Abstract
CCAAT/enhancer-binding protein α (C/ebpα) is a transcription factor that plays important roles in the regulation of hepatogenesis, adipogenesis and hematopoiesis. Disruption of the C/EBPα gene in mice leads to disturbed liver architecture and neonatal death due to hypoglycemia. However, the precise stages of liver development affected by C/ebpα loss are poorly studied. Using the zebrafish embryo as a model organism, we show that inactivation of the cebpa gene by TALENs results in a small liver phenotype. Further studies reveal that C/ebpα is distinctively required for hepatic outgrowth but not for hepatoblast specification. Lack of C/ebpα leads to enhanced hepatic cell proliferation and subsequent increased cell apoptosis. Additional loss of p53 can largely rescue the hepatic defect in cebpa mutants, suggesting that C/ebpα plays a role in liver growth regulation via the p53 pathway. Thus, our findings for the first time demonstrate a stage-specific role for C/ebpα during liver organogenesis.
Hepatogenesis in mammals has been studied extensively in recent years. In mice, liver organogenesis initiates from the ventral foregut endoderm at embryonic day 8.0 (e8.0), followed by the specification of definitive hepatoblasts. At e9.5, the hepatoblasts delaminate from the epithelium and invade the septum transversum mesenchyme (STM) to form the liver bud. Between e9.5 and e15, the liver bud undergoes rapid proliferation. Finally, the hepatoblasts differentiate into mature hepatocytes and biliary duct cells1,2. In addition, it is worth noting that the fetal liver of mammals is also a major hematopoietic organ3.
Over the last decade, the zebrafish (Danio rerio) has emerged as a favored model organism in hepatogenesis4. In zebrafish, hepatogenesis can be divided into three main stages: specification, budding/differentiation and hepatic outgrowth. Hepatoblasts originate from the anterior endoderm and can be recognized by the expression of hhex and prox1 as early as 22–24 hours post fertilization (hpf). Following this, hepatoblast differentiation occurs within the liver primordium by 50 hpf, and can be clearly distinguished as a prominent bud settling on the left side of the midline over the yolk. At the subsequent hepatic outgrowth stage beginning at 60 hpf, the liver dramatically expands because of rapid cell proliferation. By the end of outgrowth at 5 days post fertilization (dpf), the functional liver consists of a larger left lobe and a smaller right lobe5,6. While many of the transcription factors and signaling pathways essential for the first two stages have been identified5, much less is known about the genes required for hepatic outgrowth.
C/ebpα is the founding member of C/EBP family of basic leucine zipper (bZIP) transcription factors7. It exerts important biological functions in a range of cell types8. Targeted disruption of the C/EBPα gene in mice leads to abundant generation of pseudoglandular structures in the liver parenchyma during perinatal liver development9,10. The knockout mice die shortly after birth because of hypoglycemia accompanied by hyperammonemia9,10,11. Although C/EBPα is implicated in transcriptional regulation of genes for enzymes of gluconeogenesis and urea synthesis in the liver, the precise stages that are affected by C/EBPα deficiency during liver development have not been fully elucidated.
In the present study, we show that disruption of the cebpa gene using TALENs results in a small liver phenotype in zebrafish. Lack of cebpa blocks hepatic outgrowth, but does not affect liver specification. C/ebpα regulates hepatic cell proliferation and apoptosis during liver outgrowth. Furthermore, loss of p53 can largely rescue the hepatic defect in cebpa mutant embryos, suggesting a novel link between C/ebpα and the p53 pathway in the regulation of liver development.
