of two asymptomatic cases of human infection with Babesia microti (Franca, 1910) in Poland. Ann Agric Environ Med. 2015;22(1):51–4. doi: 10.5604/12321966.1141394. [DOI] [PubMed] [Google Scholar]
- 49.El-Bahnasawy MM, Khalil HH, Morsy TA. Babesiosis in an Egyptian boy aquired from pet dog, and a general review. J Egypt Soc Parasitol. 2011;41(1):99–108. [PubMed] [Google Scholar]
- 50.Shih CM, Liu LP, Chung WC, Ong SJ, Wang CC. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35(2):450–4. doi: 10.1128/jcm.35.2.450-454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shaio MF, Lin PR. A case study of cytokine profiles in acute human babesiosis. Am J Trop Med Hyg. 1998;58(3):335–7. doi: 10.4269/ajtmh.1998.58.335. [DOI] [PubMed] [Google Scholar]
- 52.Wei Q, Tsuji M, Zamoto A, Kohsaki M, Matsui T, Shiota T, et al. Human babesiosis in Japan: isolation of Babesia microti-like parasites from an asymptomatic transfusion donor and from a rodent from an area where babesiosis is endemic. J Clin Microbiol. 2001;39(6):2178–83. doi: 10.1128/JCM.39.6.2178-2183.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim JY, Cho SH, Joo HN, Tsuji M, Cho SR, Park IJ, et al. First case of human babesiosis in Korea: detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine babesia. J Clin Microbiol. 2007;45(6):2084–7. doi: 10.1128/JCM.01334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paparini A, Senanayake SN, Ryan UM, Irwin PJ. Molecular confirmation of the first autochthonous case of human babesiosis in Australia using a novel primer set for the beta-tubulin gene. Exp Parasitol. 2014;141:93–7. doi: 10.1016/j.exppara.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 55.Senanayake SN, Paparini A, Latimer M, Andriolo K, Dasilva AJ, Wilson H, et al. First report of human babesiosis in Australia. Med J Aust. 2012;196(5):350–2. doi: 10.5694/mja11.11378. [DOI] [PubMed] [Google Scholar]
- 56.Mayne PJ. Emerging incidence of Lyme borreliosis, babesiosis, bartonellosis, and granulocytic ehrlichiosis in Australia. Int J Gen Med. 2011;4:845–52. doi: 10.2147/IJGM.S27336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mayne PJ. Clinical determinants of Lyme borreliosis, babesiosis, bartonellosis, anaplasmosis, and ehrlichiosis in an Australian cohort. Int J Gen Med. 2015;8:15–26. doi: 10.2147/IJGM.S75825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou X, Xia S, Huang JL, Tambo E, Zhuge HX, Zhou XN. Human babesiosis, an emerging tick-borne disease in the People's Republic of China. Parasit Vectors. 2014;7:509. doi: 10.1186/s13071-014-0509-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou X, Li SG, Chen SB, Wang JZ, Xu B, Zhou HJ, et al. Co-infections with Babesia microti and Plasmodium parasites along the China-Myanmar border. Infect Dis Poverty. 2013;2(1):24. doi: 10.1186/2049-9957-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qi C, Zhou D, Liu J, Cheng Z, Zhang L, Wang L, et al. Detection of Babesia divergens using molecular methods in anemic patients in Shandong Province, China. Parasitol Res. 2011;109(1):241–5. doi: 10.1007/s00436-011-2382-8. [DOI] [PubMed] [Google Scholar]
- 61.Wang H, Huang F. Babesia infection in the southwest of china, a case report. Jundishapur J Microbiol. 2014;7(11):e13504. doi: 10.5812/jjm.13504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou X, Li SG, Wang JZ, Huang JL, Zhou HJ, Chen JH, et al. Emergence of human babesiosis along the border of China with Myanmar: detection by PCR and confirmation by sequencing. Emerg Microbes Infect. 2014;3(8):e55. doi: 10.1038/emi.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cursino-Santos JR, Halverson G, Rodriguez M, Narla M, Lobo CA. Identification of binding domains on red blood cell glycophorins for Babesia divergens. Transfusion. 2014;54(4):982–9. doi: 10.1111/trf.12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lobo CA. Babesia divergens and Plasmodium falciparum use common receptors, glycophorins A and B, to invade the human red blood cell. Infect Immun. 2005;73(1):649–51. doi: 10.1128/IAI.73.1.649-651.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lobo CA, Rodriguez M, Cursino-Santos JR. Babesia and red cell invasion. Curr Opin Hematol. 2012;19(3):170–5. doi: 10.1097/MOH.0b013e328352245a. [DOI] [PubMed] [Google Scholar]
