Abstract
Objective
To investigate the impact of tuberculosis (TB)-associated immune reconstitution syndrome (IRIS) upon immunological recovery and the T cell compartment after initiation of TB and antiretroviral therapy (ART).
Design and methods
We prospectively evaluated T cell immunophenotypes by flow cytometry and cytokines by Luminex assays in a subset (n=154) of highly immunosuppressed HIV+ patients with TB from the CAMELIA randomized clinical trial. We compared findings from patients who developed TB-IRIS to findings from patients who did not develop TB-IRIS. Data were evaluated with mixed effect linear regression, Kaplan-Meier estimates, and Wilcoxon rank sum tests, and q-values were calculated to control for multiple comparisons.
Results
Development of TB-IRIS was associated with significantly greater pre-ART frequencies of HLA-DR+CD45RO+CD4+, CCR5+CD4+, OX40+CD4+, and Fas+ effector memory (EM) CD8+ T cells, and significantly elevated levels of plasma IL-6, IL-1β, IL-8, and IL-10 and viral load. Post-ART initiation, EM CD4+ and Fas+ EM CD4+ T cell frequencies significantly expanded, and central memory (CM) CD4+ T cell frequencies significantly contracted in patients who experienced TB-IRIS. By week 34 post-TB treatment initiation, EM/CM CD4+ T cell ratios were markedly higher in TB-IRIS versus non-TB-IRIS patients.
Conclusions
A distinct pattern of pre-ART T cell and cytokine markers appear to poise the immune response to develop TB-IRIS. Experience of TB-IRIS is then associated with long-term remodeling of the CD4+ T cell memory compartment towards an EM-dominated phenotype. We speculate that these pre- and post-ART TB-IRIS-associated immune parameters may contribute to superior immune control of TB/HIV co-infection and better clinical outcome.
Keywords: TB, HIV, TB-IRIS, immunosuppression, ART, T cells, effector memory, CCR5+CD4+, activated T cells, CAMELIA trial
INTRODUCTION
When antiretroviral treatment (ART) is initiated in rapid succession to tuberculosis (TB) therapy in HIV/TB co-infected patients, risk of immune reconstitution inflammatory syndrome (IRIS) increases [1–4]. Typically, TB-IRIS occurs after the start of TB therapy and after initial improvement of TB symptoms [5], and is characterized by clinical deterioration after ART initiation that manifests in fever, enlarged lymph nodes, and radiological features of TB disease not associated with treatment failure due to mycobacterial resistance, poor adherence to the treatment regimen, or another opportunistic infection (OI) [5, 6].
The Cambodian Early versus Late Introduction of Antiretrovirals (CAMELIA) randomized clinical trial (ANRS1295/CIPRA KH001) demonstrated that initiation of ART at 2 weeks (early arm) as compared to 8 weeks (late arm) after TB treatment initiation significantly decreased mortality by 34% in highly immunocompromised HIV-positive adults (median CD4+ T cell count = 25/mm3) with newly diagnosed TB [1, 7]. Early ART was also associated with a significantly increased risk (2.5-fold) of TB-IRIS in the CAMELIA study [1, 7]. Notably, the survival benefit of early ART in CAMELIA was observed up to three years after the earlier ART timing intervention [1, 7].
To investigate immunological recovery in TB/HIV patients and the mechanisms underlying TB-IRIS, we nested a prospective sub-study (Cambodian Paradoxical Reaction Immune Study-T cells or “CAPRI-T” (ANRS 12164)) within the CAMELIA trial. We enrolled patients from both treatment arms at the time of their entry into the CAMELIA trial and performed extensive immunophenotypic and cytokine profiling on patient samples after TB treatment initiation (prior to the start of ART), and at several timepoints post-ART initiation up to 34 weeks after the start of TB treatment.
METHODS
Study population, design, implementation and oversight
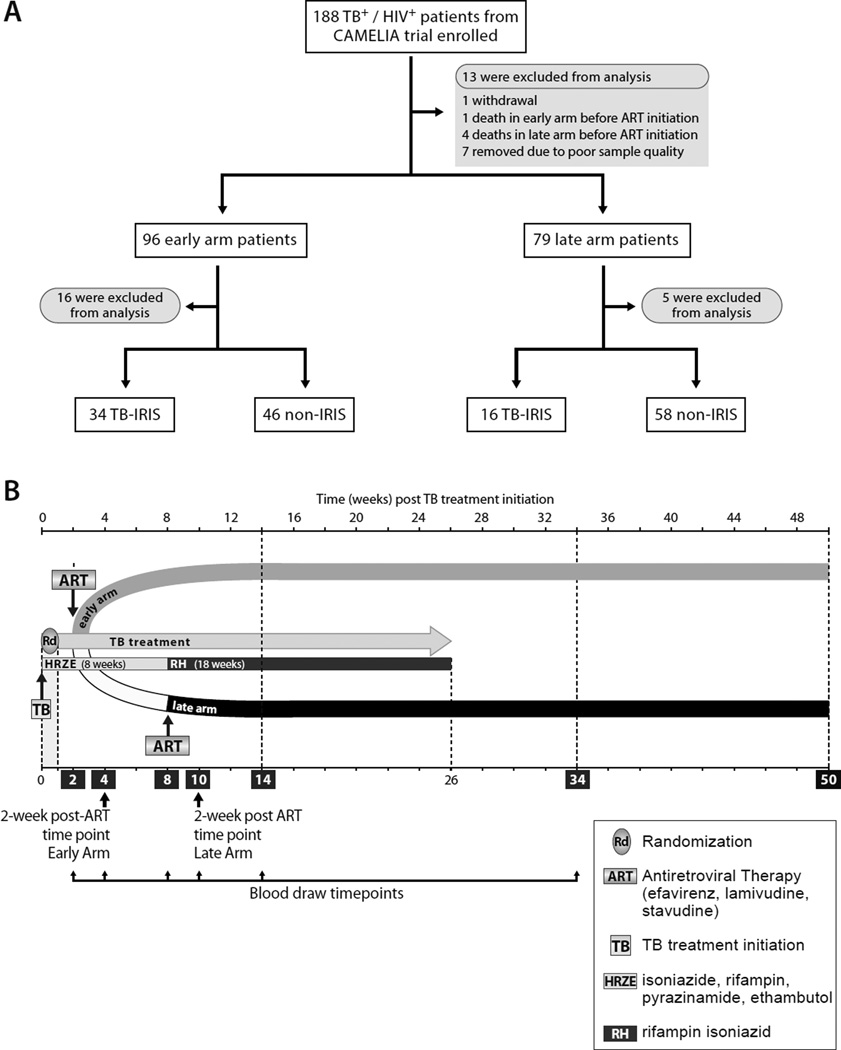
The CAMELIA trial was a prospective, multi-center, open-label superiority trial conducted in Cambodia that enrolled HIV-positive adults with a CD4+ T cell count ≤200/mm3 and newly diagnosed TB as confirmed by any clinical sample that was smear-positive for acid-fast bacilli [1]. Patients were randomly enrolled in the CAPRI-T substudy from both arms of the CAMELIA after enrollment in the CAMELIA (Fig 1A). After written informed consent was provided, 10 ml of blood was drawn at the timepoints shown in Fig 1B. CD4+T cell counts and HIV viral loads were determined at the Institut Pasteur in Cambodia as described [1]. Pre-ART clinical characteristics were taken from the CAMELIA database. See Supplementary Data for additional details.