Results
Disruption of zebrafish cebpa gene by TALENs
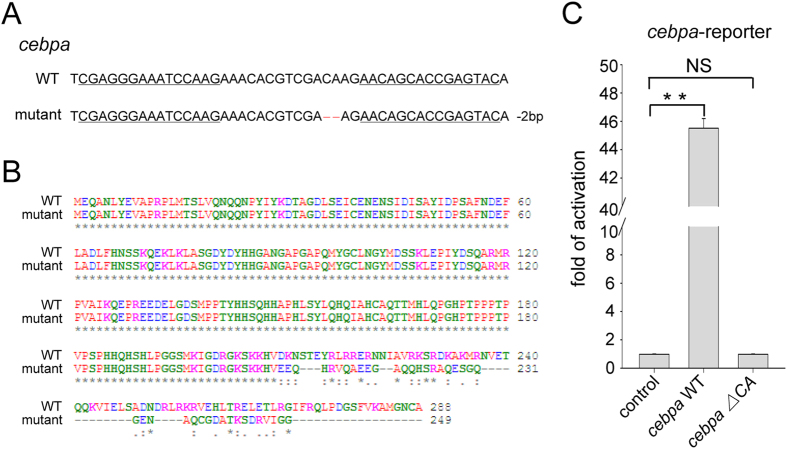
We have previously generated a cebpa mutant (cebparj31/+) using TALENs technology12. In this mutant, two nucleotides were deleted at the cebpa locus (cebpa ∆CA) (Fig. 1A), which led to frame shift at amino acid residue 209. This mutation created a premature stop codon, resulting in the synthesis of a truncated C/ebpα lacking the bZIP domain at the C-terminus (Fig. 1B). Luciferase assay showed that the mutant C/ebpα completely lost its transcriptional activity compared with the wild type (Fig. 1C), confirming inactivation of the mutant C/ebpα. The cebpa mutant zebrafish generally died around 2 to 3 weeks post fertilization.
Figure 1. Inactivation of the zebrafish cebpa gene based on TALENs.
(A) Partial nucleotide sequences of the wild type and mutant cebpa genes. The TALENs binding sites are underlined. Deletions are indicated by red dashes. (B) Amino acid sequence alignment of wild type and mutant C/ebpα proteins. (C) Luciferase activity assays were performed in 293T cells using C/ebpα constructs indicated. Renilla was used as an internal control. Data shown are the mean ± SD of three independent experiments, **P < 0.005 by student’s t-test. NS, not significant. Note that the mutant C/ebpα (ΔCA), with two nucleotide deletions, loses its transcriptional activity.
The cebpa mutant exhibits a small liver phenotype
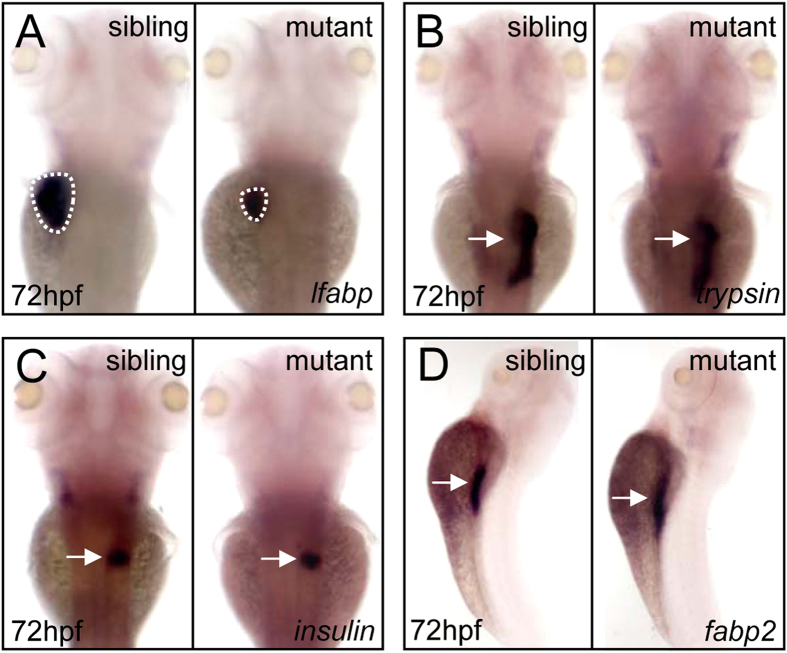
To study the function of C/ebpα in embryonic liver development, we first analyzed the expression pattern of cebpa by whole mount in situ hybridization. The results showed that cebpa expression was enriched in the developing liver (supplemental Fig. S1), consistent with the previous observation13. We then examined the expression of liver fatty acid binding protein (lfabp), a liver-specific marker14. The data revealed that the liver size of the cebpa mutant was strikingly reduced, compared to that of sibling controls at 72 hpf and 5 dpf, respectively (Fig. 2A and supplemental Fig. S2). In contrast, the development of other endoderm-derived tissues such as exocrine pancreas, endocrine pancreas and intestine was not obviously affected as determined by assessing the expression of trypsin, insulin and fatty acid binding protein 2 (fabp2), respectively (Fig. 2B–D). Together, these results suggest that cebpa is essential for liver development in zebrafish.
