Abstract
A synthesis of the 1,2-isoxazolidine fragment of the potent voltage gated sodium channel blocker, zetekitoxin AB is described. The synthesis utilizes an intramolecular nitrone –olefin 1,3-dipolar cycloaddition to establish the stereochemistry of the cis-1,2-isoxazolidine. The oxidative cleavage of an all anti-triol with the excision of the central carbon is central to using α-D-glucopyranoside as a traceless stereochemical template. This route furnishes a suitably protected synthon for the synthesis of zetekitoxin AB.
Keywords: Zetikitoxin AB; saxitoxin; dipolar cycloaddition; 1,2-isoxazolidine; nitrone
Graphical Abstract
1. Introduction
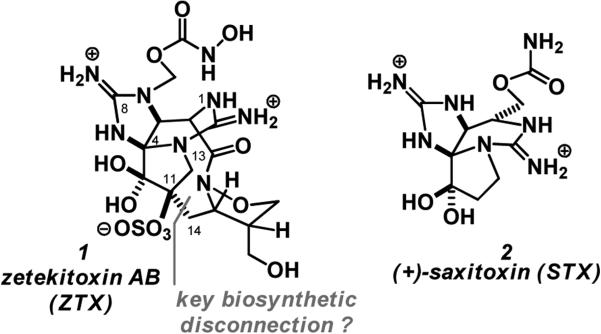
In 1969, H. S. Mosher and colleagues isolated the potent sodium ion channel blocker zetekitoxin AB (ZTX) from the skin extracts of a Panamanian golden frog, Atelopus zeteki.1 ZTX was isolated by bioassay guided fractionation for acute toxicity (LD50 = 11 μg/kg, i.p. mouse) and shown to be a very potent (pM) antagonist of the Nav, with potencies ranging from ~60-500 fold more than those for STX (2) based on tissue type and isoform distribution. In 2004, 30+ years after isolation, Yamashita and coworkers disclosed the magnificent structure of ZTX (1) using just 0.3 mg of the isolated sample.2 Similar to the related natural product saxitoxin (2), ZTX also contains a tricyclic bis-guanidine core. However, ZTX also includes a 1,2-isoxazolidine group within a 10 membered macrocyclic ring, a pendant N-hydroxycarbamate and a sulfate ester (Figure 1).2 Due to the classification of A. zeteki as an endangered species, this 0.3 mg sample remains the only purified of ZTX. Given the utility of ZTX as a probe for ion channel physiology, its unique structure and its exquisite rarity, total synthesis provides the only current avenue to secure more material for biological investigations and several groups have initiated programs in pursuit of the total synthesis of ZTX.3,4 These synthetic approaches have also revealed unique spectroscopic anomalies in the structural features in ZTX. For example, Nishikawa first noted the discrepancy of the 13C chemical shift of the amide carbonyl (C13) in ZTX (δ = 156.5 ppm) compared to most N-alkoxyamides (δ = 170-175 ppm) and showed that inductive effects of a tethered guanidinium ion in model 1,2-isoxazolidine systems could still not account for the unsusual chemical shift (δ = 167-173 ppm).4a This was further confirmed in a more elaborate bicyclic guanidine model.4b
Figure 1.
Structures of zetekitoxin AB 1 and saxitoxin 2.
Although neither report had the correct carbon framework of the 1,2-isoxazolidine fragment, it is clear that factors that affect the 13C environment are unique and suggests that ZTX will provide a rich medium for structural and synthetic studies.
This and our immediate access to STX (2) and its analogues, piqued our interest in the structure of ZTX (1).5 It is known that the pyrrolidine ring in STX is derived from arginine and thus likely the same in ZTX and further unlikely that nature elaborates this ring until late in its production.6 Since C10 in STX is oxidized one could imagine that enol/keto tautomerization produces a high enol content at C11 and this reacts with an electrophilic carbon at C14 in the biosynthesis of ZTX (Figure 1). Herein we describe the synthesis of a suitably protected 1,2-isoxazolidine derivative, with the correct carbon framework, to explore this strategy for the synthesis of ZTX.