Figure 1. Screening, enrollment, follow-up.
A. Flow chart of CAMELIA patient recruitment for the CAPRI-T sub-study. Of the 188 CAMELIA patients who provided informed consent for participation in the CAPRI-T study, 13 were excluded for the reasons shown in the figure, and an additional 21 were excluded after initial suspicion of TB-IRIS, which was not subsequently clinically validated.
B. Schema showing how the CAPRI-T sub-study was nested within the CAMELIA clinical trial and the timing of blood samples relative to initiation of TB treatment and ART. The week 2 post-ART blood collection timepoint in the early and late CAMELIA treatment arms is indicated in the figure.
Characterization of TB-IRIS
Characterization of TB-IRIS was a secondary objective of the CAMELIA trial and has been described elsewhere [1, 7]. See Supplementary Data for details.
Ethics Statement
The CAPRI-T and CAMELIA study protocols and consent forms were approved by the National Ethics Committee of Cambodia, the NIH (CSRC), and institutional review boards of the Institut Pasteur, France, and the Immune Disease Institute, (now the Program in Cellular and Molecular Medicine, Children’s Hospital) of Boston, MA, USA. All work was conducted according to the principles expressed in the Declaration of Helsinki.
T cell immunophenotyping
Phenotypic studies were performed on freshly isolated whole blood. After staining with antibodies, cells were processed by standard lyse/wash procedure and acquired on a four-color BD FACScalibur II cytometer (BD, Paris, France) on site at the Institut Pasteur in Cambodia. All flow cytometry data were analyzed using Flow-Jo 8.8.4 software (Tree Star Inc). Lymphocytes gated by light scattering were verified to be >90% pure using standard CD14/CD45 back-gating methods [8], and samples were excluded if this threshold was not met. Panels of antibody conjugates used for staining are shown in Suppl. Table 1, and a representative example of gating strategy is shown in Suppl. Fig 1.
Plasma cytokine measurements
Plasma levels of IL-1β, IL-2, IL-4, IL-6, IL-7, IL-8, IL-10, IL-12, IL-17, GMCSF, IFN-γ and TNF were quantified with Bio-Plex (Bio-Rad) and Luminex (Life Technologies) assay systems. Assays were performed in duplicate, with company-provided controls, to minimize intra- and inter-assay variation.
Statistics
Mixed effect linear regression was used to characterize progression of the phenotypic markers under ART, with time 0 being ART initiation; the effects of TB-IRIS and of the early or late arm on both phenotype frequency at ART initiation and changes in its progression were investigated. Phenotypic markers were first checked for normal distribution using Kernel density plots, and a square root transformation was used when the distribution was not normal. Based on non-parametric representations of the markers’ progression over time that were inferred from the regression models we validated that the progression looked linear. The Wilcoxon rank sum test was performed on all immunophenotypes at week 34 post-TB treatment initiation (week 32 post-ART in the early arm and week 26 post-ART in the late arm, respectively) between TBIRIS and non-TB-IRIS patients. Supplementary Tables 2–5 shows p- and q-values for the different analyses.
Association of specific phenotypes with the risk of occurrence of TB-IRIS was described using Kaplan-Meier estimates after stratifying by phenotype frequency (≤ versus > median at ART initiation) and after comparison between groups was done by log-rank test.
Statistical analyses were performed using the Stata 11 (Stata Corporation, College Station, Texas, USA) and the FDR (false discovery rate) correction was performed using the R software (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org).
RESULTS
Impact of timing of ART, viral load and CD4+ T cell count on TB-IRIS
Of the 154 patients included in the CAPRI-T study, 50 developed clinically validated TB-IRIS secondary to TB treatment initiation (Fig. 1A). We note that all other potential causes of IRIS (aside from TB) were ruled out (Supplementary data). [1, 7]. None of the 104 non-TB-IRIS patients exhibited signs or symptoms of TB-IRIS at any time during their clinical course. TB-IRIS occurred at a median of 12 days (inter-quartile range (IQR): 7–24) post-ART initiation and occurred at similar times post-ART in both CAMELIA treatment arms (median of 11.5 and 16 days, respectively; p=0.27). TB-IRIS was significantly more common in the early (34/80; 42%) versus late (16/74; 22%) (p=0.003) treatment arm, consistent with findings in the overall CAMELIA cohort [1, 7].
At ART initiation, the CD4+ T cell count in TB-IRIS patients and non-TBIRIS patients was similar (median: 22/mm3 (IQR: 11–52) and 32/mm3 (IQR: 15–71), respectively; p=0.10), as was body mass index (BMI) (median: 17.6 (IQR 16–19) and median: 17.8 (IQR 16–20), respectively; p=0.88). However, pre-ART viral load was significantly higher in TB-IRIS patients versus non-TB-IRIS patients (median: 5.9 (IQR: 5.4–6.4) vs. 5.6 (IQR: 5.2–6.1) log copies/ml, respectively; p=0.04). Other baseline characteristics of TB-IRIS and non-TB-IRIS patients in the CAPRI-T study were similar, consistent with the overall CAMELIA cohort (Suppl. Table 6) [7].
TB-IRIS and activated and regulatory T cells
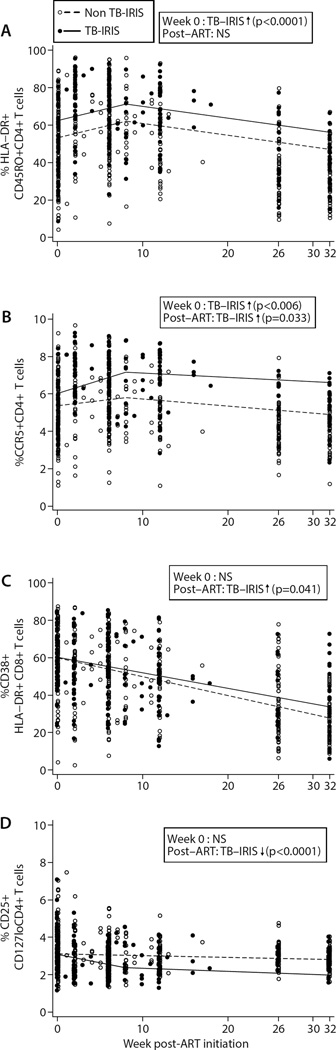
Prior to ART initiation, patients who went on to develop TB-IRIS had a significantly greater frequency of activated (CD45RO+HLA-DR+) CD4+ T cells compared to non-TB-IRIS patients (p<0.0001) (Fig 2A). This subset of cells remained significantly higher in the TB-IRIS group at week 34 post-TB therapy initiation (p=0.0003), which was week 32 and week 26 post-ART in the early and late CAMELIA treatment arms, respectively (Fig 1B). Pre-ART, TB-IRIS patients also had significantly higher proportions of CCR5+CD4+ T cells (p=0.006) (Fig 2B), which remained markedly higher at week 34 relative to the non-TB-IRIS group (p<0.0001)(Fig 2B). By contrast, proportions of activated CD8+ (HLADR+ CD38+) T cells at the start of ART were not significantly different in the two groups (p=0.91), although there was a greater post-ART decrease in activated CD8+ T cell frequency in non-TB-IRIS patients (p=0.041; Fig 2C).
