SUMMARY
Tumors are incredibly diverse and contain many different subpopulations of cells. The cancer stem cell (CSC) subpopulation is responsible for many aspects of tumorigenesis and has been shown to play an important role in melanoma development, progression, drug resistance and metastasis. However, it is becoming clear that tumor cell populations are dynamic and can be influenced by many factors, such as signals from the tumor microenvironment and somatic evolution. This review will present the current understanding of CSCs and the challenges of identifying and characterizing this dynamic cell population. The known characteristics and functions of melanoma stem cells, and the potential for therapeutic targeting of these cells in melanoma, will be discussed.
KEYWORDS : cancer stem cells, melanoma, melanoma stem cells, targeting cancer stem cells, tumor cell plasticity, tumor heterogeneity, tumor initiating cells
Practice points.
Tumors are heterogeneous and contain many diverse cell populations.
Cancer stem cells (CSCs) are subpopulations of cells within tumors that are self-renewing and important for tumor initiation, progression, immunoevasion, drug resistance and metastasis.
CSC populations are dynamic and influenced by signals from the tumor microenvironment and somatic evolution, making them challenging to identify and characterize.
Melanoma stem cell (MSC) populations have been characterized and associated with tumor progression, immunoevasion, drug resistance and metastasis.
Initial treatment strategies targeting MSCs are promising, but a better understanding of CSC dynamics is needed to determine the best methods for successfully targeting CSCs therapeutically.
Melanoma is a malignant tumor of melanocytes which typically arises in the skin. In the USA, the incidence of melanoma has been increasing for 30 years, and melanoma is now the third and fourth most prevalent cancer in men and women, respectively [1]. Melanoma accounts for the minority of skin cancer diagnoses, but the majority of skin cancer deaths [2]. The treatment of advanced or metastatic melanoma is particularly challenging, and there is a high propensity for patients to relapse and become resistant to the current therapeutic agent [3]. Therefore, there is an urgent need for a better understanding of melanoma tumor biology and the possible mechanisms behind poor patient response to therapeutics.
Over the last few decades, it has become clear that cancers are composed of heterogeneous cell populations with phenotypic and functional differences. Various mechanisms have been proposed to explain cancer cell heterogeneity, including speculation that a stem cell-like subpopulation in tumors plays a vital role in cancer progression, drug resistance and tumor regrowth. However, despite the significant amount of interest in this field, defining and characterizing this population of cells has been challenging in some cancers, and the presence of a proposed ‘cancer stem cell (CSC)’ population was debated [4]. In this review, we will discuss the current understanding of CSCs and outline the complexities and challenges facing this rapidly developing field. We will highlight the identification and phenotype of melanoma stem cells (MSCs) specifically, and discuss the therapeutic potential of targeting this subpopulation in melanoma.
Cancer stem cells
• Definition
Stem cells are biologically unique cells essential for the generation, maintenance and repair of tissues. Under physiologic conditions, stem cells form the apex of a hierarchy that gives rise to more rapidly proliferating progenitor cells, which develop into nonproliferative differentiated cells that fulfill the functions of a specific organ [5]. CSCs are tumor cells that have stem cell-like characteristics and are defined by two fundamental stem cell properties, self-renewal and differentiation. Self-renewal is the ability of a stem cell population to maintain itself, and differentiation is the ability of a stem cell population to generate specialized types of differentiated progeny cells. All tumor cells that meet these criteria, regardless of origin or lineage, are considered to be CSCs [6].
• Identification
The gold standard assay to confirm the presence of CSCs which meet the criteria of self-renewal and differentiation is serial transplantation of tumor cells in an animal model [6]. In this assay, suspected CSCs are isolated from tumors and injected into serial generations of mice. The frequency of tumor initiating cells (TICs) in each population is determined, and only tumor populations containing CSCs will be able to continually form tumors and recapitulate the original tumor after serial implantation. It must be emphasized that not all TICs are CSCs: TICs form tumors for a limited period of time, for a longer time or eternally. It must also be emphasized that sustained tumor growth or self-renewal is not equivalent to rapid tumor growth or rapid proliferation of tumor cells [6]. Only those cells that fulfill the criteria for CSCs (tumorigenesis with self-renewal and differentiation in serial transplantation in mice) are CSCs. Although serial transplantation in mice is the standard assay for assessing the presence of CSCs in a tumor cell population, it has several drawbacks. Because the tumor microenvironment and the stem cell niche are highly important in the regulation and activation of CSCs, implanting human tumor cells into a mouse microenvironment most likely impacts the characteristics and phenotypes of the cells. In addition, the mouse model and the method of cell injection can greatly influence the estimated TIC frequency in a population. Despite these limitations and uncertainties in TIC frequency measurements, the fact that syngeneic transplantation of mouse tumors into immunocompetent mice has supported the CSC model provides reassurance that the CSC model is not merely an artifact of xenotransplantation [7–9].
While CSCs are defined by their functional properties, they are generally identified and traced using fluorescence-activated cell sorting based on the expression of specific cell-surface markers. However, there is increasing evidence that some CSC markers may not be generalizable to all tumors, and that patient to patient variability may exist. There have been discrepancies in the correlation between surface marker expression and tumorigenicity in several studies, even those within the same cancer type. Early studies in acute myeloid leukemia suggested that the CD34+/CD38- phenotype could differentiate tumorigenic from nontumorigenic fractions (CD34- or CD38+) [10]. However, more recent studies have also identified TIC activity in CD34+/CD38+, CD34- and CD38+ leukemia cell populations [11–13]. Similarly, a study in ovarian cancer found that while CD133+ cell populations had enriched TICs in many tumors, tumors from other patients contained both CD133+ and CD133- TICs, or exclusively CD133- TICs [14]. These data should not be used to reject the existence of CSCs in these tumors. Rather, these studies suggest that CSC populations are dynamic and more heterogeneous than previously thought.