2. Results and discussion
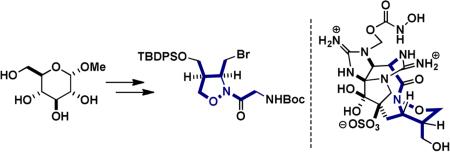
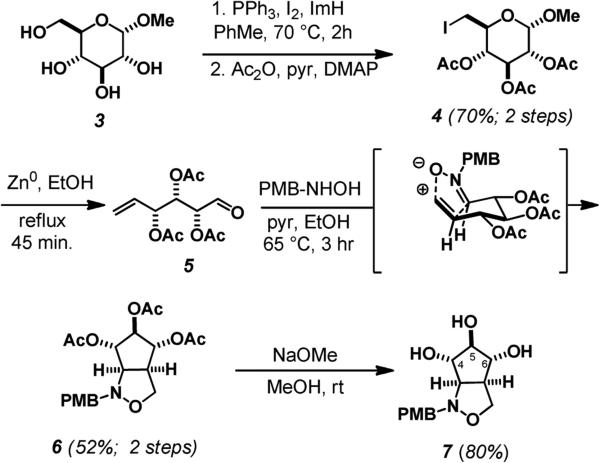
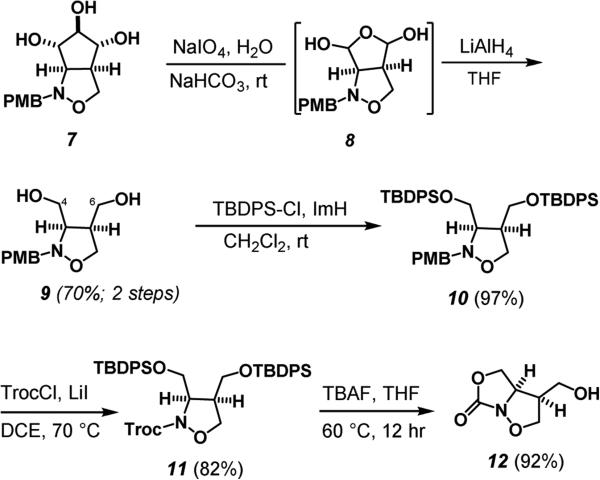
Our synthetic approach toward the 1,2-isooxazolidine fragment began by regioselectively converting the primary hydroxyl group of methyl α-D-glucopyranoside (3) to the iodide and acetylation of the remaining alcohols to give α-D-2,3,4 triacetoxy-6-deoxy-6-iodoglucopyranoside 4 in 70% yield.6 According to methods developed by Bernet and Vasella, reductive fragmentation of 4 with zinc in refluxing ethanol gave the aldehyde 5.7 Without further purification, the aldehyde was condensed with p-methoxybenzylhydroxylamine to afford the nitrone. Heating in ethanol triggered an intramolecular nitroneolefin [3+2] cycloaddition, proceeding through a predictable transition state in which all acetates adopt an equatorial arrangement, to afford the cis-fused bicyclic isoxazolidine 6 in 52% yield as a single stereoisomer.7,8 Deacetylation of 6 was carried out with sodium methoxide to afford the product triol 7 in 80% yield (Scheme 1).8
Scheme 1.
The key 1,3-dipolar cycloaddition.
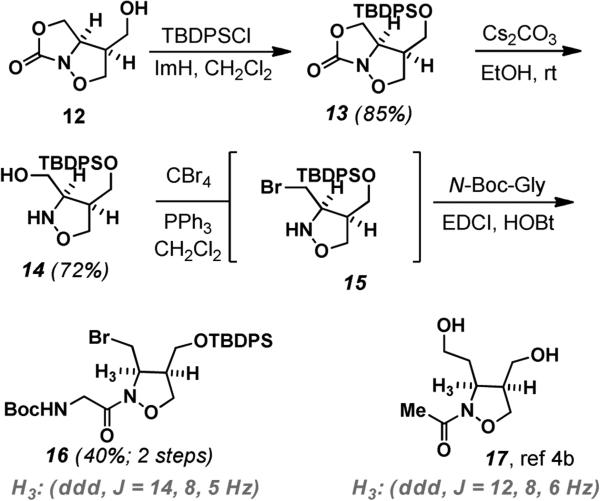
Having set the requisite cis-stereochemistry of the isoxazolidine we focused our attention on the cleavage of anti-triol 7 with the excision of C5 to remove the initial stereochemical information provided by the pyranoside. After exploring various conditions, sodium periodate and NaHCO3 gave an unstable bis-aldehyde hydrate 8 as a mixture of diastereomers. In-situ reduction with LiAlH4 reduction at room temperature afforded the 3,4-dihydroxymethyl isoxazolidine 9 in 70% overall yield.9,10 Attempts to differentially protect the C4 and C6 alcohols at this point proved unsuccessful. Oxidative strategies to cyclize the C6 alcohol on the benzylic carbon of the PMB group were also unsuccessful, leading to decomposition of the substrate. An alternative strategy would engage the C6 alcohol and the amine as an oxazolidinone. Protection of two primary hydroxyl groups with tert-butyldiphenylsilyl chloride gave the di-TBDPS protected species 10 in 97% yield. Von Braun type cleavage of the PMB group via the reaction with 2,2,2-trichloroethoxycarbonyl chloride (TrocCl) gave the Troc protected 1,2-isoxazolidine derivative 11 in 82% yield (Scheme 3). The Troc group allowed us to effectively distinguish the two primary hydroxyl groups by selectively trapping one of the primary hydroxyl groups as a cyclic carbamate. This was accomplished by treatment of 11 with tetra-n-butylammonium fluoride to give 12 in 92% yield.