Figure 2. Disruption of cebpa gene reduces liver size.
(A–D) WISH assay of lfabp (A), trypsin (B), insulin (C) and fabp2 (D) at 72 hpf, respectively. Dashed lines circle the boundary of the liver. (A–C), dorsal views, anterior to the top. (D) lateral view, dorsal to the right.
C/ebpα is required for hepatic outgrowth but not for hepatoblast specification
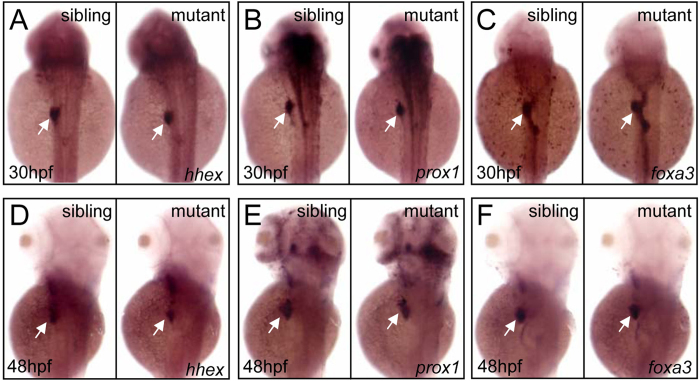
The failure of liver development in the cebpa mutant could be attributable to defects in hepatoblast specification from endodermal cells or budding prior to hepatic outgrowth. To test which stage had been affected, we examined the expression of hhex and prox1, representing the earliest markers for hepatoblasts15,16. Data showed that these genes displayed similar expression patterns in the liver primordia of both sibling and mutant embryos at 30 hpf (completion of specification) (Fig. 3A,B) and 48 hpf (completion of budding) (Fig. 3D–E). Moreover, the expression of foxa3, a pan-endodermal marker, was also unaffected in the mutant embryos (Fig. 3C,F). Therefore, these results, together with that of lfabp, indicate that C/ebpα is not required for liver specification but for liver expansion growth during hepatogenesis.
Figure 3. cebpa is dispensable for liver specification or budding.
(A–F) WISH assay of hhex (A and D), prox1 (B and E) and foxa3 (C and F) at 30 and 48 hpf, respectively. (A–F), dorsal views, anterior to the top. White arrows indicate liver primordium.