• Phenotypes & functions
Similar to the role of physiological stem cells in maintaining tissues and organs, the main function of CSCs is to generate and maintain tumors [6]. This ability to constantly give rise to tumors from a small number of cells is hypothesized to be one of the main mechanisms for relapse and tumor regrowth.
As mentioned earlier, CSCs are defined by their two most important functions, self-renewal and differentiation. CSCs can undergo symmetric division to generate two daughter cells: either two CSCs or two progenitor cells (non-CSC daughter cells with different fates), which may be capable of rapid proliferation and bulk tumor generation [15]. CSCs can also undergo asymmetric division to generate two daughter cells that are not identical to each other: one CSC and one non-CSC daughter cell. This ability to undergo either symmetric or asymmetric division is thought to play an important role in the ability of a tumor to adapt and survive within the microenvironment.
Other phenotypes of CSCs include dormancy, chemoresistance, immunoevasion and metastasis [16]. Dormancy is a quiescent state that CSCs can reside in before proliferating in a suitable environment. Although the mechanisms of dormancy are complex and not yet fully understood, dormancy is associated with metastasis, immunoevasion and drug resistance of cancer cells [17], and understanding the dormancy of CSCs may provide insight into these phenotypes.
CSCs are also reported to be capable of vascular mimicry. Vascular mimicry is the process by which aggressive tumor cells form nonendothelial microvascular channels, essentially mimicking the normal angiogenesis process of blood vessel formation by endothelial cells [18]. These channels are thought to connect to traditional blood and lymphatic vessels and potentially serve as routes for tumor cells to metastasize. An association between CSC populations and enhanced vascular mimicry ability has now been described for several cancer types, including breast [18], glioma [19,20], melanoma [21] and renal cell carcinoma [22], suggesting this process is a functional phenotype of CSCs.
Tumor heterogeneity & CSC dynamics
• Models for tumor heterogeneity
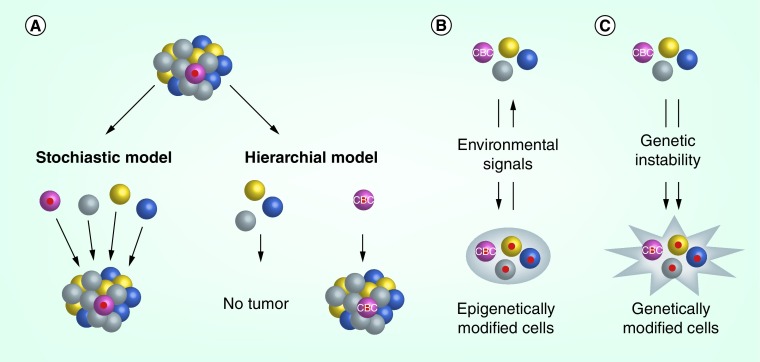
Although it has been known for decades that tumors are not homogeneous, a recent next generation sequencing study has revealed that tumors have a much higher degree of genetic intratumoral heterogeneity than previously thought, and the phenotypic diversity of these subpopulations may contribute to the ability of the tumor to quickly adapt and survive chemotherapeutic treatment [23]. Two dominant models have emerged to address the issue of tumor heterogeneity, the stochastic model and the hierarchical model (Figure 1A). The stochastic model argues that all cells within a tumor harbor similar tumorigenic and self-renewing potential, and that heterogeneity results from a process of clonal selection by which individual tumor cells acquire genetic or epigenetic changes. These changes are often driven by the tumor microenvironment, and are the result of extrinsic factors such as hypoxic stress, immune attack, metabolic stress and drug/toxic exposures, which confer phenotypic differences among tumor cells and impart a survival advantage or disadvantage [24]. In contrast to the stochastic model, the hierarchical model proposes that CSCs sit at the top of the hierarchy and establish tumor cell heterogeneity by giving rise to differentiated progenitor cells. This model suggests that the generation of daughter cells is unidirectional, therefore the CSC population within the tumor is finite and its depletion would result in elimination of the tumor. Although this model has also been referred to as the CSC model, this nomenclature is confusing because it is often misused to define CSCs. As described in the previous section, CSCs are defined by their functional properties of self-renewal and differentiation, thus they may not always follow this hierarchical model. While these two models appear distinct, the reality is that the stochastic and hierarchical models are not mutually exclusive, and both models are over-simplified because tumor heterogeneity may be attributed to multiple sources, as described in the following section.
Figure 1. . Cancer stem cells are dynamic.
(A) Two models have been proposed to explain tumor heterogeneity, but neither model accounts for the plasticity and somatic evolution of tumor cells. The stochastic model for tumor heterogeneity proposes that all tumor cells have the potential to be tumor-initiating stem cells. The hierarchical model for tumor heterogeneity proposes that tumors have a fixed population of cancer stem cells (CSCs), which are the only tumor cells capable of generating a tumor. (B) Epigenetic changes can occur in tumor cells in response to the tumor microenvironment and other stress factors. These changes can be reversible, and may only be transient in nature. (C) Genetic instability is an inherent property of tumor cells, and over time tumor cells may develop irreversible changes in DNA such as mutations, deletions or amplifications. While these genetic changes can occur in CSCs and non-CSCs, the changes will only be propagated and persist if they are in the CSC population, or if stem cell properties (self-renewal and differentiation) are acquired in non-CSCs because of these genetic changes.
