Abstract
Prairie vole males typically display robust preferences for affiliation with their respective mates that indicate the expression of a pair-bond. However, it recently has been shown that the strength of a male vole’s pair-bond can differ depending on the reproductive status of his mate. In the present study, we examined the possibility that female-controlled pacing of the mating sequence could alter males’ affiliative behaviors in a partner-preference test by affecting reproductive success. We expected an earlier onset of mating and thus earlier onset of pregnancy would occur if females controlled the pace of mating, in turn, reinforcing males’ preference for their familiar mates vs for a stranger. We found that female-pacing did not affect latency to mating, mating duration, or any of our other measures of social or mating behaviors. Further, female paced-mating did not alter reproductive success as indicated by litter size. We conclude that female-paced mating in prairie voles does not impact the formation, consolidation and/or expression of a pair-bond, either directly or indirectly, by their male partners.
Introduction
Preference for affiliative contact with a familiar individual is a fundamental part of social bonding in general, and of pair-bonding in particular. Prairie voles [Microtus ochrogaster (Wagner)] are well-known for displaying such affiliative bonds and thus have been used extensively in studies of the neurochemical, hormonal, and behavioral changes that underlie social bonding (Aragona and Wang 2004). Early studies focused on the presence or absence of mating as a critical factor in “pair-bond” formation (c.f., Carter et al. 1988), but those studies typically manipulated mating behavior of the animals via gonadectomy and/or hormonal treatments (c.f., Insel and Hulihan 1995). More recent studies, however, have suggested that natural variation in the normal hormonal and neurochemical processes that underlie prairie vole mating and social bonding can significantly affect the outcome of social affiliation testing.
This variation first came to light in a study of changes in the central nervous system associated with prairie vole pair-bonding by Aragona et al. (Aragona et al. 2006). In that study, only males from pairs in which the female was pregnant after two weeks of cohabitation were included in the analysis, but differences in pregnancy status (i.e., how far advanced the pregnancy was) between pairs were not taken into account. Surprisingly, although the female mates exhibited some evidence of pregnancy after two weeks of cohabitation with a male, some of the males in those pairs did not show the neural reorganization that is necessary for pair-bond expression. Those results suggest that the timing of the onset of his mate’s pregnancy may affect the formation, consolidation, or expression of a pair-bond on the part of the male. Further support for this possibility was found in studies showing that both partner-preference expression and stranger-oriented aggression (social behaviors normally associated with pair-bonding) are heavily dependent on how soon after pairing female reproductive activation and pregnancy occurs (Curtis 2010, Resendez et al. 2012).
Mating behaviors of both members of a reproductive pair may contribute to successful pregnancy (Gray et al. 1974, Coopersmith and Erskine 1994). Among rodents, the temporal patterns and numbers of mounts, intromissions, and ejaculations by the male can influence both egg fertilization, and the endocrine changes associated with pregnancy (Adler 1969). Female behavior also contributes to pregnancy success. Among female mice, reproductive success (number of litters and pup survival) and offspring fitness was higher for those that chose the male with which they mated (Drickamer et al. 2000). Furthermore, when female rats control the frequency and temporal pattern of copulations (female-paced mating) reproductive success is enhanced as indicated by larger litter sizes (Coopersmith and Erskine 1994).
The male prairie vole’s role in mating success is even more extensive than are those of male rats and mice. Female prairie voles do not display a spontaneous puberty. Rather, olfactory stimuli associated with the male induce a surge in circulating estrogen which, in turn, induces sexual receptivity on the part of the female. Only then does copulation occur which, again in turn, induces ovulation (Carter et al. 1987). However, as noted above, the latency to onset of sexual receptivity and mating can significantly affect subsequent male responses to his mate. Thus, factors that affect the latency to mating can have an inordinate impact on prairie vole pair-bonding. At this point, although there is evidence of female-mate-choice in prairie voles (Pierce and Dewsbury 1991), it is not known whether female-pacing of mating in this species affects reproductive success which, in turn, can influence male affiliative behavior. Thus, in the present study, we tested the hypothesis that female-paced mating can affect affiliative behaviors of their male partners. We predicted that female-pacing would reduce the latency to mating and/or other reproductive parameters, and thus change the males’ affiliative behaviors. We first examined the effects of female-pacing on several reproductive parameters and then tested male behavior in a partner-preference paradigm.