- 66.Montero E, Rafiq S, Heck S, Lobo CA. Inhibition of human erythrocyte invasion by Babesia divergens using serine protease inhibitors. Mol Biochem Parasitol. 2007;153(1):80–4. doi: 10.1016/j.molbiopara.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 67.Montero E, Rodriguez M, Gonzalez LM, Lobo CA. Babesia divergens: identification and characterization of BdHSP-20, a small heat shock protein. Exp Parasitol. 2008;119(2):238–45. doi: 10.1016/j.exppara.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 68.Montero E, Rodriguez M, Oksov Y, Lobo CA. Babesia divergens apical membrane antigen 1 and its interaction with the human red blood cell. Infect Immun. 2009;77(11):4783–93. doi: 10.1128/IAI.00969-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rodriguez M, Alhassan A, Ord RL, Cursino-Santos JR, Singh M, Gray J, et al. Identification and Characterization of the RouenBd1987 Babesia divergens Rhopty-Associated Protein 1. PLoS One. 2014;9(9):e107727. doi: 10.1371/journal.pone.0107727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Repnik U, Gangopadhyay P, Bietz S, Przyborski JM, Griffiths G, Lingelbach K. The apicomplexan parasite Babesia divergens internalizes band 3, glycophorin A and spectrin during invasion of human red blood cells. Cell Microbiol. 2015 doi: 10.1111/cmi.12422. [DOI] [PubMed] [Google Scholar]
- 71.Hunfeld KP, Brade V. Zoonotic Babesia: possibly emerging pathogens to be considered for tick-infested humans in Central Europe. Int J Med Microbiol. 2004;293(Suppl 37):93–103. doi: 10.1016/s1433-1128(04)80014-7. [DOI] [PubMed] [Google Scholar]
- 72.Mylonakis E. When to suspect and how to monitor babesiosis. Am Fam Physician. 2001;63(10):1969–74. [PubMed] [Google Scholar]
- 73.Krause PJ, McKay K, Gadbaw J, Christianson D, Closter L, Lepore T, et al. Increasing health burden of human babesiosis in endemic sites. Am J Trop Med Hyg. 2003;68(4):431–6. [PubMed] [Google Scholar]
- 74.Filstein MR, Benach JL, White DJ, Brody BA, Goldman WD, Bakal CW, et al. Serosurvey for human babesiosis in New York. J Infect Dis. 1980;141(4):518–21. doi: 10.1093/infdis/141.4.518. [DOI] [PubMed] [Google Scholar]
- 75.Krause PJ, Telford SR, 3rd, Ryan R, Hurta AB, Kwasnik I, Luger S, et al. Geographical and temporal distribution of babesial infection in Connecticut. J Clin Microbiol. 1991;29(1):1–4. doi: 10.1128/jcm.29.1.1-4.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.White DJ, Talarico J, Chang HG, Birkhead GS, Heimberger T, Morse DL. Human babesiosis in New York State: Review of 139 hospitalized cases and analysis of prognostic factors. Arch Intern Med. 1998;158(19):2149–54. doi: 10.1001/archinte.158.19.2149. [DOI] [PubMed] [Google Scholar]
- 77.Bruning G. Localization of NADPH diaphorase, a histochemical marker for nitric oxide synthase, in the mouse spinal cord. Acta Histochem. 1992;93(2):397–401. doi: 10.1016/S0065-1281(11)80109-1. [DOI] [PubMed] [Google Scholar]
- 78.Wozniak EJ, Lowenstine LJ, Hemmer R, Robinson T, Conrad PA. Comparative pathogenesis of human WA1 and Babesia microti isolates in a Syrian hamster model. Lab Anim Sci. 1996;46(5):507–15. [PubMed] [Google Scholar]
- 79.Gerber MA, Shapiro ED, Krause PJ, Cable RG, Badon SJ, Ryan RW. The risk of acquiring Lyme disease or babesiosis from a blood transfusion. J Infect Dis. 1994;170(1):231–4. doi: 10.1093/infdis/170.1.231. [DOI] [PubMed] [Google Scholar]
- 80.Gubernot DM, Lucey CT, Lee KC, Conley GB, Holness LG, Wise RP. Babesia infection through blood transfusions: reports received by the US Food and Drug Administration, 1997-2007. Clin Infect Dis. 2009;48(1):25–30. doi: 10.1086/595010. [DOI] [PubMed] [Google Scholar]
- 81.Cushing M, Shaz B. Transfusion-transmitted babesiosis: achieving successful mitigation while balancing cost and donor loss. Transfusion. 2012;52(7):1404–7. doi: 10.1111/j.1537-2995.2012.03746.x. [DOI] [PubMed] [Google Scholar]
- 82.Cursino-Santos JR, Alhassan A, Singh M, Lobo CA. Babesia: impact of cold storage on the survival and the viability of parasites in blood bags. Transfusion. 2014;54(3):585–91. doi: 10.1111/trf.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tonnetti L, Eder AF, Dy B, Kennedy J, Pisciotto P, Benjamin RJ, et al. Transfusion-transmitted Babesia microti identified through hemovigilance. Transfusion. 2009 doi: 10.1111/j.1537-2995.2009.02317.x. [DOI] [PubMed] [Google Scholar]