• Is the cancer stem cell state static?
Under physiologic conditions, the path from a stem cell to a differentiated cell is predominantly unidirectional and irreversible. In vivo lineage tracing experiments of tumor cells have revealed the presence of finite CSCs and supported the idea that CSCs are a fixed population of biologically unique tumor cells (Figure 1A) [7–9,25]. However, differentiated cells or committed progenitor cells could be reprogrammed and dedifferentiated to stem cells by genetic interventions or microenvironmental changes [26,27]. These findings support the idea that stemness may not be restricted to a distinct pool of cells and could be acquired epigenetically and genetically. Similarly, in certain circumstances, ‘cancer stemness’ may be a fluid existence whereby tumor cells can both acquire and lose stemness (Figure 1B & C) [16,28].
During cancer progression, tumor cells are subject to epigenetic and genetic changes due to exposure to extrinsic factors in the tumor microenvironment. Upon influence from the tumor microenvironment or experimental manipulations, unidirectional differentiation may be lost and differentiated cells may be reprogrammed and dedifferentiated to a stem-like state (Figure 1B). For example, in lung cancer, cancer-associated fibroblasts regulate lung CSCs by secreting IGF-II, which induces dedifferentiation and acquisition of stem-like properties [29]. Independent of extrinsic factors, tumor cells may also mutate, differentiate and dedifferentiate over time because of their inherent genetic instability [30,31], through which stem-like features may be acquired (Figure 1C). Collectively, these factors may contribute to the phenotypic and functional plasticity and diversity of cancer cells.
Melanoma stem cells
• Identification
In 2005, Herlyn and colleagues identified a subpopulation of CD20+ cells in metastatic human melanoma with stem cell-like properties [32]. Frank and colleagues are credited with the discovery of a MSC population characterized by the expression of the ATP-binding cassette (ABC) transporter ABCB5 [25]. These cells demonstrated the capacity for self-renewal and differentiation with serial transplantation in the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice. Since this report, CD271 [33,34] and ALDH [35,36] have been shown to characterize phenotypically distinct MSC populations with self-renewal and differentiation capacities.
These markers are also common to other cancers. For example, ABCB5 identifies a drug resistant tumor cell population in colorectal cancer patients [37], and CD271 has been identified as a CSC marker for glioblastoma [38] and ovarian cancer [14]. In addition to serving as a MSC marker, ALDH is a detoxifying enzyme which oxidizes retinal to retinoic acid and regulates many genes associated with cell proliferation and differentiation. This finding enables a functional assay measuring ALDH activity (Aldefluor assay) to be used for the identification of MSCs instead of relying on cell-surface markers that are susceptible to experimental conditions such as enzymatic treatment [36]. Increased ALDH activity has also been reported in hematopoietic stem cells [39] and leukemic stem cells [40] as well as breast [41], prostate [42], pancreatic [43], colon [44] and ovarian [45] CSCs. Furthermore, these markers (ABCB5, CD271 and ALDH) have been shown to identify overlapping populations of MSCs [21,36].
• Phenotype-switching & plasticity of melanoma cells
Recently, Roesch and colleagues described the dynamic expression of JARID1B and its role in melanoma maintenance [46]. JARID1B is a histone demethylase linked to tissue development, cancer and normal stem cell biology. Although the authors did not conduct serial xenotransplantation procedures in immunocompromised mice to assess MSC status, JARID1B+ cells gave rise to rapidly proliferating progeny recapitulating the parental heterogeneity. However, different from previously described CSC markers, knockdown of JARID1B first promoted tumor growth, then followed by exhaustion, suggesting that JARID1B is not associated with rapid tumor growth but is strongly associated with tumor maintenance and propagation. JARID1B was expressed in a small subpopulation of slow-cycling melanoma cells and its expression was dynamically regulated by a hypoxic microenvironment, proposing an alternative concept of tumor heterogeneity with a dynamic subpopulation of TICs.
Cheli and colleagues have also reported the dynamic regulation of MITF [47] and demonstrated the downregulation of MITF by hypoxia in melanoma [48]. MITF regulates the phenotypic plasticity from poorly differentiated and highly metastatic cells (MITF-low) to fast growing progeny (MITF-high). The dynamic regulation of JARID1B and MITF illustrates the principle of phenotype-switching in melanoma. Phenotype-switching is a term which describes the process by which tumor cells switch between differentiated, proliferating and stem cell-like states [49]. Phenotype switching may be triggered by conditions in the tumor microenvironment (i.e., hypoxia) when there is a lowered threshold imposed by genetic and epigenetic changes. It remains unclear how long cancer cells maintain these acquired phenotypes and with what frequency this occurs, but phenotype switching may contribute to tumor heterogeneity. In tumors where phenotype-switching is prevalent, a hierarchical organization may be difficult to establish. It should be noted, however, that the absence of a hierarchical organization or the presence of a shallow hierarchy does not preclude the existence of CSCs within a given tumor. As previously discussed, CSCs are defined by their capacity for self-renewal and differentiation, and tumor cells that fulfill these criteria may exist in tumors independent of the degree of hierarchical organization.