cebpa deficiency results in enhanced hepatic cell proliferation and subsequent increased apoptosis
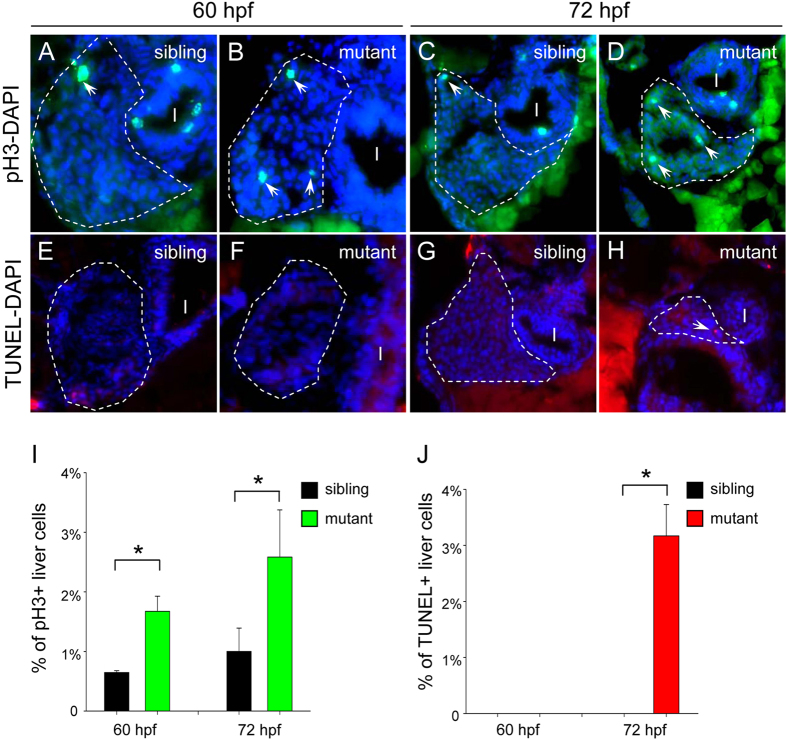
The small liver phenotype in the cebpa mutant could potentially result from abnormal cell proliferation and/or apoptotic cell death. We next examined the terminal fate of the cebpa-deficient hepatic cells using immunostaining. Firstly, using an antibody against phospho-histone H3 (pH3), a marker of cell proliferation, we found that the pH3-positive hepatic cells exhibited a 2.5-fold increase in the cebpa mutant sectioned embryos at 60 and 72 hpf, respectively (Fig. 4A–D,I). Next, we examined apoptotic events using the TUNEL assay. The mutant hepatic cells underwent significant activation of cell apoptosis at 72 hpf, whereas almost no apoptotic cells were detected in the developing liver of sibling controls or at earlier developmental stage (Fig. 4E–H,J). Collectively, these results suggest that loss of C/ebpα results in enhanced hepatic cell proliferation and subsequent increased cell apoptosis which may account for the small liver phenotype.
Figure 4. Loss of cebpa leads to enhanced hepatic cell proliferation and subsequent increased apoptosis.
(A–H) Hepatic cell proliferation and apoptosis were determined by pH3 staining and TUNEL assay in 60 and 72 hpf embryos, respectively. The sections were counterstained with DAPI to label the nucleus. Dashed lines circle the boundary of the liver. White arrows indicate pH3 or TUNEL positive cells, respectively. In each case or at each time-point, more than 5 sections from at least three sibling control or cebpa mutant fish were examined. (I) intestine. (I,J) Quantification of hepatic cell proliferation and apoptosis, respectively. Data shown are the mean ± SD, n ≥ 3, *P < 0.05 by student’s t-test.