Materials and Methods
All animal usage in this study was approved by the Oklahoma State University Center for Health Sciences Institutional Animal Care and Use Committee. The animals used in this study were adult (≥ 60 days of age) male and female prairie voles from a captive breeding colony descended from a southern Illinois population (F4–F5 generation relative to the last out-crossing with wild stock). Animals were maintained under a long-day photoperiod (14:10) and colony room temperature was maintained at 21 °C. Food (Purina rabbit chow) and water were available ad libitum. Breeding pairs were housed in 20x25x45cm cages containing 1–2 cm of corn cob bedding. Breeders were supplemented with black-oil sunflower seeds and ~10 cm of Timothy hay was placed in their cages to provide environmental enrichment and nesting places. After weaning at 19–20 days of age, voles were housed as same-sex pairs in 10x17x28 cm cages with corncob bedding until used in experiments. Weaned males were housed in a room separate from that containing females and breeding pairs.
Unrelated opposite-sex pairs of voles were assigned to one of three groups and placed into an apparatus consisting of two parallel cages (10x17x28 cm) connected by a tube (7.5x16 cm). Both cages contained food and water sources. In the first group (n = 7 pairs), females were tethered to restrict their movements to one of the two cages, while the males were free to move at will between the occupied and empty cages. In the second group (n = 10 pairs), the males were tethered and the females were free to move between cages. The third group (n = 9 pairs) consisted of pairs in which both animals were free to move about in the apparatus. All pairs were video-recorded using low-light cameras for the first 72 hours after pairing for later assessment of latency to mating and duration of mating period. A red light was used to provide sufficient illumination for video-recording during the lights-off period. In addition, the animals’ locomotor activity during the first 24 hours after pairing was measured using customized computer software (R. Henderson, Florida State University) that monitors a series of light beams across the connecting tubes to record movements of the untethered animals between the cages. This program records the number of transits between cages and the amount of time spent by the subjects in each cage. After the initial 72 hour video-recording period, each pair was placed in a clean cage and returned to the general colony room. After a further 11 days of cohabitation, males were tested for evidence of pair-bonds with their partners.
A partner-preference test is used to assess selective affiliation and is used routinely to examine pair-bonding in voles (Williams et al. 1992). The apparatus for the partner-preference test consists of a central cage (10x17x28) joined by tubes (7.5x16 cm) to two identical parallel cages. One of these latter cages contained the familiar female partner with which the male had cohabited for two weeks, while the other cage contained an unrelated, reproductively-intact, sexually-naïve, age-matched female with which the male had no prior contact. Both females were tethered to restrict their movements to their separate cages and thus had no direct contact with each other. All cages contained food and water. The subject male was released into the central cage and had unfettered access to all cages for 3h, and his movements among the cages were monitored as outlined above. Throughout the test, the interactions of the animals were video-recorded for detailed behavioral analysis. Variables included the time spent by the male in each cage and number of transits between cages (measures of general activity to ensure that treatments do not affect locomotor behavior), and the amount of time the male spent in quiet, direct contact with each female (a measure of affiliative behavior). A partner-preference was inferred if the males spent significantly more time in contact with their partners than they did with the strangers.
Following behavioral testing, females were euthanized via CO2 asphyxiation and their uteri were examined for evidence of pregnancy. Pregnancy status was graded as previously described (Curtis 2010) using a five-point scale defining a gradient ranging from no evidence of reproductive activation to pregnancy sufficiently advanced to indicate that mating occurred within the first 48 hours after pairing. In addition, we noted the number of pups and their locations within the uterine horns. For pregnancies that were at very early stages, we noted the number and placement of implantation sites as indicated by dark red spots within the otherwise pink uteri. Finally, to assess whether female-pacing behavior could be related to differences in litter size associated with mating latency as has been noted for Microtus pennsylvanicus (Ord) (meadow voles) (Meek and Lee 1993), we retroactively examined data from 177 prairie vole pairs that were used in this and other studies. We compared litter size from pairs in which fetus size indicated early onset of mating, late onset of mating, and intermediate onset of mating relative to initial pairing.
Except for the Probability Test described below, statistical analyses were performed using Statistica software (Statsoft). Statistical assumptions were tested using Lavenes’s test for homogeneity of variance and half-normal plots for normality. Between groups treatment effects on physiological or behavioral measures were evaluated using one- or two-way ANOVAs with sex and tethering status as factors. Repeated measures ANOVA was used when measures were not independent (e.g., time split between multiple cages). Significant (p < 0.05) main effects or interactions were probed further using Student-Neuman-Keuls pair-wise comparisons. Group comparisons of mating parameters were made using the Fisher-Freeman-Halton Probability Test (http://vassarstats.net/fisher2x3.html). Data are presented as mean ± SE.