• Variability in TIC frequency & possible explanations
Initial xenotransplantation assays to determine TIC frequency were conducted using NOD/SCID mice. These studies supported the hypothesis that TICs are rare in most human tumors including melanoma [25,50–52]. However, when Quintana and colleagues utilized a highly immunocompromised NOD/SCID IL-2Rγnull (NSG) mouse model in human melanoma, they reported a markedly increased frequency of tumorigenic cells, such that one in four human melanoma cells were tumorigenic [53]. Subsequent to this report, other groups have compared TIC frequency in NOD/SCID and NSG mice in human melanoma [21,33,35–36] and other solid organ malignancies [54]. These groups have confirmed increased tumorigenicity in NSG mice, but have not been able to validate the magnitude of the increase described by Quintana and colleagues.
It is speculated that, in addition to the type of mouse model, other variations in xenotransplantation assays are responsible for variations in TIC frequency, including source of tumor materials, lack of standardized methodologies for the dissociation and isolation of cancer cells and sampling bias of a heterogenous tumor [4,34,55–57]. The methods and findings of select xenotransplantation studies are compared in Table 1, highlighting differences in experimental methodologies which could account for variable TIC frequency data. The type of enzymes used for tumor cell dissociation, such as collagenases, and the incubation time differ between studies. Also, the method of tumor cell isolation after cell dissociation (in order to eliminate macrophages and other nontumor cells) varies, where some studies use positive selection to label and select human tumor cells and others use negative selection to label and remove mouse (nontumor) cells. TIC frequency has also been shown to be influenced by the site of injection [14] and the 3D biophysical microenvironment at the injection site [58]. Some studies have added Matrigel, a 3D matrix of basement compounds, to the cells prior to injection. A recent proteome array analysis of standard Matrigel revealed it contains many more biologically active proteins than had been previously reported [59]. High-protein Matrigel is characterized by even higher concentrations of protein and is formulated and marketed to further enhance tumorigenesis [60].
Table 1. . Tumor initiating cell frequency measurements in melanoma .
| TIC frequency | Tumor dissociation | Isolation | Xenotransplantation | Ref. | |||
|---|---|---|---|---|---|---|---|
| Trypsin | Collagenase type | Incubation time | Positive/negative selection | High-protein Matrigel | Mice | ||
| Quintana et al. | [53] | ||||||
| 1 in 111,000 | Yes | IV | 20 min | + | Yes | NOD/SCID | |
| 1 in 9 | Yes | IV | 20 min | + | No | NSG | |
| Boonyaratanakornkit et al. | [35] | ||||||
| 1 in 18,000–1,851,000 | No | Collagenase | 1.5–2 h | - | No | NOD/SCID | |
| 1 in 142–418 (unfractionated) | |||||||
| 1 in 4 (ALDH+) | No | Collagenase | 1.5–2 h | - | No | NSG | |
| 1 in 469-601 (ALDH-) | |||||||
| Luo et al. | [36] | ||||||
| 1 in 520 (unfractionated) | |||||||
| 1 in 100–150 (ALDH+) | No | I | 2 h | - | No | NOD/SCID | |
| 1 in 2910–8370 (ALDH-) | |||||||
| 1 in 100 (ALDH+) | No | I | 2 h | - | No | NSG | |
| 1 in 830 (ALDH-) | |||||||
| Schatton et al. | [25] | ||||||
| 1 in 158,170 (ABCB5+) | No | I and II | 3 h | - | No | Nude or NOD/SCID | |
| 1 in 11,152,529 (ABCB5-) | |||||||
NOD: Nonobese diabetic; NSG: NOD/SCID IL-2Rγnull; SCID: Severe combined immunodeficiency; TIC: Tumor initiating cells
These studies clearly illustrate the influence of experimental conditions on TIC frequency. Together with the fact that TICs are not synonymous to CSCs with sustained tumor growth, it is reasonable to conclude that the increased frequency of TICs reported by Quintana and colleagues does not disprove the presence of a special subpopulation of tumor cells, CSCs, in melanoma.
• Therapeutic strategies for MSCs
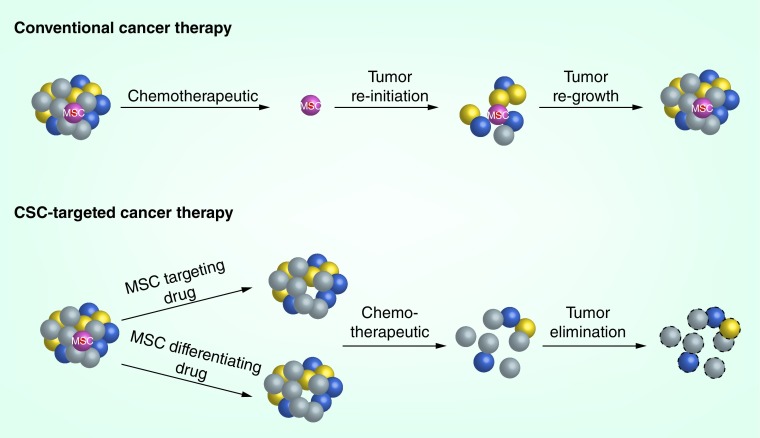
It has been proposed that chemotherapy and radiation therapy target only the rapidly dividing non-CSCs which comprise the bulk of the tumor, and that quiescent CSCs may remain viable after these treatments because of intrinsic survival mechanisms and chemoresistance [4,6]. MSCs may be associated with the so-called ‘side population’ (SP) phenotype, which is the ability to efficiently efflux drugs via ABC transporter proteins, ABCB1 and ABCB5 [61,62]. ABC transporters limit intracellular accumulation of drug, thereby conferring resistance to multiple chemotherapeutic agents. The CSCs remaining after treatment are then capable of revitalizing the tumor, and the relapsing tumor becomes drug resistant due to the selective pressure exerted by the drug (Figure 2). Adoptive transfer of cytotoxic T lymphocytes (CTLs) in melanoma induces TNF-α-mediated loss of melanocytic antigens and upregulation of MSC marker CD271 on melanoma cells [63], which in turn suppresses CTL activation, leading to poor treatment response [64]. Therefore, targeting CSC populations in melanoma and other cancers is of paramount importance.