- 84.Tonnetti L, Thorp AM, Reddy HL, Keil SD, Goodrich RP, Leiby DA. Riboflavin and ultraviolet light reduce the infectivity of Babesia microti in whole blood. Transfusion. 2012 doi: 10.1111/j.1537-2995.2012.03791.x. [DOI] [PubMed] [Google Scholar]
- 85.Winter KM, Johnson L, Kwok M, Vidovic D, Hyland RA, Mufti N, et al. Red blood cell in vitro quality and function is maintained after S-303 pathogen inactivation treatment. Transfusion. 2014;54(7):1798–807. doi: 10.1111/trf.12545. [DOI] [PubMed] [Google Scholar]
- 86.Sun T, Tenenbaum MJ, Greenspan J, Teichberg S, Wang RT, Degnan T, et al. Morphologic and clinical observations in human infection with Babesia microti. J Infect Dis. 1983;148(2):239–48. doi: 10.1093/infdis/148.2.239. [DOI] [PubMed] [Google Scholar]
- 87.Demeter Z, Palade EA, Balogh E, Jakab C, Farkas R, Tanczos B, et al. Postmortem small babesia-like morphology of Babesia canis - short communication. Acta Vet Hung. 2011;59(4):427–32. doi: 10.1556/AVet.2011.029. [DOI] [PubMed] [Google Scholar]
- 88.Teal AE, Habura A, Ennis J, Keithly JS, Madison-Antenucci S. A new real-time PCR assay for improved detection of the parasite Babesia microti. J Clin Microbiol. 2012;50(3):903–8. doi: 10.1128/JCM.05848-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bloch EM, Lee TH, Krause PJ, Telford SR, 3rd, Montalvo L, Chafets D, et al. Development of a real-time polymerase chain reaction assay for sensitive detection and quantitation of Babesia microti infection. Transfusion. 2013 doi: 10.1111/trf.12098. [DOI] [PubMed] [Google Scholar]
- 90.Wilson M, Glaser KC, Adams-Fish D, Boley M, Mayda M, Molestina RE. Development of droplet digital PCR for the detection of Babesia microti and Babesia duncani. Exp Parasitol. 2015;149:24–31. doi: 10.1016/j.exppara.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vannier E, Krause PJ. Babesiosis in China, an emerging threat. Lancet Infect Dis. 2015;15(2):137–9. doi: 10.1016/S1473-3099(14)71062-X. [DOI] [PubMed] [Google Scholar]
- 92.Vannier E, Krause PJ. Update on babesiosis. Interdiscip Perspect Infect Dis. 2009;2009:984568. doi: 10.1155/2009/984568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brasseur P, Lecoublet S, Kapel N, Favennec L, Ballet JJ. Quinine in the treatment of Babesia divergens infections in humans. Eur J Clin Microbiol Infect Dis. 1996;15(10):840–1. doi: 10.1007/BF01701533. [DOI] [PubMed] [Google Scholar]
- 94.Krause PJ, Lepore T, Sikand VK, Gadbaw J, Jr., Burke G, Telford SR, 3rd, et al. Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med. 2000;343(20):1454–8. doi: 10.1056/NEJM200011163432004. [DOI] [PubMed] [Google Scholar]
- 95.Wormser GP, Prasad A, Neuhaus E, Joshi S, Nowakowski J, Nelson J, et al. Emergence of resistance to azithromycin-atovaquone in immunocompromised patients with Babesia microti infection. Clin Infect Dis. 2010;50(3):381–6. doi: 10.1086/649859. [DOI] [PubMed] [Google Scholar]
- 96.Nathavitharana RR, Mitty JA. Diseases from North America: focus on tick-borne infections. Clin Med. 2015;15(1):74–7. doi: 10.7861/clinmedicine.14-6-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Krause PJ, Spielman A, Telford SR, 3rd, Sikand VK, McKay K, Christianson D, et al. Persistent parasitemia after acute babesiosis. N Engl J Med. 1998;339(3):160–5. doi: 10.1056/NEJM199807163390304. [DOI] [PubMed] [Google Scholar]
- 98.Iguchi A, Matsuu A, Matsuyama K, Hikasa Y. The efficacy of artemisinin, artemether, and lumefantrine against Babesia gibsoni in vitro. Parasitol Int. 2015;64(2):190–3. doi: 10.1016/j.parint.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Cuesta I, Gonzalez LM, Estrada K, Grande R, Zaballos A, Lobo CA, et al. High-Quality Draft Genome Sequence of Babesia divergens, the Etiological Agent of Cattle and Human Babesiosis. Genome Announc. 2014;2 doi: 10.1128/genomeA.01194-14. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cornillot E, Hadj-Kaddour K, Dassouli A, Noel B, Ranwez V, Vacherie B, et al. Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res. 2012;40(18):9102–14. doi: 10.1093/nar/gks700. [DOI] [PMC free article] [PubMed] [Google Scholar]