Results
Demographics and non-social behavior during cohabitation
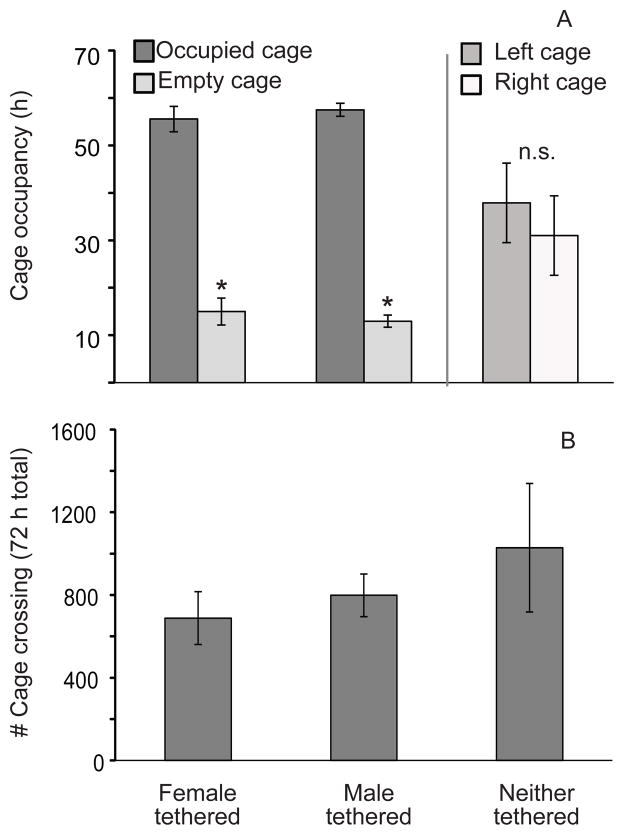
Animals in the three groups did not differ in age at the start of the experiment (group means ranged from 87 ± 5 to 100 ± 11 days of age; all p-values for pair-wise comparisons ≥ 0.36). Not surprisingly, tethering one of the animals affected the amount of time its partner spent in each of the cages during the initial 72 hours of cohabitation (Fig. 1A; significant tethering x cage interaction F2,17 = 5.98, p < 0.02). When females were tethered, their male partners spent an average of 55.6 ± 2.7 of the 72 hours in the cage containing the female and only 15.0 ± 2.8 hours in the empty cage. These values did not differ from those of females when their male partners were tethered (57.5 ± 1.4 hours and 13.0 ± 1.3 hours, respectively). When neither animal was tethered, neither cage was favored. The average number of crossings between cages for males (688 ± 128) and females (799 ± 103) when their respective partners were tethered were not different. Locomotor activity data from one male was excluded as an outlier (> 4 standard deviations above the group mean). Although there were no statistically significant effects whether or not that male was included, exclusion of that animal allows a more accurate portrayal of locomotor behavior during the initial 72 hours of cohabitation (Fig. 1B). The average number of cage crossings for the untethered pairs was somewhat higher (1028 ± 311) than for pairs in which one animal was tethered; however, the differences were not significantly different. Two males slipped out of their tethers but both did so shortly before (3 and 5 hours, respectively) the end of the 72 h cohabitation period. Since mating by these pairs had already ended, this small amount of time without restriction was not considered sufficient to warrant excluding them from the study.
Figure 1.
Non-social behaviors exhibited by newly-paired prairie voles with various restrictions on freedom of movement among cages. A) Amount of time spent in each half of a two-cage apparatus by the male vole when the female was tethered to restrict her movements to a single cage; by the female when the male’s movements were restricted, and when neither animals’ movements were restricted. The asterisk indicates significantly less time spent in the unoccupied cage than in the cage were the partner was tethered (p < 0.001); n.s. indicates no significant group differences. B) Number of cage crossings by male voles with tethered female mates, by females with tethered male mates, and by pairs in which neither animal was tethered
Female pacing and mating behavior or success
Three pairs (two pairs in which the male was tethered and one pair in which neither animal was tethered) did not mate during the initial 72 hour cohabitation period. Examination of the females from these three pairs at the end of the two-week cohabitation revealed one female that displayed no evidence of female reproductive activation, and two females that displayed evidence of mating within the last 2–3 days of the two-week cohabitation period. Several other pairs mounded bedding such that some details of their behaviors could not be fully assessed, but all of these pairs were scored as “maximally” pregnant at the end of the experiment. All other pairs were observed to mate during the first 72 hours after pairing. The overall latency to first mating (39.7 ± 5.5 hours) was quite similar to that previously reported for reproductively intact female prairie voles (39.5 ± 1.3 hours; (Curtis 2010)) and there were no significant differences among the three tethering groups (F2,18 = 0.62, p = 0.55). The duration of mating (19.3 ± 2.4 hours) also was similar to that previously reported (16.6 ± 2.5 hours; (Curtis 2010)) and, again there were no group differences (F2,24 = 0.62, p = 0.27). Finally there were no differences in the numbers of mating bouts among pairs that mated.