Figure 2. . Strategies for targeting melanoma stem cells.
Conventional cancer therapy using chemotherapeutics eliminates the rapidly proliferating, differentiated tumor cells, but does not target the quiescent and slowly proliferating MSC population. The MSCs reinitiate tumor formation and lead to regrowth of the tumor. CSC-targeted therapy would aim to eliminate the MSC population, by either targeting the MSCs directly or using differentiating drugs. Once the stem cell population is removed, traditional treatment will eliminate the differentiated cells and the tumor will not be able to regenerate itself.
CSC: Cancer stem cell; MSC: Melanoma stem cell.
To date, targeted approaches against MSC marker proteins have shown some therapeutic promise. ABCB5 imparts an in vitro and in vivo survival advantage to melanoma cells upon exposure to dacarbazine and the BRAF inhibitor vemurafenib [61], and systemic administration of anti-ABCB5 antibody inhibited melanoma tumorigenesis in nude mice [25]. Silencing of ALDH1A (a family member of the MSC marker ALDH) led to decreased cell viability, reduced tumorigenesis, and decreased chemoresistance in melanomas [36]. In other studies, adoptive transfer of CTLs engineered with a chimeric antigen receptor for CD20 and HMW-MAA eradicated melanomas by eliminating a minor subset of CD20+ melanoma cells [65]. Knockdown of JARID1B was associated with initial acceleration of tumor growth followed by exhaustion, highlighting the function of JARID1B in tumor maintenance and propagation [46]. Collectively, these findings suggest that drugs which target MSCs may act synergistically with existing cytotoxic agents, making combinatorial treatment involving MSCs an attractive therapeutic strategy (Figure 2). We have used a combination of a BCL-2 inhibitor and the retinoid derivative fenretinide, and have shown decreased percentage of ALDHhigh cells, inhibited self-renewal capacity and reduced tumorigenesis [66].
Despite the early success of studies targeting MSC markers, we know that MSCs are dynamically regulated and induced at times of cellular stress. Conceivably, existing melanoma treatments may result in the reprogramming of differentiated cells into a transient MSC population which drives tumor maintenance and propagation. Identifying and targeting all MSC populations is vital to achieving sustained remission. Because the dynamic nature of MSCs makes them a moving target, it may not be feasible to identify and target all MSC subpopulations. An alternative approach is to deplete the MSC pool using drugs that induce terminal differentiation. All transretinoic acid is a drug that has been used to treat acute promyelocytic leukemia for decades, and it works by inducing the maturation of immature promyelocytes to mature granulocytes which causes spontaneous apoptosis [67]. All transretinoic acid has been recently shown to inhibit growth of head and neck CSCs by suppressing Wnt/β-catenin pathway [68]. BMP4 induces terminal differentiation of glioblastoma stem cells, leading to reduced TIC frequency and tumor growth [69]. BMP4 also promotes the terminal differentiation, apoptosis and chemosensitization of colorectal cancer stem cells [70]. The success of differentiation-inducing drugs in other cancers makes this a promising area of future study in the MSC field (Figure 2).
Finally, another therapeutic strategy against MSCs is targeting the oncogenic phenotypes specifically promoted by the MSCs rather than targeting the MSC populations directly. Vascular mimicry is an oncogenic phenotype recently associated with CSCs in other cancer types, and it is a well-documented phenotype that has been observed in aggressive melanoma cells. Mary Hendrix and her group describe a process by which poorly differentiated melanoma cells express endothelium-associated genes involved in angiogenesis, leading to vascular mimicry [71]. Vascular mimicry has also been associated with invasiveness and enhanced metastatic potential in melanomas [72], and if vascular mimicry is an important phenotype of MSCs, targeting this downstream function may impair tumor growth and metastasis promoted by MSCs. Indeed, in vivo targeting of VEGFR-1 blocks the development of ABCB5+ vascular mimicry and inhibits tumor growth in melanoma [21]. Nodal, an embryonic morphogen from the TGF-β family, is also associated with the formation of vascular channels in melanoma [73]. Researchers have demonstrated that aggressive melanoma cells secrete Nodal, and its inhibition leads to reduced expression of VE-cadherin and an impaired ability of melanoma cells to form vascular networks. Nodal inhibition also reduces melanoma cell tumorigenicity and promotes reversion of melanoma cells to the melanocyte phenotype, highlighting the role of Nodal signaling in melanoma cell plasticity.
Recent insights into the metastatic process show that dissemination of tumor cells occurs very early in tumorigenesis, even before full transformation of primary tumors [74–76], and that disseminated or circulating tumor cells display CSC markers [76–78]. Therefore, alternative strategy to treat cancer would be therapeutic targeting of CSCs or circulating tumor cells at an early stage before metastasis to prevent the development of clinical manifestation of metastasis, which would reduce cancer morbidity and mortality.