Figure 2. Markers of CD4+ and CD8+ T cell activation and regulatory activity in TB-IRIS and non-TB-IRIS patients.
T cell immunophenotypes were obtained on whole blood samples. Lines depict mean progression over time deduced from mixed effect linear regression models in each patient group for the T cell subset shown. TB-IRIS patients (filled circles and solid line) and non-TB-IRIS patients (open circles and dashed line) are shown at the actual time of sample analysis post-ART initiation. Significant differences obtained from regression analysis in the frequency of each T cell subset at week 0 of ART and/or its rate of change post-ART initiation are indicated in each figure (see supplementary tables 2 and 3 for full list of p- and q-values). NS= not significant; up arrow = higher in TB-IRIS; down arrow = lower in TB-IRIS. For CCR5+CD4+ T cells and CD4+ Tregs, regression plots were generated with square root transformation due to non-normal distribution. A) Activated (CD45RO+HLA-DR+) CD4+ T cells; B) CCR5+CD4+ T cells; C) Activated (CD38+HLA-DR+) CD8+ T cells; D) CD4+ regulatory T cells. So the spread of values in each subgroup (TB-IRIS vs. non-TB-IRIS) can be better appreciated, the two patient groups are shown immediately adjacent to one another at weeks 0, 2, 6, 8, 26, and 32 post-ART in Suppl. Figs 4A–D.
To determine whether regulatory CD4+ T cell (CD4+ Treg) frequencies differed between TB-IRIS and non-TB-IRIS patients we employed surface staining to enumerate CD25+CD127lo CD4+ T cells [9–11]. CD4+ Treg frequencies were similar in the two groups prior to ART initiation (p=0.72; Fig 2D). However, patients who experienced TB-IRIS exhibited a much greater post-ART rate of decline in CD4+ Treg frequency (p<0.0001), resulting in a significantly lower proportion of CD4+ Tregs in TB-IRIS patients as compared to non-TB-IRIS patients at week 34 post-TB therapy initiation (p=0.034) (Fig 2D).
TB-IRIS and T cell memory
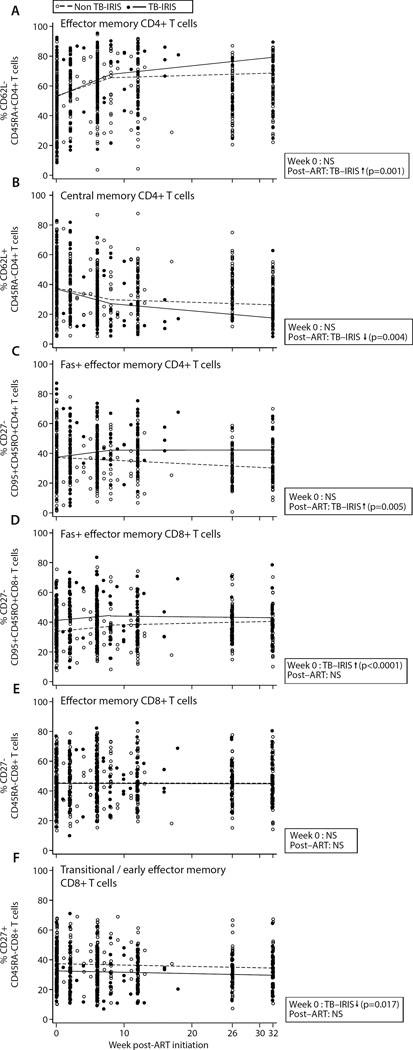
To evaluate the impact of TB-IRIS on the reconstitution of antigen experienced T cell subsets, we next studied changes in effector memory (EM) and central memory (CM) CD4+ T cell proportions and their association with TBIRIS. At ART initiation, there was no appreciable difference between EM (CD62L-CD45RA-) and CM (CD62L+CD45RA-) CD4+ T cell frequencies in patients who did or did not develop TB-IRIS (p=0.96 and p=0.48, respectively) (Figs 3A and B). Strikingly, however, EM and CM CD4+ T cell frequencies diverged significantly in the two patient groups after ART initiation. The EM CD4+ T cell frequency rose, and the CM CD4+ T cell frequency declined, at significantly greater rates in TB-IRIS patients (p=0.001 and p=0.004, respectively), and by week 34 TB-IRIS patients had significantly higher EM CD4+ T cell proportions (p=0.002) and significantly lower CM CD4+ T cell proportions (p=0.011) (Fig 3A and 3B).
Figure 3. Markers of CD4+ and CD8+ T cell memory differentiation in TBIRIS and non-TB-IRIS patients.
T cell immunophenotypes were obtained on whole blood samples. Lines depict mean progression over time deduced from mixed effect linear regression models in each patient group for the T cell subset shown. TB-IRIS patients (filled circles and solid line) and non-TB-IRIS patients (open circles and dashed line) are shown at the actual time of sample analysis post-ART initiation. Significant differences obtained from regression analysis in the frequency of each T cell subset at week 0 of ART and/or its rate of change post-ART initiation are indicated in each figure (see supplementary tables 2 and 3 for full list of p- and qvalues). NS= not significant; up arrow = higher in TB-IRIS; down arrow = lower in TB-IRIS A) Effector memory (CD62L-CD45RA-) CD4+ T cells; B) Central memory (CD62L+CD45RA−) CD4+ T cells; C) Fas+ effector memory (CD27−CD95+CD45RO+) CD4+ T cells; D) Fas+ effector memory (CD27−CD95+CD45RO+) CD8+ T cells; E) Effector memory (CD27−CD45RA−) CD8+ T cells; F) Transitional/early effector memory (CD27+CD45RA−) CD8+ T cells. So the spread of values in each subgroup (TB-IRIS vs. non-TB-IRIS) can be better appreciated, the two patient groups are shown immediately adjacent to one another at weeks 0, 2, 6, 8, 26, and 32 post-ART in Suppl. Figs 5A–F.
The frequency of Fas+ (CD95+) EM CD4+ T cells (CD27-CD45RO+), indicative of late-stage EM cells, was also similar in non-TB-IRIS and TB-IRIS patients at ART initiation (p=0.40). Post-ART, however, the frequency of Fas+ EM CD4+ T cells rose significantly in TB-IRIS patients (p=0.005) and at week 34 was markedly higher in this group (p=0.003) (Fig 3C).