p53 pathway activation in the cebpa mutant
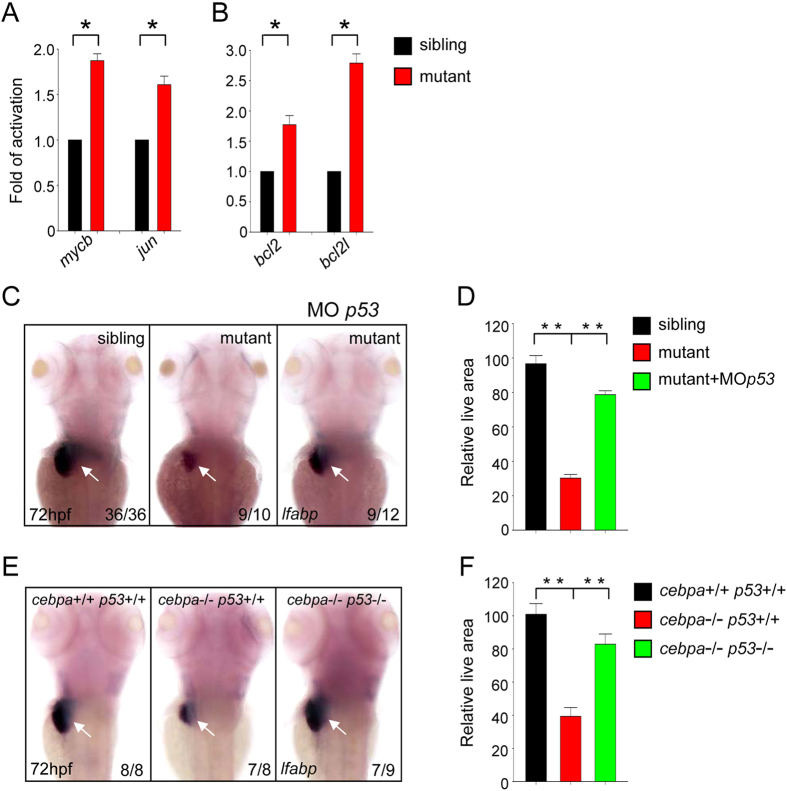
To study the molecular mechanisms that may underlie the small liver phenotype in the cebpa mutant, we examined the expression levels of genes which are involved in cell proliferation and apoptosis. It was previously shown that the expression of c-myc and c-jun were induced in the liver of C/EBPα knockout mice10. In agreement with this, we also detected elevated expression of mycb and jun in the cebpa mutant zebrafish embryos (Fig. 5A), underscoring the evolutionary conserved role of these genes in the regulation of normal liver development. Importantly, we also observed that the expression of bcl2 and bcl2l were significantly increased in these mutant embryos (Fig. 5B), supporting the notion that an increased portion of the cebpa-deficient hepatic cells were in an apoptotic state. It is well known that the p53 signaling pathway plays a key role in controlling cell proliferation and apoptosis17. The aberrant cell proliferation and apoptosis observed in the cebpa mutant suggests that p53 pathway is likely activated. In order to test this, we knocked down p53 using morpholino (MO) in the cebpa mutant embryos. As expected, the hepatic defect of the cebpa mutant embryos could be efficiently rescued by p53 knockdown (Fig. 5C,D). To further confirm the role of p53 in this process, we next generated cebpa and p53 double mutant zebrafish. The data showed that additional loss of p53 could restore normal liver development in cebpa-deficient embryos (Fig. 5 E,F). Moreover, the defects of cell proliferation and apoptosis could be also largely recovered in the cebpa and p53 double mutant (supplemental Fig. S3). Thus, these results demonstrate that the p53 pathway is indeed involved in C/ebpα-dependent hepatic outgrowth.
Figure 5. The p53 signaling pathway is activated in cebpa-deficient developing liver.
(A–B) Quantitative PCR analysis of the expression of cell proliferation and apoptosis-related genes in 72 hpf embryos. Data shown are the mean ± SD, n ≥ 3, *P < 0.05 by student’s t-test. (C,E) WISH assay of lfabp at 72 hpf. Loss of p53 could rescue the hepatic defect in cebpa mutant embryos. Dorsal views with anterior to the top. (D,F) The relative liver area measured in (C,E) respectively. The result shown is fold difference compared with the level (set to 100) detected in control embryos (mean ± SD, n ≥ 3, **P < 0.005 by student’s t-test).
Discussion
Targeted disruption of the C/EBPα gene in mice leads to disturbed liver architecture and results in neonatal death due to hypoglycemia9,10. However, the exact stages affected by C/EBPα deficiency during liver development have not been analyzed in detail. Furthermore, the fetal liver of mammals is also a hematopoietic organ, and hepatogenesis and hematopoiesis are intertwined. Therefore, dysfunction of one process may prevent the proper development of the other. C/EBPα not only plays important roles in hepatogenesis, but also is required for hematopoiesis18, which obfuscates its role in these two processes. To assess the independent function of C/EBPα in liver development, we used the popular model organism, zebrafish, due to the fact that embryonic hematopoiesis does not take place in the liver. Here, we found that the expression of cebpa was enriched in the developing liver of zebrafish. Inactivation of the cebpa gene by TALENs led to a small liver phenotype. Detailed analysis revealed that C/ebpα was required for hepatic outgrowth but not for hepatoblast specification during liver development.