Conclusion & future perspective
Advances in the cancer stem cell field are revealing the highly dynamic and complex nature of this cell population in tumors. It is apparent that the original models attempting to describe tumor cell heterogeneity fail to account for the many intrinsic and extrinsic factors affecting tumor heterogeneity and CSC lineage. Although this plasticity makes it challenging to accurately define, characterize and measure CSC populations, several studies have confirmed the presence of these populations in melanoma, and have shown an enhanced tumorigenic potential ability for these cells.
In order to develop effective therapeutics against CSCs, we must better understand the plasticity and genetic instability of these cells and the role played by the microenvironment, stress factors and somatic evolution. Establishing accurate methods of analyzing CSCs in tumors is also very important, as this will influence the direction of therapeutic strategies against this population in melanomas and other cancers. Despite all of these complexities, initial studies targeting MSC markers and phenotypes show promise, and MSC directed therapeutics may provide a much needed breakthrough in melanoma treatment.
Acknowledgements
The authors apologize to all their colleagues whose important work could not be directly cited.
Footnotes
Financial & competing interests disclosure
This study was supported in part by a research grant from the Veterans Affairs Merit Review Award (to M Fujita). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Desantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics 2014. CA Cancer J. Clin. 2014;64(4):252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Atlanta: American Cancer Society; 2014. Cancer Facts & Figures 2014.www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf [Google Scholar]
- 3.Holderfield M, Deuker MM, McCormick F, McMahon M. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat. Rev. Cancer. 2014;14(7):455–467. doi: 10.1038/nrc3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreso A, Dick JE. Evolution of the cancer stem cell model. Cell Stem Cell. 2014;14(3):275–291. doi: 10.1016/j.stem.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993;81(11):2844–2853. [PubMed] [Google Scholar]
- 6.Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells – perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]; •• This AACR workshop established a consensus definition for CSCs capable of self-renewal and differentiation into the daughter cells.
- 7.Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488(7412):522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driessens G, Beck B, Caauwe A, Simons BD, Blanpain C. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488(7412):527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schepers AG, Snippert HJ, Stange DE, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337(6095):730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 10.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367(6464):645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 11.Taussig DC, Miraki-Moud F, Anjos-Afonso F, et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood. 2008;112(3):568–575. doi: 10.1182/blood-2007-10-118331. [DOI] [PubMed] [Google Scholar]
- 12.Taussig DC, Vargaftig J, Miraki-Moud F, et al. Leukemia-initiating cells from some acute myeloid leukemia patients with mutated nucleophosmin reside in the CD34- fraction. Blood. 2010;115(10):1976–1984. doi: 10.1182/blood-2009-02-206565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarry JE, Murphy K, Perry R, et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J. Clin. Invest. 2011;121(1):384–395. doi: 10.1172/JCI41495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart JM, Shaw PA, Gedye C, Bernardini MQ, Neel BG, Ailles LE. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc. Natl Acad. Sci. USA. 2011;108(16):6468–6473. doi: 10.1073/pnas.1005529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441(7097):1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 16.Beck B, Blanpain C. Unravelling cancer stem cell potential. Nat. Rev. Cancer. 2013;13(10):727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 17.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer. 2007;7(11):834–846. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu TJ, Sun BC, Zhao XL, et al. CD133+ cells with cancer stem cell characteristics associates with vasculogenic mimicry in triple-negative breast cancer. Oncogene. 2013;32(5):544–553. doi: 10.1038/onc.2012.85. [DOI] [PubMed] [Google Scholar]
- 19.Dong J, Zhao Y, Huang Q, et al. Glioma stem/progenitor cells contribute to neovascularization via transdifferentiation. Stem Cell Rev. 2011;7(1):141–152. doi: 10.1007/s12015-010-9169-7. [DOI] [PubMed] [Google Scholar]
- 20.Cheng L, Huang Z, Zhou W, et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell. 2013;153(1):139–152. doi: 10.1016/j.cell.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frank NY, Schatton T, Kim S, et al. VEGFR-1 expressed by malignant melanoma-initiating cells is required for tumor growth. Cancer Res. 2011;71(4):1474–1485. doi: 10.1158/0008-5472.CAN-10-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruno S, Bussolati B, Grange C, et al. CD133+ renal progenitor cells contribute to tumor angiogenesis. Am. J. Pathol. 2006;169(6):2223–2235. doi: 10.2353/ajpath.2006.060498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlinger M, Rowan AJ, Horswell S, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Using whole-exome multiregion spatial sequencing, this study revealed a high degree of spatially separated intratumor genetic heterogeneity consistent with branched evolutionary tumor growth. Single tumor biopsy samples may not be representative of the entire tumor.
- 24.Bissell MJ, Hines WC. Why don't we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat. Med. 2011;17(3):320–329. doi: 10.1038/nm.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schatton T, Murphy GF, Frank NY, et al. Identification of cells initiating human melanomas. Nature. 2008;451(7176):345–349. doi: 10.1038/nature06489. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This study demonstrates that ABCB5 enriches tumorigenic melanoma cells. This is the first report of a CSC population in melanoma tumors.