The proportion of Fas+ EM (CD27-CD45RA-) CD8+ T cells was significantly higher in TB-IRIS patients at ART initiation (p<0.0001; Fig 3D), although the overall EM CD8+ T cell frequency was similar between the groups at week 0 (p=0.06). The proportion of CD8+ T cells with a CM/early EM or ‘transitional’ phenotype [12–14] (CD27+CD45RA-) was also significantly lower in TB-IRIS patients (p=0.017) (Fig 3F). By week 34 there were no differences between TB-IRIS and non-TB-IRIS patients in proportions of Fas+ EM CD8+ T cells (p=0.075), EM CD8+ T cells (p=0.067), or CM/early EM transitional memory CD8+ T cells (p=0.072).
TB-IRIS and T cell co-stimulatory signals
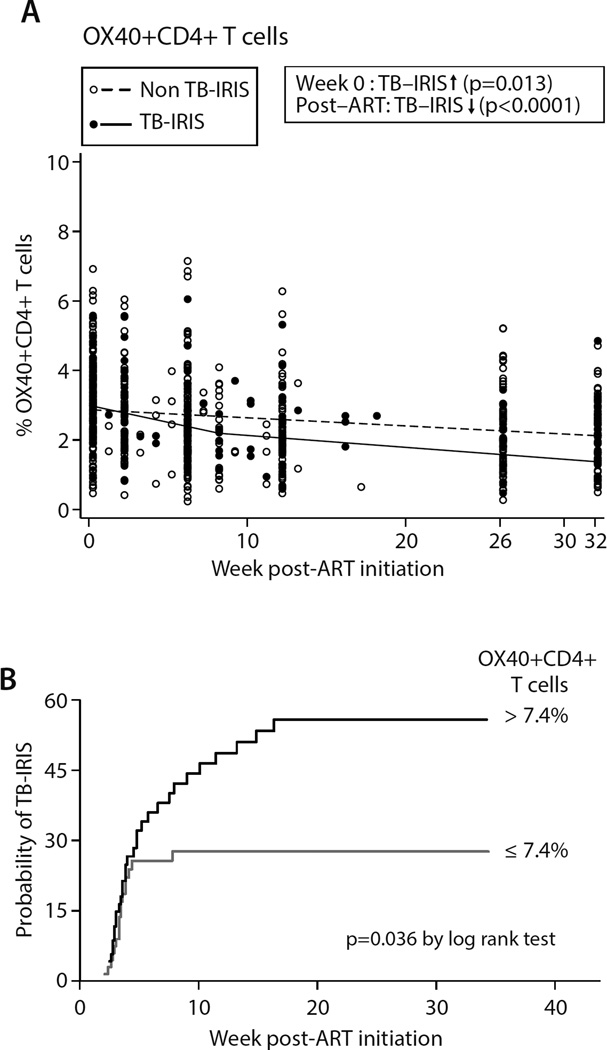
We next evaluated a panel of CD4+ or CD8+ co-stimulatory markers. We found no differences in proportions of ICOS+CD4+ cells or CD28+CD8+ or PD-1+CD8+ cells prior to ART or at week 34 (Suppl. Figs 2A–C). By contrast, OX40+CD4+ T cell proportions were significantly elevated (p=0.013) at ART initiation in TB-IRIS relative to non-TB-IRIS patients (Fig 4A), and post-ART this cellular subpopulation declined to a much greater extent in the TB-IRIS group, leading to similar OX40+CD4+ T cell frequencies in the two groups (p=0.15).
Figure 4. Differences in OX40+CD4+ T cell frequencies between TB-IRIS and non-TB-IRIS patients.
OX40+CD4+ T cell frequencies were measured in whole blood samples. Lines depict mean progression over time deduced from mixed effect linear regression models. TB-IRIS patients (filled circles and solid line) and non-TB-IRIS patients (open circles and dashed line) are shown at the actual time of sample analysis post-ART initiation in the left panel. Significant differences obtained from regression analysis in the frequency of OX40+CD4+ T cells at week 0 of ART and/or its rate of change post-ART initiation are indicated (see supplementary tables 2 and 3 for full list of p- and q-values). Up arrow = higher in TB-IRIS; down arrow = lower in TB-IRIS. Since OX40+CD4+ T cell frequencies were not normally distributed they were plotted using square root transformation. So the spread of values in each subgroup (TB-IRIS vs. non-TB-IRIS) can be better appreciated, the two patient groups are shown immediately adjacent to one another at weeks 0, 2, 6, 8, 26, and 32 post-ART in Suppl. Fig 6.
B. Plot depicting Kaplan–Meier estimates for probability of developing TBIRIS depending on pre-ART frequency of OX40+CD4+ T cells. Black line: patients with >7.4% OX40+CD4+ T cells at ART initiation; grey line: patients with ≤ 7.4% OX40+CD4+ T cells at ART initiation.
When we divided the 154 patients into two groups based on median OX40+CD4+ T cell frequency at ART initiation (median=7.4%), Kaplan-Meier analysis revealed that a pre-ART OX40+CD4+ T cell frequency >7.4% was associated with a significantly greater risk of developing TB-IRIS as compared to a frequency ≤7.4% (p=0.036) (H.R=1.8, 95% C.I. 1.03–3.36) (Fig. 4B). No other cellular phenotype was associated with TB-IRIS risk after similar stratification by median pre-ART phenotype frequency.
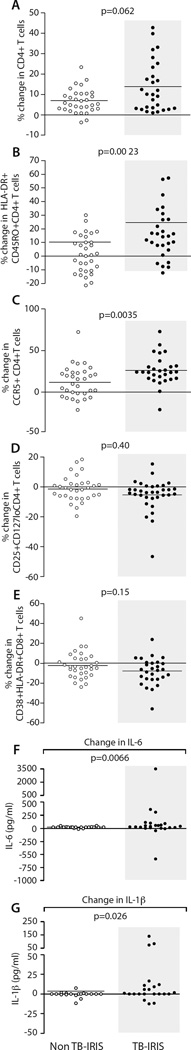
Cellular and cytokine changes between ART initiation and TB-IRIS
In order to evaluate early post-ART immune parameters associated with TB-IRIS development, we evaluated changes from week 0 of ART to the time of TB-IRIS in a randomly selected subgroup of TB-IRIS patients (n=32) who experienced TB-IRIS within 3 weeks (median of 10 days, IQR: 6–14) post-ART. As controls, we evaluated the same parameters in a randomly selected subgroup (n=28) of non-TB-IRIS patients who had a week 2 post-ART timepoint available (see Supplementary data). The net increase in CD4+ T cell frequency was similar between the two groups (Fig 5A); however, the net increase in HLA-DR+CD45RO+ and CCR5+ CD4+ T cell frequencies from ART initiation to the TB-IRIS timepoint was significantly greater (p=0.0023 and p=0.0035, respectively) than to the 2-week post-ART timepoint in non-TB-IRIS patients (Figs 5B and 5C). No differences in activated CD8+ and CD4+ Treg frequency increases were detected (Figs 5D and 5E). Among other subsets examined, only CD8+ T cells, but not CD4+ T cells, bearing a CD27+CD95-CD45RO− phenotype increased to a greater extent in the TB-IRIS group versus the non-TB-IRIS group (p=0.0085) (Suppl. Figs 3A–B).
Figure 5. Net changes in cellular phenotypes and plasma cytokines early post-ART in TB-IRIS and non-TB-IRIS patients.