An increasing number of reports have implicated C/EBPα as a suppressor of cell proliferation8. In support of this idea, we found that loss-of-function of C/ebpα induced hepatic proliferation in the developing liver of zebrafish. Interestingly, we also detected increased hepatic cell apoptosis in the cebpa mutant embryos at later developmental stage. These ambivalent results suggest that the cebpa-deficient hepatic cells seemed to be in an inappropriate proliferative state and then underwent apoptosis, eventually resulting in the small liver phenotype. It is not a rare phenomenon in the liver development, since disruption of Apc (adenomatous polyposis coli) in the liver of mice also leads to increased hepatocyte proliferation and apoptosis, which may be caused by elevated DNA damage, accumulation of p53 and increased levels of anaphase bridges19. Additionally, in the partial hepatectomy (PH)-induced mouse liver regeneration study, Nur77 knock-out livers exhibited enhanced hepatocyte proliferation coincided with hepatocyte apoptosis20. Further studies are required to investigate the switch between cell proliferation and apoptosis regulated by C/ebpα, such as the role of C/ebpα in controlling chromosome segregation and genomic stability.
The p53 pathway is composed of a network of genes responding to a variety of intrinsic and extrinsic stress signals. Activation of the p53 protein induces cell cycle arrest, cellular senescence or apoptosis17,21. p53, as a well-known tumor suppressor, also plays a critical role during organogenesis, including hepatogenesis. In zebrafish defhi429 (digestive-organ expansion factor) mutant, the expression of ∆113p53, a newly identified isoform of p53, was selectively up-regulated within the mutant digestive organs, and then triggered the arrest of cell proliferation, resulting in compromised organ growth. Furthermore, knock-down of p53 and Δ 113p53 levels could rescue the developmental defects of the mutants22,23. Moreover, the Def-p53 pathway was also involved in scar formation at the amputation site after PH in zebrafish24. Here, we showed that the p53 pathway was activated in the cebpa-deficient embryos, which may be triggered by the aberrant cell proliferation, and additional loss of p53 could largely rescue the hepatic defects in the cebpa mutants. However, p53 might not be a direct target gene of C/ebpα in the liver organogenesis, since the transcriptional level of p53 has no obvious changes in the cebpa mutants compared with sibling controls (supplemental Fig. S4). It will be of interest in future studies to determine how C/ebpα regulates p53 activities in the developing liver.
Taken together, we hereby provide novel evidence that C/ebpα is specifically required for hepatic outgrowth via the p53 pathway, and accordingly have expanded our understanding of liver development. Moreover, these new findings may help to identify new targets for therapeutic manipulation in the treatment of liver failure and liver cancer.
Methods
Zebrafish
Zebrafish maintenance and staging were performed as described previously25. The zebrafish facility and study were approved by the Institutional Review Board of the Institute of Health Sciences, Shanghai Institutes of Biological Sciences, Chinese Academy of Sciences (Shanghai, China) and the methods were carried out in accordance with the approved guidelines. The cebparj31/+ and p53zdf1/zdf1 mutants were used in this study12,26.
Generation of constructs
The zebrafish cebpa∆CA was generated by genomic PCR with the following primers: Forward 5’ CCGGAATTCATGGAGCAAGCAAACCTCTACGAGG 3’, Reverse 5’ CCGCTCGAGTTAAGCGCAGTTGCCCATGGCTTTGAC 3’, then cloned into the pCS2+ vector.
Luciferase reporter assay
293T cells were transfected with the indicated plasmids using Effectene Transfection Reagent (QIAGEN). Tetramer of the CEBP site of human GCSFR was inserted into the promoterless luciferase vector pTK81-luc and used as a reporter plasmid27. Cells were harvested 36 hours after transfection and luciferase activities were analyzed using the Dual Luciferase Reporter Assay Kit (Promega), according to the manufacturer’s protocols. Luciferase activity was normalized to Renilla activity.