- 26.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 27.Barroca V, Lassalle B, Coureuil M, et al. Mouse differentiating spermatogonia can generate germinal stem cells in vivo . Nat. Cell Biol. 2009;11(2):190–196. doi: 10.1038/ncb1826. [DOI] [PubMed] [Google Scholar]
- 28.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501(7467):328–337. doi: 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen WJ, Ho CC, Chang YL, et al. Cancer-associated fibroblasts regulate the plasticity of lung cancer stemness via paracrine signalling. Nat. Commun. 2014;5:3472. doi: 10.1038/ncomms4472. [DOI] [PubMed] [Google Scholar]
- 30.Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396(6712):643–649. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 31.Sjoblom T, Jones S, Wood LD, et al. The consensus coding sequences of human breast and colorectal cancers. Science. 2006;314(5797):268–274. doi: 10.1126/science.1133427. [DOI] [PubMed] [Google Scholar]
- 32.Fang D, Nguyen TK, Leishear K, et al. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 33.Boiko AD, Razorenova OV, Van De Rijn M, et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature. 2010;466(7302):133–137. doi: 10.1038/nature09161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Civenni G, Walter A, Kobert N, et al. Human CD271-positive melanoma stem cells associated with metastasis establish tumor heterogeneity and long-term growth. Cancer Res. 2011;71(8):3098–3109. doi: 10.1158/0008-5472.CAN-10-3997. [DOI] [PubMed] [Google Scholar]
- 35.Boonyaratanakornkit JB, Yue L, Strachan LR, et al. Selection of tumorigenic melanoma cells using ALDH. J. Invest. Dermatol. 2010;130(12):2799–2808. doi: 10.1038/jid.2010.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo Y, Dallaglio K, Chen Y, et al. ALDH1A isozymes are markers of human melanoma stem cells and potential therapeutic targets. Stem Cells. 2012;30(10):2100–2113. doi: 10.1002/stem.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilson BJ, Schatton T, Zhan Q, et al. ABCB5 identifies a therapy-refractory tumor cell population in colorectal cancer patients. Cancer Res. 2011;71(15):5307–5316. doi: 10.1158/0008-5472.CAN-11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumor initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 39.Hess DA, Meyerrose TE, Wirthlin L, et al. Functional characterization of highly purified human hematopoietic repopulating cells isolated according to aldehyde dehydrogenase activity. Blood. 2004;104(6):1648–1655. doi: 10.1182/blood-2004-02-0448. [DOI] [PubMed] [Google Scholar]
- 40.Pearce DJ, Taussig D, Simpson C, et al. Characterization of cells with a high aldehyde dehydrogenase activity from cord blood and acute myeloid leukemia samples. Stem Cells. 2005;23(6):752–760. doi: 10.1634/stemcells.2004-0292. [DOI] [PubMed] [Google Scholar]
- 41.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Den Hoogen C, Van Der Horst G, Cheung H, et al. High aldehyde dehydrogenase activity identifies tumor-initiating and metastasis-initiating cells in human prostate cancer. Cancer Res. 2010;70(12):5163–5173. doi: 10.1158/0008-5472.CAN-09-3806. [DOI] [PubMed] [Google Scholar]
- 43.Rasheed ZA, Yang J, Wang Q, et al. Prognostic significance of tumorigenic cells with mesenchymal features in pancreatic adenocarcinoma. J. Natl Cancer Inst. 2010;102(5):340–351. doi: 10.1093/jnci/djp535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carpentino JE, Hynes MJ, Appelman HD, et al. Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Res. 2009;69(20):8208–8215. doi: 10.1158/0008-5472.CAN-09-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Silva IA, Bai S, McLean K, et al. Aldehyde dehydrogenase in combination with CD133 defines angiogenic ovarian cancer stem cells that portend poor patient survival. Cancer Res. 2011;71(11):3991–4001. doi: 10.1158/0008-5472.CAN-10-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roesch A, Fukunaga-Kalabis M, Schmidt EC, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141(4):583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article reports the dynamic expression of JARI1B in melanoma and highlights potential contributions to melanoma heterogeneity and CSC plasticity.
- 47.Cheli Y, Giuliano S, Botton T, et al. Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene. 2011;30(20):2307–2318. doi: 10.1038/onc.2010.598. [DOI] [PubMed] [Google Scholar]
- 48.Cheli Y, Giuliano S, Fenouille N, et al. Hypoxia and MITF control metastatic behaviour in mouse and human melanoma cells. Oncogene. 2012;31(19):2461–2470. doi: 10.1038/onc.2011.425. [DOI] [PubMed] [Google Scholar]
- 49.Hoek KS, Goding CR. Cancer stem cells versus phenotype-switching in melanoma. Pigment Cell Melanoma Res. 2010;23(6):746–759. doi: 10.1111/j.1755-148X.2010.00757.x. [DOI] [PubMed] [Google Scholar]
- 50.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study demonstrates that CD44+CD24- enriches tumorigenic breast cancer cells. This is the first report of a CSC population in solid tumors.
- 51.Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67(3):1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 52.Ricci-Vitiani L, Lombardi DG, Pilozzi E, et al. Identification and expansion of human colon-cancer-initiating cells. Nature. 2007;445(7123):111–115. doi: 10.1038/nature05384. [DOI] [PubMed] [Google Scholar]
- 53.Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumor formation by single human melanoma cells. Nature. 2008;456(7222):593–598. doi: 10.1038/nature07567. [DOI] [PMC free article] [PubMed] [Google Scholar]; • This study shows that about 25% of human melanoma cells are capable of forming tumors in NOD/SCID IL2Rγ(null) mice, suggesting TICs may not be rare in human melanoma.