The Wilcoxon rank sum test was used to compare the net change in cellular phenotype frequency (n=28) and plasma cytokine level (n=19) in non-TBIRIS patients from week 0 to week 2 and cellular phenotype frequency (n=32) and plasma cytokine level (n=23) in TB-IRIS patients from week 0 to the TB-RIIS event. P and q values for these analyses are presented in Suppl. Tables 4 and 5. A) CD3+CD4+ T cells; B) HLA-DR+CD45RO+CD4+ T cells; C) CCR5+CD4+ T cells; D) HLA-DR+CD38+CD8+ T cells; E) CD4+ regulatory T cells; F) plasma IL-6; and G) plasma IL-1β
We also measured plasma levels of 12 different cytokines by Luminex, which revealed that the net increases in IL-6 and IL-1β were significantly greater at the TB-IRIS event than at the 2-week post-ART timepoint in non-TB-patients (IL-6: p=0.0066 and IL-1β: p=0.026 (Figs 5F and 5G). Other cytokine differences included: (i) IL-1β, IL-6, IL-8 and IL-10 higher prior to ART and at the TB-IRIS timepoint; (ii) IL-17, IL-12, and TNF similar at ART initiation but higher at the time of TB-IRIS; and (iii) IL-2, IL-7, GM-CSF and IFN-γ similar at all timepoints (Suppl. Fig 3 and Supplementary Data).
DISCUSSION
We have identified distinct immunological patterns that differentiate TBIRIS and non-TB-IRIS patients both prior to ART and post-ART. Pre-ART, significantly higher frequencies of cellular subsets associated with T cell activation, including HLA-DR+/CD45RO+, CCR5+, and OX40+ CD4+ T cells and Fas+ EM CD8+ T cells were associated with TB-IRIS. Furthermore, prior to ART initiation, higher viral loads and significantly elevated levels of circulating IL-1β, IL-6, IL-8 and IL-10 were present in patients who developed TB-IRIS. Post-ART, EM CD4+ T cells and Fas+ EM CD4+ T cells increased, and CM CD4+ T cells decreased in TB-IRIS patients relative to non-TB-IRIS patients, and these differences remained evident even two months after completion of TB treatment and over 4 months after the vast majority of TB-IRIS events, when the last blood collection timepoint occurred.
Our ability to detect previously unidentified immunological parameters associated with risk of TB-IRIS, TB-IRIS pathogenesis, and post-TB-IRIS immune reconstitution was facilitated by our study design. The nesting of the prospective CAPRI-T study within the CAMELIA trial provided strategic advantages over other studies that were observational or retrospective in nature. These advantages included: (i) the ability to prospectively evaluate patients who went on to develop TB-IRIS and ‘control’ patients who avoided TB-IRIS from a single large cohort of treatment-naïve patients of similar ethnicity, all with profound HIV-associated immunosuppression and newly diagnosed AFB smearpositive TB; (ii) clinical TB-IRIS validation or exclusion by an experienced clinical team; and (iii) the sampling design, which included long-term follow-up, allowing us to evaluate the impact of TB-IRIS on ART-mediated T cell reconstitution.
Previous studies have reported increased global CD4+ T cell activation at the time of, or following, TB-IRIS [15, 16], or during IRIS precipitated by diverse opportunistic infections [17]. This is the first report, however, to demonstrate that activated CD4+ T cell frequencies are elevated prior to ART in TB+/HIV+ patients who go on to develop TB-IRIS. Furthermore, this pre-ART CD4+ T cell activation was accompanied by a significantly higher OX40+CD4+ T cell frequency, and the latter phenotype was predictive of TB-IRIS risk. We also found that the activated CD4+ T cell frequency rises more dramatically post-ART in the TB-IRIS group, confirming a previous report [16]. Taken together, these findings underscore the critical role of CD4+ T cells in the development of TB-IRIS, and clearly demonstrate that the pre-ART CD4+ T cell compartment is distinct in the subset of TB+/HIV+ patients who subsequently develop TB-IRIS. In agreement with other reports [16, 20, 21], pre-ART CD4+ Treg proportions were similar in both TB-IRIS and non-TB-IRIS patients, although there was a relatively greater post-ART decline in this CD4+ subpopulation in TB-IRIS patients.
Our finding that an elevated pre-ART CCR5+CD4+ T cell frequency was also associated with TB-IRIS development, combined with the relatively higher pre-ART viral loads in TB-IRIS patients, provides a novel link between pre-ART CCR5+CD4+ T cell levels, viral load, and TB-IRIS occurrence. Although a recent small study reported that CCR5+CD4+ T cell proportions were higher in TB-IRIS versus non-TB-IRIS patients at week 6 post-ART [22], only 7 TB-IRIS patients were analyzed, and there was no indication when TB-IRIS occurred in these patients relative to ART initiation. In our patient cohort, which included 50 TBIRIS patients, we found that CCR5+CD4+ T cell proportions increased dramatically in the first weeks post-ART relative to non-TB-IRIS patients, and remained significantly higher six months later. CCR5 is a critical homing receptor for Th1 cells to peripheral inflammatory sites, including the lungs and the central nervous system [23–26]. Thus, the rapid post-ART rise in CCR5+CD4+ T cell frequency in TB-IRIS patients may help explain certain clinical manifestations of TB-IRIS, including pleural effusion and neurological symptoms [4, 7, 27, 28]. In addition, since CCR5 is a major co-receptor for HIV [29], the higher pre-ART CCR5+ CD4+ T cell frequency in patients who develop TB-IRIS may help drive the higher viral loads observed in these patients.
Although other innate immune cell types, including NK cells and γ/δ T cells, have been linked to TB-IRIS development [15, 31], it is becoming increasingly clear that myeloid cells play a major part in this syndrome [32]. Our finding that plasma IL-1β levels are elevated pre-ART and increase significantly post-ART initiation in TB-IRIS patients relative to non-TB-IRIS patients provides the first clear indication that this critical pro-inflammatory mediator plays a role in TB-IRIS. We also found that circulating IL-6 levels were higher prior to ART in the TB-IRIS group and increased more dramatically in the TB-IRIS patients once ART began, and that plasma IL-8, IL-12, and TNF (which is also produced by activated T cells [33]) were all significantly higher at the time of TB-IRIS, confirming previous reports that found higher plasma levels of these proinflammatory mediators prior to ART and/or at the time of TB-IRIS.
While other studies have found elevated MTb antigen-induced IFN-γ production by T cells from TB-IRIS patients stimulated ex vivo [15, 16, 22, 42, 44– 46], and higher levels of IFN-γ in plasma of TB-IRIS patients [42], we saw no difference in plasma IFN-γ levels between TB-IRIS and non-TB-IRIS patients. We did observe that circulating IL-10 levels were significantly higher in TB-IRIS patients both pre-ART and at the time of TB-IRIS, similar to what was observed in a South African patient cohort [42, 43]. Thus, the relatively elevated IL-10 levels in the TB-IRIS group might have suppressed IFN-γ production.