Morpholino
The morpholino oligonucleotide (MO) of p53 (TCTTGGCTGTCGTTTTGCGCCATTG) was used as previously described28.
Whole-mount mRNA in situ hybridization
Digoxigenin-labeled antisense RNA probes were transcribed from linearized constructs using T3, T7 or Sp6 polymerase (Roche). Whole-mount mRNA in situ hybridization was performed as described previously18. The probes were detected using alkaline phosphatase (AP)-coupled anti-digoxigenin Fab fragment antibody (Roche) with BCIP/NBT staining (Vector Laboratories). The probes used in this study included: cebpa, lfabp, trypsin, insulin, fabp2, hhex, prox1 and foxa3.
Phospho-histone H3 (pH3) immunostaining and TUNEL assay
Embryos were fixed in 4% paraformaldehyde at 4 °C overnight. For sectioning, the embryos were embedded in OCT compound (SAKURA) and cryosectioned into 14μm slices. After blocking with 10% FBS for 1 hr at room temperature, the sections were incubated with rabbit anti-pH3 antibody (1:100 dilution, Santa Cruz) at 4 °C overnight. Secondary antibody of Alexa Fluor 488 conjugated anti-rabbit IgG (Invitrogen) was then incubated for 1 hr at room temperature. The sections were counterstained with DAPI (Vector Labs) to label cell nuclei.
Terminal transferase UTP nick end labeling (TUNEL) was carried out on cryosections using the In Situ Cell Death Detection Kit, TMR red (Roche) according to the manufacturer’s recommendations.
Quantitative PCR
Total RNA was extracted from the head region containing liver dissected from embryos at 72 hpf, and the trunks of the embryos were used for genotyping. Quantitative PCR was performed using a LightCycler 1.5 (Roche) following the manufacturer’s protocol. Primers are listed in supplemental Table S1.
Additional Information
How to cite this article: Yuan, H. et al. CCAAT/enhancer-binding protein a is required for hepatic outgrowth via the p53 pathway in zebrafish. Sci. Rep. 5, 15838; doi: 10.1038/srep15838 (2015).
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Jinrong Peng and Dr. Xiaodong Shu for kindly providing plasmids. We appreciate Dr. Paul Gavine for proofreading this manuscript. We are also grateful for Yi Chen and Yi Jin for their excellent technical support. This work was supported by the National Natural Science Foundation of China (31371479), the Natural Science Foundation of Shanghai City (13ZR1425800) and the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning.
Footnotes
Author Contributions H.Y. designed the research, performed experiments, analyzed data and wrote the manuscript; B.W. and X.H.L. performed experiments; C.G., R.M.Y., L.X.W., S.J.C., Z.C. and H.d.T. provided suggestions on experimental design and analyzed data; J.Z. and J.Z. designed experiments, analyzed data and wrote the manuscript.