Female pacing did not alter any of the measures of mating success. When the male was tethered (i.e., mating was female-paced), 9/11 pairs mated successfully; when the female was tethered, 7/7 pairs mated successfully; and 8/9 pairs mated successfully when neither animal was tethered. These ratios were not statistically different (PA = PB = 0.76). Seventeen of twenty-seven pairs achieved pregnancy within approximately 48 hours, seven pairs within approximately 96 hours and only three pairs showed no signs of sexual activation. In the present study, females that controlled the pace of mating carried a mean of 3.5 ± 0.7 pups at time of sacrifice. This value was not different from those for females that were tethered (4.1 ± 0.6 pups) or for females when neither animal was tethered (3.6 ± 0.6 pups). Examination of litter size (n = 177 litters) as a function of the timing of mating onset revealed no effect of mating onset (early mating onset 4.15 ± 0.10 pups; intermediate mating onset 4.31 ± 0.23 pups; late mating onset 4.71 ± 0.32 pups).
Female pacing and male partner-preference performance
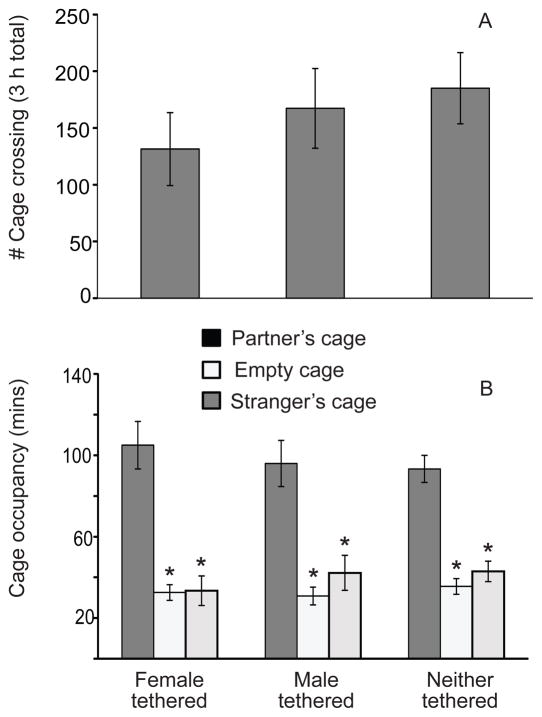
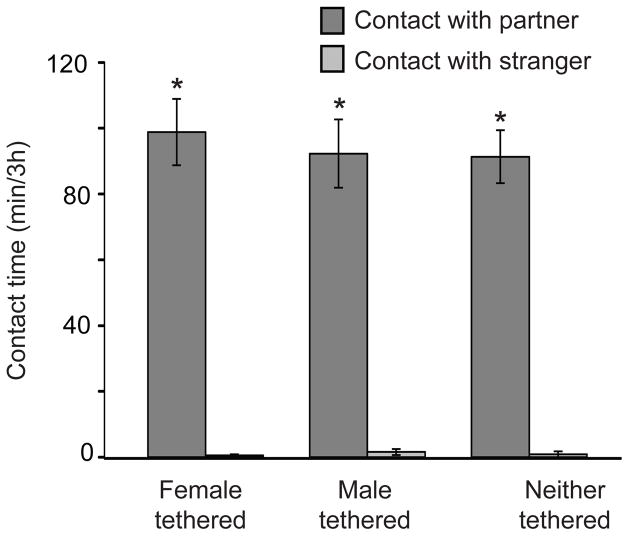
Overall, measures of non-social behaviors during the partner-preference test did not differ between males in each of the three treatment groups during the partner-preference tests. Locomotor activity, as measured by the number of crossings between cages, did not differ between groups (Fig. 2A). The patterns of cage occupancy (Fig. 2B) also did not differ among the three tethering groups. Regardless of group, males spent significantly more time in their partner’s cage than in either the stranger’s cage or the empty cage during the test (F2,48 = 41.74, p < 0.001; p < 0.02 for all pair-wise comparisons). Time spent in the empty cage and the cage in which the stranger was tethered did not differ. Males from all three groups spent significantly more time in quiet direct contact with their respective mates than they did with the strangers (Fig. 3; F1,23 = 326.12, p < 0.001; p-values for all pair-wise comparisons < 0.001, effect size = 0.93), but the amounts of contact with the respective partners did not differ between groups (F2,23 = 0.23, p = 0.80; p-values for all pair-wise comparisons > 0.43).