Scheme 3.
Synthesis of a peptide coupling substrate.
Re-silylation of the C4 hydroxyl group as the TBDPS ether gave the protected cyclic carbamate 13 in 85% yield (Scheme 3). Base mediated hydrolysis (Cs2CO3 in EtOH) of cyclic carbamate 13 furnished the amino alcohol 14 in 72% yield. Using N-Bocglycine as a surrogate for the saxitoxin core, we attempted a coupling with the amino alcohol 14 using EDCI, HOBt or HATU. This resulted in a mixture of undesired isoxazolidineglycine ester and amide. Whether this mixture arises from direct acylation of the alcohol or a facile N→O acyl migration from the isoxazolidine is unclear but argued for activation of the C6 hydroxyl group prior to peptide coupling. Treatment of 14 with carbon tetrabromide and triphenylphosphine provided the aminobromide 15 which was unstable when isolated. Coupling of this intermediate, in situ, with N-Boc-Gly-OH using standard EDCI, HOBt coupling conditions afforded the protected isoxazolidineglycine amide 16 in 40% overall yield.
We had some concern with the stability of the sensitive bis-aldehyde hydrate 8 and the potential for epimerization. Comparison of the coupling constants for H3 in our final product 16 (H3: J = 14, 8, 5 Hz) with Nishikawa's N-acetylisoxazolidine 17 ((H3: J = 12, 8, 6 Hz) indicates that the cis-stereochemistry has been maintained. The 13C chemical shift of the isoxazolidine amide carbonyl in 16 is 171.0 ppm, consistent with the previously published models4a,b and suggest that if the structure of ZTX is correct, a significant decoupling of the isoxazolidine nitrogen non-bonding electrons from the carbonyl, or complex shielding phenomenon imposed by the rigid three dimensional structure of ZTX, significantly effects this carbonyl.
In conclusion we have developed a synthesis of the 1,2-isoxazolidine fragment of ZTX. Our synthesis features an intramolecular nitrone-olefin cycloaddition to establish the cis-fused isoxazolidine. This strategy uses α-D-glucopyranoside as a sterochemical template to relay the absolute stereochemistry required for this fragment. The oxidative cleavage of an all anti-triol with excision of the central carbon serves to remove all of the stereochemical information presented in the original template. This approach allows us to prepare gram quantities of 14, a suitably protected synthon to explore a C11 - C14 coupling strategy for the synthesis of ZTX.
Supplementary Material
Scheme 2.
Synthesis of a differentially protected diol.
Acknowledgments
We are grateful to the NIH, General Medical Sciences (R01 GM090082) for financial support. We are grateful to Dr. Paul R. Sebahar for insightful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary data (experimental procedures, characterization data for new compounds) associated with this article can be found, in the online version.
References and notes
- 1.a Shindelman J, Mosher HS, Fuhrman FA. Toxicon. 1969;7:315. doi: 10.1016/0041-0101(69)90031-2. [DOI] [PubMed] [Google Scholar]; b Fuhrman FA, Fuhrman GJ, Mosher HS. Science. 1969;165:1376. doi: 10.1126/science.165.3900.1376. [DOI] [PubMed] [Google Scholar]
- 2.Yotsu-Yamashita M, Kim YH, Dudley SC, Jr., Choudhary G, Pfahnl A, Oshima Y, Daly JW. Proc. Natl. Acad. Sci. USA. 2004;101:4346. doi: 10.1073/pnas.0400368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peasrson AD, Williams RM. Tetrahedron. 2014;70:7942. doi: 10.1016/j.tet.2014.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a Nishikawa T, Wang C, Akimoto T, Koshino H, Nagasawa K. Asain J. Org. Chem. 2014;3:1308. [Google Scholar]; b Nishikawa T, Urabe D, Isobe M. Heterocycles. 2009;79:379. [Google Scholar]
- 5.Bhonde VR, Looper RE. J. Am. Chem. Soc. 2011;133:20172. doi: 10.1021/ja2098063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller JN, Pongdee R. Tetrahedron Lett. 2013;54:3185. doi: 10.1016/j.tetlet.2013.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernet B, Vasella A. Helv. Chim. Acta. 1979;62:1990. [Google Scholar]
- 8.a Ferrier RJ, Furneaux RH, Prasit P, Tyler PC. J. Chem. Soc., Perkin Trans. 1983;1:1621. [Google Scholar]; b Dransfield PJ, Moutel S, Shipman M, Sik V. J. Chem. Soc., Perkin Trans. 1999;1:3349. [Google Scholar]
- 9.Ogawa S, Orihara M. Carbohydrate Research. 1989;189:323. [Google Scholar]
- 10.Baumgartner H, O'Sullivan AC, Schneider J. Heterocycles. 1997;45:1537. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.