References
- Lu J. W. et al. Liver development and cancer formation in zebrafish. Birth Defects Res C Embryo Today. 93, 157–172 (2011). [DOI] [PubMed] [Google Scholar]
- Si-Tayeb K., Lemaigre F. P. & Duncan S. A. Organogenesis and development of the liver. Dev Cell. 18, 175–189 (2010). [DOI] [PubMed] [Google Scholar]
- Orkin S. H. & Zon L. I. Hematopoiesis: an evolving paradigm for stem cell biology. Cell. 132, 631–644 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T. & Peng J. Liver development in zebrafish (Danio rerio). J Genet Genomics. 36, 325–334 (2009). [DOI] [PubMed] [Google Scholar]
- Chu J. & Sadler K. C. New school in liver development: lessons from zebrafish. Hepatology. 50, 1656–1663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins B. J. & Pack M. Zebrafish models of human liver development and disease. Compr Physiol. 3, 1213–1230 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 17, 318–324 (2007). [DOI] [PubMed] [Google Scholar]
- Johnson P. F. Molecular stop signs: regulation of cell-cycle arrest by C/EBP transcription factors. J Cell Sci. 118, 2545–2555 (2005). [DOI] [PubMed] [Google Scholar]
- Wang N. D. et al. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 269, 1108–1112 (1995). [DOI] [PubMed] [Google Scholar]
- Flodby P., Barlow C., Kylefjord H., Ahrlund-Richter L. & Xanthopoulos K. G. Increased hepatic cell proliferation and lung abnormalities in mice deficient in CCAAT/enhancer binding protein alpha. J Biol Chem. 271, 24753–24760 (1996). [DOI] [PubMed] [Google Scholar]
- Kimura T. et al. Hypoglycemia-associated hyperammonemia caused by impaired expression of ornithine cycle enzyme genes in C/EBPalpha knockout mice. J Biol Chem. 273, 27505–27510 (1998). [DOI] [PubMed] [Google Scholar]
- Yuan H. et al. Sumoylation of CCAAT/enhancer-binding protein alpha is implicated in hematopoietic stem/progenitor cell development through regulating runx1 in zebrafish. Sci Rep. 5, 9011 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons S. E. et al. Molecular cloning, genetic mapping, and expression analysis of four zebrafish c/ebp genes. Gene. 281, 43–51 (2001). [DOI] [PubMed] [Google Scholar]
- Huang H. et al. Mypt1-mediated spatial positioning of Bmp2-producing cells is essential for liver organogenesis. Development. 135, 3209–3218 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace K. N., Yusuff S., Sonntag J. M., Chin A. J. & Pack M. Zebrafish hhex regulates liver development and digestive organ chirality. Genesis. 30, 141–143 (2001). [DOI] [PubMed] [Google Scholar]
- Ober E. A., Verkade H., Field H. A. & Stainier D. Y. Mesodermal Wnt2b signalling positively regulates liver specification. Nature. 442, 688–691 (2006). [DOI] [PubMed] [Google Scholar]
- Vousden K. H. & Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2, 594–604 (2002). [DOI] [PubMed] [Google Scholar]
- Yuan H. et al. Sumoylation of CCAAT/enhancer-binding protein alpha promotes the biased primitive hematopoiesis of zebrafish. Blood. 117, 7014–7020 (2011). [DOI] [PubMed] [Google Scholar]
- Meniel V. et al. Apc and p53 interaction in DNA damage and genomic instability in hepatocytes. Oncogene. 34, 1–12 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y. et al. Accelerated partial hepatectomy-induced liver cell proliferation is associated with liver injury in Nur77 knockout mice. Am J Pathol. 184, 3272–3283 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vousden K. H. p53: death star. Cell. 103, 691–694 (2000). [DOI] [PubMed] [Google Scholar]
- Chen J. et al. Loss of function of def selectively up-regulates Delta113p53 expression to arrest expansion growth of digestive organs in zebrafish. Genes Dev. 19, 2900–2911 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. et al. p53 Isoform delta113p53 is a p53 target gene that antagonizes p53 apoptotic activity via BclxL activation in zebrafish. Genes Dev. 23, 278–290 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Chen J., Xiong J. W. & Peng J. Haploinsufficiency of Def activates p53-dependent TGFbeta signalling and causes scar formation after partial hepatectomy. PloS one. 9, e96576 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Ballard W. W., Kimmel S. R., Ullmann B. & Schilling T. F. Stages of embryonic development of the zebrafish. Dev Dyn. 203, 253–310 (1995). [DOI] [PubMed] [Google Scholar]
- Berghmans S. et al. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci USA. 102, 407–412 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T. X. et al. Dominant-interfering C/EBPalpha stimulates primitive erythropoiesis in zebrafish. Exp Hematol. 35, 230–239 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H. et al. Small ubiquitin-related modifier paralogs are indispensable but functionally redundant during early development of zebrafish. Cell Res. 20, 185–196 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.