Our findings that elevated CCR5+CD4+ and OX40+CD4+ T cell frequencies, and circulating IL-1β and IL-6 levels, are present pre-ART in patients who go on to develop TB-IRIS point to possible therapeutic interventions to reduce TB-IRIS incidence and/or severe or complicated clinical presentations of TB-IRIS. Although the recently completed CADIRIS trial found no benefit from inclusion of the CCR5 blocker maraviroc at ART initiation in reducing IRIS incidence due to multiple etiologies, the patients included in this trial had CD4 counts >100/mm3 and viral loads as low 103 copies/ml [47]. It is possible therefore that CCR5 antagonists would have some benefit in patients with more advanced immunosuppression and higher pre-ART CCR5+CD4+ T cell frequencies, like those analyzed here. We note that IL-1β and IL-6 antagonists are also in clinical use for diverse inflammatory conditions [48], and OX40/OX40L antagonists are currently in development [49].
Due to our study design we could evaluate the impact of TB-IRIS on ART-mediated T cell reconstitution up to two months after TB therapy completion/TB cure. Here, we have shown that EM and Fas+ EM CD4+ T cell frequencies expanded, and CM CD4+ T cell frequencies contracted, at significantly greater rates post-ART in TB-IRIS patients versus non-TB-IRIS patients, and that these remained evident at week 34. This strongly suggests that the TB-IRIS event is associated with a dramatic and persistent shift in EM/CM CD4+ T cell ratios either as a precipitant or as a reflection of this inflammatory host response. Our findings differ from a previous retrospective study of patients with IRIS caused by diverse opportunistic pathogens, which found similar kinetics of EM CD4+ T cell expansion/contraction between IRIS and non-IRIS patients post-ART [17].
TB-IRIS incidence was significantly higher in CAPRI-T patients from the early treatment arm (ART at 2 weeks post-TB treatment) versus the late treatment arm (ART at 8 weeks post-TB treatment), as it was in the overall CAMELIA cohort [1, 7]. We speculate that the steep post-ART rise in EM/CM CD4+ T cell ratio in the TB-IRIS group, which was especially apparent after week 8 when the vast majority of TB-IRIS cases had resolved, reflected an MTb-specific response, and that this response was amplified in early treatment arm patients due to relatively higher bacterial burden at the time of ART initiation. In this scenario, early ART facilitated the expansion of MTb-specific CD4+ T cell clones in patients who were already predisposed to respond strongly to infection once immune reconstitution began, as reflected by their enhanced pre-ART CD4+ T cell activation profiles. By contrast, delay of ART to 8 weeks in patients with a similarly “primed” CD4+ T cell compartment led to a more muted and less robust MTb-specific response due to the relatively lower bacterial burden by this timepoint, which may have been exacerbated by greater CD4+ T cell death due to the absence of ART for 6 additional weeks in the context of very high viremia. Intriguingly, EM CD4+ T cells are elevated in individuals with latent TB as compared to BCG-vaccinated individuals, consistent with this memory subpopulation playing a critical role in the long-term control of TB reactivation [56]. Indeed, it has been argued that induction of “frontline” EM T cells that can respond to pathogens that establish chronic infections at sites of entry (like the lung for MTb) should be a major goal of HIV, malaria, and TB vaccines [57].
Taken together, it is interesting to speculate that the post-ART/TB-IRIS shift of the CD4+ T cell memory compartment to an EM-dominated phenotype may help in controlling acute TB infection during the early stages of ART-mediated immune restoration and help in conferring long-term enhanced protection from MTb reinfection/reactivation/relapse. In this scenario, TB-IRIS is a clinical manifestation at the end of a spectrum of desirable MTb-driven innate and CD4+ T cell responses that are associated with better treatment outcome in TB/HIV co-infected patients.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are indebted to Wallis Annenberg and John Moores whose generosity made it possible to initiate and to complete this work. This work was supported by grants from the ANRS (12164), the NIH (R21AI076023), the Annenberg Foundation, AERAS, and AmFAR with support from NIAID/NIH-IeDEA (U01AI069907-07), and a gift from John Moores. We are grateful to Francoise Barré-Sinoussi for her help, advice, and support over the years. We thank Eric Nerrienet, Claire Rekacewicz, Daniel Scott, Gianfranco Pancino, Jean-Louis Sarthou, and Charles Mayaud for helpful discussions and advice as we began the study, and Jean-Louis Sarthou for his long time support of the Harvard team’s collaboration with the Institut Pasteur du Cambodge. We thank the CAMELIA study team, including the site nurses, doctors, and health workers, and Vincent Deubel and the staff at the Institut Pasteur du Cambodge. We thank Larry Fox, Rod Hoff, Jane Bupp, and Brigitte Bazin for their support and helpful discussions from the beginning of CAMELIA onward and the staff of the Cambodian Health Committee, especially Sam Sophan, for his contributions to and support of the work. We thank Leslie Kalish of Children’s Hospital Boston and the Harvard Catalyst Biostatistical Consulting program (NIH UL1 TR001102) for expert statistical advice and help as we started the analysis. We also thank Jerry Sadoff for his early support and Judy Lieberman for helpful comments on the manuscript. Finally, we are deeply indebted to the patients who took part in this study, who generously offered their participation to help the scientific community find solutions to AIDS and TB.
Footnotes
A subset of the results was presented at the 2013 CROI Conference, Atlanta Georgia, March 2013.