Figure 2.
Non-social behaviors exhibited by male prairie voles during partner-preference testing. A) Locomotor activity by males whose mates were tethered during initial 72 hours of a two-week cohabitation; by males who were themselves tethered during the early part of the cohabitation; and by males in which neither the male nor his mate were tethered during cohabitation. B) Amount of time spent in each part of a three-cage apparatus by males in each of the three groups described in (A). The asterisks indicate significantly less time spent in the empty cage or the stranger’s cage than in the cage containing the familiar partner (p < 0.02).
Figure 3.
Social contact exhibited by male prairie voles during a partner-preference test. The asterisk indicates significantly more time spent in close, quiet contact with the familiar partner than with the stranger (p < 0.02).
Discussion
Pair-bonding by male prairie voles is strongly influenced by reproductive compatibility with their mates. Males display stronger partner-preferences and higher levels of stranger-oriented aggression if their mates become pregnant within about 48–72 hours after pairing than do males with partners that do not become pregnant or whose pregnancies are delayed (Curtis 2010, Resendez et al. 2012). Importantly, this behavioral variation appears to be tied to successful pregnancy rather than to the timing of copulation per se. Males that are paired with estrogen-primed, ovarectomized (and thus sexually receptive but incapable of pregnancy) females do not express partner-preferences despite copulating within the appropriate timeframe (Curtis 2010). Thus, other factors that affect reproductive success need to be explored.
Previous studies examining vole mating patterns generally have allowed both animals of a pair to move freely about the enclosure. The inability of a female to completely isolate herself from the male is a conspicuous limitation of these studies. For example, Corona et al. (2011) concluded that male behavior contributed to the pace of mating in meadow voles; however, in that experiment the female was not given the option of separating herself from the male entirely. Although female prairie voles often will reject the advances of a male (unpublished observation), this behavior does not afford the same magnitude of control by the female as when she can freely approach and withdraw from a restrained male. In the present study, we variously manipulated freedom of access of each member of mixed-sex pairs of prairie voles to their respective mates. In one group, the males’ movements were restricted, thus permitting the females to set the pace of mating, i.e., female-pacing. In this group, the females could control the temporal pattern of social interactions with the male, the onset of mating, and the frequency of mating behaviors such as mounts and intromissions and, ultimately, ejaculations by the males. It might be expected that the females in this group would experience a lower level of stress relative to those in the other groups. In addition to the female-paced group, there were groups in which the females had less control over the pace of mating and thus, potentially, experienced higher stress levels. In one of these groups, neither animal was tethered which may have permitted the females to have some modicum of control of mating by providing at least some possibility for them to move away from the males. The final group consisted of pairs in which the females were tethered and, thus, had the least amount of control over interactions with the male, which in turn may produce the highest stress levels. Since stress interferes with pair-bond formation in female voles (DeVries et al. 1996), non-paced mating could indirectly affect male behavior by altering female responses during partner-preference testing. Further, in rats, female-paced mating is rewarding (Jenkins and Becker 2003). If a similar response occurs in prairie voles, females may find paced-mating to be more of a positive experience, in addition to it being less stressful, than if the pace of mating is uncontrollable, again impacting female responses to the male during behavioral testing. Neither appears to be the case.