- 54.Ishizawa K, Rasheed ZA, Karisch R, et al. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell. 2010;7(3):279–282. doi: 10.1016/j.stem.2010.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ghadially R. The role of stem and circulating cells in cancer metastasis. J. Surg. Oncol. 2011;103(6):555–557. doi: 10.1002/jso.21807. [DOI] [PubMed] [Google Scholar]
- 56.Girouard SD, Murphy GF. Melanoma stem cells: not rare, but well done. Lab. Invest. 2011;91(5):647–664. doi: 10.1038/labinvest.2011.50. [DOI] [PubMed] [Google Scholar]
- 57.Fukunaga-Kalabis M, Roesch A, Herlyn M. From cancer stem cells to tumor maintenance in melanoma. J. Invest. Dermatol. 2011;131(8):1600–1604. doi: 10.1038/jid.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu C, Liu Y, Xu XX, et al. Potential effect of matrix stiffness on the enrichment of tumor initiating cells under three-dimensional culture conditions. Exp. Cell. Res. 2015;330(1):123–134. doi: 10.1016/j.yexcr.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 59.Talbot NC, Caperna TJ. Proteome array identification of bioactive soluble proteins/peptides in Matrigel: relevance to stem cell responses. Cytotechnology. 2014 doi: 10.1007/s10616-014-9727-y. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.BD Biosciences. BD Matrigel Matrix High Concentration Product Sheet. 2003. www.stemcell.so/images/upload/pdf/514ac150c7043.pdf
- 61.Luo Y, Ellis LZ, Dallaglio K, et al. Side population cells from human melanoma tumors reveal diverse mechanisms for chemoresistance. J. Invest. Dermatol. 2012;132(10):2440–2450. doi: 10.1038/jid.2012.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wouters J, Stas M, Gremeaux L, et al. The human melanoma side population displays molecular and functional characteristics of enriched chemoresistance and tumorigenesis. PLoS ONE. 2013;8(10):e76550. doi: 10.1371/journal.pone.0076550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Landsberg J, Kohlmeyer J, Renn M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature. 2012;490(7420):412–416. doi: 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 64.Furuta J, Inozume T, Harada K, Shimada S. CD271 on melanoma cell is an IFN-gamma-inducible immunosuppressive factor that mediates downregulation of melanoma antigens. J. Invest. Dermatol. 2014;134(5):1369–1377. doi: 10.1038/jid.2013.490. [DOI] [PubMed] [Google Scholar]
- 65.Schmidt P, Kopecky C, Hombach A, Zigrino P, Mauch C, Abken H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc. Natl Acad. Sci. USA. 2011;108(6):2474–2479. doi: 10.1073/pnas.1009069108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mukherjee N, Reuland SN, Lu Y, et al. Combining a BCL2 inhibitor with the retinoid derivative fenretinide targets melanoma cells Including melanoma initiating cells. J. Invest. Dermatol. 2014;135(3):842–850. doi: 10.1038/jid.2014.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang ME, Ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72(2):567–572. [Google Scholar]
- 68.Lim YC, Kang HJ, Kim YS, Choi EC. All-trans-retinoic acid inhibits growth of head and neck cancer stem cells by suppression of Wnt/beta-catenin pathway. Eur. J. Cancer. 2012;48(17):3310–3318. doi: 10.1016/j.ejca.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Piccirillo SG, Reynolds BA, Zanetti N, et al. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumor-initiating cells. Nature. 2006;444(7120):761–765. doi: 10.1038/nature05349. [DOI] [PubMed] [Google Scholar]
- 70.Lombardo Y, Scopelliti A, Cammareri P, et al. Bone morphogenetic protein 4 induces differentiation of colorectal cancer stem cells and increases their response to chemotherapy in mice. Gastroenterology. 2011;140(1):297–309. doi: 10.1053/j.gastro.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Maniotis AJ, Folberg R, Hess A, et al. Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry. Am. J. Pathol. 1999;155(3):739–752. doi: 10.1016/S0002-9440(10)65173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hendrix MJ, Seftor EA, Hess AR, Seftor RE. Vasculogenic mimicry and tumour-cell plasticity: lessons from melanoma. Nat. Rev. Cancer. 2003;3(6):411–421. doi: 10.1038/nrc1092. [DOI] [PubMed] [Google Scholar]
- 73.Topczewska JM, Postovit LM, Margaryan NV, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat. Med. 2006;12(8):925–932. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 74.Husemann Y, Geigl JB, Schubert F, et al. Systemic spread is an early step in breast cancer. Cancer Cell. 2008;13(1):58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 75.Klein CA. Parallel progression of primary tumours and metastases. Nat. Rev. Cancer. 2009;9(4):302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 76.Weng D, Penzner JH, Song B, Koido S, Calderwood SK, Gong J. Metastasis is an early event in mouse mammary carcinomas and is associated with cells bearing stem cell markers. Breast Cancer Res. BCR. 2012;14(1):R18. doi: 10.1186/bcr3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aktas B, Tewes M, Fehm T, Hauch S, Kimmig R, Kasimir-Bauer S. Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res. BCR. 2009;11(4):R46. doi: 10.1186/bcr2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reid AL, Millward M, Pearce R, et al. Markers of circulating tumor cells in the peripheral blood of patients with melanoma correlate with disease recurrence and progression. Br. J. Dermatol. 2013;168(1):85–92. doi: 10.1111/bjd.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]