REFERENCES
- 1.Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365:1471–1481. doi: 10.1056/NEJMoa1013911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havlir DV, Kendall MA, Ive P, Kumwenda J, Swindells S, Qasba SS, et al. Timing of antiretroviral therapy for HIV-1 infection and tuberculosis. N Engl J Med. 2011;365:1482–1491. doi: 10.1056/NEJMoa1013607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, et al. Integration of antiretroviral therapy with tuberculosis treatment. N Engl J Med. 2011;365:1492–1501. doi: 10.1056/NEJMoa1014181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luetkemeyer AF, Kendall MA, Nyirenda M, Wu X, Ive P, Benson CA, et al. Tuberculosis Immune Reconstitution Inflammatory Syndrome in A5221 STRIDE: Timing, Severity and Implications for HIV-TB programs. J Acquir Immune Defic Syndr. 2013 doi: 10.1097/QAI.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meintjes G, Lawn SD, Scano F, Maartens G, French MA, Worodria W, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8:516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meintjes G, Rabie H, Wilkinson RJ, Cotton MF. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med. 2009;30:797–810. x. doi: 10.1016/j.ccm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Laureillard D, Marcy O, Madec Y, Chea S, Chan S, Borand L, et al. Paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome after early initiation of antiretroviral therapy in a randomized clinical trial. AIDS. 2013;27:2577–2586. doi: 10.1097/01.aids.0000432456.14099.c7. [DOI] [PubMed] [Google Scholar]
- 8.Loken MR, Brosnan JM, Bach BA, Ault KA. Establishing optimal lymphocyte gates for immunophenotyping by flow cytometry. Cytometry. 1990;11:453–459. doi: 10.1002/cyto.990110402. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saison J, Demaret J, Venet F, Chidiac C, Malcus C, Poitevin-Later F, et al. CD4+CD25+CD127- assessment as a surrogate phenotype for FOXP3+ regulatory T cells in HIV-1 infected viremic and aviremic subjects. Cytometry B Clin Cytom. 2013;84:50–54. doi: 10.1002/cyto.b.21047. [DOI] [PubMed] [Google Scholar]
- 12.Romero P, Zippelius A, Kurth I, Pittet MJ, Touvrey C, Iancu EM, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J Immunol. 2007;178:4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 13.Tomiyama H, Takata H, Matsuda T, Takiguchi M. Phenotypic classification of human CD8+ T cells reflecting their function: inverse correlation between quantitative expression of CD27 and cytotoxic effector function. Eur J Immunol. 2004;34:999–1010. doi: 10.1002/eji.200324478. [DOI] [PubMed] [Google Scholar]
- 14.Takata H, Takiguchi M. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J Immunol. 2006;177:4330–4340. doi: 10.4049/jimmunol.177.7.4330. [DOI] [PubMed] [Google Scholar]
- 15.Bourgarit A, Carcelain G, Samri A, Parizot C, Lafaurie M, Abgrall S, et al. Tuberculosis-associated immune restoration syndrome in HIV-1-infected patients involves tuberculin-specific CD4 Th1 cells and KIR-negative gammadelta T cells. J Immunol. 2009;183:3915–3923. doi: 10.4049/jimmunol.0804020. [DOI] [PubMed] [Google Scholar]
- 16.Meintjes G, Wilkinson KA, Rangaka MX, Skolimowska K, van Veen K, Abrahams M, et al. Type 1 helper T cells and FoxP3-positive T cells in HIV-tuberculosis-associated immune reconstitution inflammatory syndrome. Am J Respir Crit Care Med. 2008;178:1083–1089. doi: 10.1164/rccm.200806-858OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonelli LR, Mahnke Y, Hodge JN, Porter BO, Barber DL, DerSimonian R, et al. Elevated frequencies of highly activated CD4+ T cells in HIV+ patients developing immune reconstitution inflammatory syndrome. Blood. 2010;116:3818–3827. doi: 10.1182/blood-2010-05-285080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishii N, Takahashi T, Soroosh P, Sugamura K. OX40-OX40 ligand interaction in T-cell-mediated immunity and immunopathology. Adv Immunol. 2010;105:63–98. doi: 10.1016/S0065-2776(10)05003-0. [DOI] [PubMed] [Google Scholar]
- 19.Yu Q, Yue FY, Gu XX, Schwartz H, Kovacs CM, Ostrowski MA. OX40 ligation of CD4+ T cells enhances virus-specific CD8+ T cell memory responses independently of IL-2 and CD4+ T regulatory cell inhibition. J Immunol. 2006;176:2486–2495. doi: 10.4049/jimmunol.176.4.2486. [DOI] [PubMed] [Google Scholar]
- 20.Seddiki N, Sasson SC, Santner-Nanan B, Munier M, van Bockel D, Ip S, et al. Proliferation of weakly suppressive regulatory CD4+ T cells is associated with over-active CD4+ T-cell responses in HIV-positive patients with mycobacterial immune restoration disease. Eur J Immunol. 2009;39:391–403. doi: 10.1002/eji.200838630. [DOI] [PubMed] [Google Scholar]
- 21.Zaidi I, Peterson K, Jeffries D, Whittle H, de Silva T, Rowland-Jones S, et al. Immune reconstitution inflammatory syndrome and the influence of T regulatory cells: a cohort study in The Gambia. PLoS One. 2012;7:e39213. doi: 10.1371/journal.pone.0039213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vignesh R, Kumarasamy N, Lim A, Solomon S, Murugavel KG, Balakrishnan P, et al. TB-IRIS after initiation of antiretroviral therapy is associated with expansion of preexistent Th1 responses against Mycobacterium tuberculosis antigens. J Acquir Immune Defic Syndr. 2013;64:241–248. doi: 10.1097/QAI.0b013e31829f6df2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin S, Rottman JB, Myers P, Kassam N, Weinblatt M, Loetscher M, et al. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J Clin Invest. 1998;101:746–754. doi: 10.1172/JCI1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sallusto F, Kremmer E, Palermo B, Hoy A, Ponath P, Qin S, et al. Switch in chemokine receptor expression upon TCR stimulation reveals novel homing potential for recently activated T cells. Eur J Immunol. 1999;29:2037–2045. doi: 10.1002/(SICI)1521-4141(199906)29:06<2037::AID-IMMU2037>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 25.Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. P.N.A.S. USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giunti D, Borsellino G, Benelli R, Marchese M, Capello E, Valle MT, et al. Phenotypic and functional analysis of T cells homing into the CSF of subjects with inflammatory diseases of the CNS. J Leukoc Biol. 2003;73:584–590. doi: 10.1189/jlb.1202598. [DOI] [PubMed] [Google Scholar]
- 27.Pepper DJ, Marais S, Maartens G, Rebe K, Morroni C, Rangaka MX, et al. Neurologic manifestations of paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome: a case series. Clin Infect Dis. 2009;48:e96–e107. doi: 10.1086/598988. [DOI] [PubMed] [Google Scholar]
- 28.Leone S, Nicastri E, Giglio S, Narciso P, Ippolito G, Acone N. Immune reconstitution inflammatory syndrome associated with Mycobacterium tuberculosis infection: a systematic review. Int J Infect Dis. 2010;14:e283–e291. doi: 10.1016/j.ijid.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 29.Verhofstede C, Nijhuis M, Vandekerckhove L. Correlation of coreceptor usage and disease progression. Curr Opin HIV AIDS. 2012;7:432–439. doi: 10.1097/COH.0b013e328356f6f2. [DOI] [PubMed] [Google Scholar]
- 30.Sierra-Madero J, Tierney A, Rasool M, Azzoni L, Sere I, Andrade-Villaneuva J. Efficacy and safety of Maraviroc to prevent immune reconstitution inflammatory syndrome in high-rsk subjects initiating ART: 24 week results of a randomized, placebo-controlled trial (Abstract 182LB). 20th Conference on Retroviruses and Opportunistic Infections (CROI 2013); Atlanta, GA. 2013. [Google Scholar]
- 31.Pean P, Nerrienet E, Madec Y, Borand L, Laureillard D, Fernandez M, et al. Natural killer cell degranulation capacity predicts early onset of the immune reconstitution inflammatory syndrome (IRIS) in HIV-infected patients with tuberculosis. Blood. 2012;119:3315–3320. doi: 10.1182/blood-2011-09-377523. [DOI] [PubMed] [Google Scholar]
- 32.Tran HT, Van den Bergh R, Vu TN, Laukens K, Worodria W, Loembe MM, et al. The role of monocytes in the development of Tuberculosis-associated Immune Reconstitution Inflammatory Syndrome. Immunobiology. 2014;219:37–44. doi: 10.1016/j.imbio.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Goldfeld AE, Strominger JL, Doyle C. Human tumor necrosis factor a gene regulation in phorbol ester stimulated T and B cell lines. J. Exp. Med. 1991;174:73–81. doi: 10.1084/jem.174.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narendran G, Andrade BB, Porter BO, Chandrasekhar C, Venkatesan P, Menon PA, et al. Paradoxical tuberculosis immune reconstitution inflammatory syndrome (TB-IRIS) in HIV patients with culture confirmed pulmonary tuberculosis in India and the potential role of IL-6 in prediction. PLoS One. 2013;8:e63541. doi: 10.1371/journal.pone.0063541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goovaerts O, Jennes W, Massinga-Loembe M, Ceulemans A, Worodria W, Mayanja-Kizza H, et al. LPS-binding protein and IL-6 mark paradoxical tuberculosis immune reconstitution inflammatory syndrome in HIV patients. PLoS One. 2013;8:e81856. doi: 10.1371/journal.pone.0081856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chakrabarti LA, Boucherie C, Bugault F, Cumont MC, Roussillon C, Breton G, et al. Biomarkers of CD4+ T cell activation as risk factors for tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2014 doi: 10.1097/QAD.0000000000000311. [DOI] [PubMed] [Google Scholar]
- 37.Conesa-Botella A, Meintjes G, Coussens AK, van der Plas H, Goliath R, Schutz C, et al. Corticosteroid therapy, vitamin D status, and inflammatory cytokine profile in the HIV-tuberculosis immune reconstitution inflammatory syndrome. Clin Infect Dis. 2012;55:1004–1011. doi: 10.1093/cid/cis577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morlese JF, Orkin CM, Abbas R, Burton C, Qazi NA, Nelson MR, et al. Plasma IL-6 as a marker of mycobacterial immune restoration disease in HIV-1 infection. AIDS. 2003;17:1411–1413. doi: 10.1097/00002030-200306130-00025. [DOI] [PubMed] [Google Scholar]
- 39.Stone SF, Price P, Keane NM, Murray RJ, French MA. Levels of IL-6 and soluble IL-6 receptor are increased in HIV patients with a history of immune restoration disease after HAART. HIV Med. 2002;3:21–27. doi: 10.1046/j.1464-2662.2001.00096.x. [DOI] [PubMed] [Google Scholar]
- 40.Boulware DR, Hullsiek KH, Puronen CE, Rupert A, Baker JV, French MA, et al. Higher levels of CRP, D-dimer, IL-6, and hyaluronic acid before initiation of antiretroviral therapy (ART) are associated with increased risk of AIDS or death. J Infect Dis. 2011;203:1637–1646. doi: 10.1093/infdis/jir134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boulware DR, Meya DB, Bergemann TL, Wiesner DL, Rhein J, Musubire A, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7:e1000384. doi: 10.1371/journal.pmed.1000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tadokera R, Meintjes G, Skolimowska KH, Wilkinson KA, Matthews K, Seldon R, et al. Hypercytokinaemia accompanies HIV-tuberculosis immune reconstitution inflammatory syndrome. Eur Respir J. 2011;37:1248–1259. doi: 10.1183/09031936.00091010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tadokera R, Wilkinson KA, Meintjes GA, Skolimowska KH, Matthews K, Seldon R, et al. Role of the interleukin 10 family of cytokines in patients with immune reconstitution inflammatory syndrome associated with HIV infection and tuberculosis. J Infect Dis. 2013;207:1148–1156. doi: 10.1093/infdis/jit002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bourgarit A, Carcelain G, Martinez V, Lascoux C, Delcey V, Gicquel B, et al. Explosion of tuberculin-specific Th1-responses induces immune restoration syndrome in tuberculosis and HIV co-infected patients. Aids. 2006;20:F1–F7. doi: 10.1097/01.aids.0000202648.18526.bf. [DOI] [PubMed] [Google Scholar]
- 45.Tan DB, Lim A, Yong YK, Ponnampalavanar S, Omar S, Kamarulzaman A, et al. TLR2-induced cytokine responses may characterize HIV-infected patients experiencing mycobacterial immune restoration disease. AIDS. 2011;25:1455–1460. doi: 10.1097/QAD.0b013e328348fb18. [DOI] [PubMed] [Google Scholar]
- 46.Elliott JH, Vohith K, Saramony S, Savuth C, Dara C, Sarim C, et al. Immunopathogenesis and diagnosis of tuberculosis and tuberculosis-associated immune reconstitution inflammatory syndrome during early antiretroviral therapy. J Infect Dis. 2009;200:1736–1745. doi: 10.1086/644784. [DOI] [PubMed] [Google Scholar]
- 47.Sierra-Madero J, Ellenberg SS, Rassool MS, Tierney A, Belaunzaran-Zamudio PF, Lopez-Martinez A, et al. Effect of the CCR5 antagonist maraviroc on the occurrence of immune reconstitution inflammatory syndrome in HIV (CADIRIS): a double-blind, randomised, placebo-controlled trial. The Lancet HIV. 2014;1:e60–e67. doi: 10.1016/S2352-3018(14)70027-X. [DOI] [PubMed] [Google Scholar]
- 48.Choy EH, Kavanaugh AF, Jones SA. The problem of choice: current biologic agents and future prospects in RA. Nat Rev Rheumatol. 2013;9:154–163. doi: 10.1038/nrrheum.2013.8. [DOI] [PubMed] [Google Scholar]
- 49.Croft M, Benedict CA, Ware CF. Clinical targeting of the TNF and TNFR superfamilies. Nat Rev Drug Discov. 2013;12:147–168. doi: 10.1038/nrd3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Singh V, Jain S, Gowthaman U, Parihar P, Gupta P, Gupta UD, et al. Co-administration of I-1+IL-6+TNF-alpha with Mycobacterium tuberculosis infected macrophages vaccine induces better protective T cell memory than BCG. PLoS One. 2011;6:e16097. doi: 10.1371/journal.pone.0016097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khoruts A, Osness RE, Jenkins MK. IL-1 acts on antigen-presenting cells to enhance the in vivo proliferation of antigen-stimulated naive CD4 T cells via a CD28-dependent mechanism that does not involve increased expression of CD28 ligands. Eur J Immunol. 2004;34:1085–1090. doi: 10.1002/eji.200324170. [DOI] [PubMed] [Google Scholar]
- 53.Leal IS, Smedegard B, Andersen P, Appelberg R. Interleukin-6 and interleukin-12 participate in induction of a type 1 protective T-cell response during vaccination with a tuberculosis subunit vaccine. Infect Immun. 1999;67:5747–5754. doi: 10.1128/iai.67.11.5747-5754.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saijo S, Asano M, Horai R, Yamamoto H, Iwakura Y. Suppression of autoimmune arthritis in interleukin-1-deficient mice in which T cell activation is impaired due to low levels of CD40 ligand and OX40 expression on T cells. Arthritis Rheum. 2002;46:533–544. doi: 10.1002/art.10172. [DOI] [PubMed] [Google Scholar]
- 55.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, et al. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adekambi T, Ibegbu CC, Kalokhe AS, Yu T, Ray SM, Rengarajan J. Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PLoS One. 2012;7:e36046. doi: 10.1371/journal.pone.0036046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J.R. Statist. Soc. B. 1995;57:289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.