Although female behaviors associated with mating do not appear to alter subsequent male behavior directly, the possibility remains that female-pacing may affect reproductive outcomes, and thus affect male behavior indirectly. Again, this does not appear to be the case. Neither any of the indices of non-social behavior nor any of the measures of reproductive success differed among the three treatment groups. Even when the male was tethered and the female had complete control of social interaction, the amount of time she spent in the cage containing the male did not differ from that occurring when the female was tethered and the male was in control of the interactions. Similarly, female movements among the cages did not differ from those of males. Female-pacing did not affect the proportion of pairs that mated, and did not affect latency to onset of mating or duration of mating. In fact, the latter two measures were remarkably similar to those previously reported for this species (Curtis 2010). Finally, our hypothesis that female-paced mating could affect subsequent male behavior by altering fertility was not supported since there were no group differences in litter size, nor was there a difference in litter size as a function of latency to pregnancy onset.
A few caveats must be mentioned. We did not attempt to manipulate the timing, duration, number of intromissions, or the number of mating bouts. Thus, the present study differs from earlier similar mating studies that, for example, limited mating to one copulatory sequence (Coopersmith and Erskine 1994). The females in the present study were nulliparous and it is unclear how parity (Dewsbury et al. 1979) may interact with female-pacing in voles. However, at the time of parturition, female prairie voles likely have already formed social bonds and, thus, reduced mating success in post-partum pairs may not affect their subsequent social behavior. This is an aspect of social behavior that has not been examined in prairie voles and further study may provide insights into the stability of prairie vole pair-bonds.
Finally, the present study examined the effects of female pacing on male behavior. This begs the obvious question – Why not test females? The answer is found in the study by Resendez et al. (2012) which showed that the effects of pregnancy status on pair-bond expression are sexually dimorphic in prairie voles. In that study, stranger-oriented aggressive behavior by males was positively correlated with their mates’ pregnancy status; however, no relationship between pregnancy status and aggression was displayed by females (Resendez et al. 2012). Thus, it appears that differential timing of pregnancy onset does not affect pair bond formation in females as it does in males, so we focused our efforts on examining male behavior. Overall, the results of this study suggest that female paced-mating behavior does not directly or indirectly affect the behavior of her mate during subsequent partner-preference testing. In a previous paper (Curtis 2010), we suggested that non-volatile pheromonal signals from the female that carry information regarding the status of her pregnancy strongly influence male preference behavior. We find nothing in the results of the present study to refute that suggestion.
Acknowledgments
Funding for this project was provided in part by the National Institute for Child Health and Development (HD48462), by the National Institute for General Medical Sciences (GM110593), and by intramural funding from Oklahoma State University Center for Health Sciences
Contributor Information
Kelly McCracken, Email: kelly.mccracken@okstate.edu.
Robert Lewis, Email: robert.lewis11@okstate.edu.
Literature Cited
- Adler NT. Effects of the male’s copulatory behavior on successful pregnancy of the female rat. J Comp Physiol Psychol. 1969;69:613–622. doi: 10.1037/h0028244. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–139. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. The prairie vole (Microtus ochrogaster): an animal model for behavioral neuroendocrine research on pair bonding. ILAR J. 2004;45:35–45. doi: 10.1093/ilar.45.1.35. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Thompson EG, Carlstead K. Effects of hormonal, sexual, and social history on mating and pair bonding in prairie voles. Physiol Behav. 1988;44:691–697. doi: 10.1016/0031-9384(88)90049-2. [DOI] [PubMed] [Google Scholar]
- Coopersmith C, Erskine MS. Influence of paced mating and number of intromissions on fertility in the laboratory rat. J Reprod Fert. 1994;102:451–458. doi: 10.1530/jrf.0.1020451. [DOI] [PubMed] [Google Scholar]
- Corona R, Larriva-Sahd J, Paredes RG. Paced-mating increases the number of adult new born cells in the internal cellular (granular) layer of the accessory olfactory bulb. PLOS One. 2011;6:e19380. doi: 10.1371/journal.pone.0019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis JT. Does fertility trump monogamy? Anim Behav. 2010;80:319–328. doi: 10.1016/j.anbehav.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, Carter CS. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proc Natl Acad Sci U S A. 1996;93:11980–11984. doi: 10.1073/pnas.93.21.11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewsbury DA, Evans RL, Webster DG. Pregnancy initiation in postpartum estrus in three species of Muroid rodents. Horm Behav. 1979;13:1–8. doi: 10.1016/0018-506x(79)90030-8. [DOI] [PubMed] [Google Scholar]
- Drickamer LC, Gowaty PA, Holmes CM. Free female mate choice in house mice affects reproductive success and offspring viability and performance. Anim Behav. 2000;59:371–378. doi: 10.1006/anbe.1999.1316. [DOI] [PubMed] [Google Scholar]
- Gray GD, Zerylnic M, Davis HN, Dewsbury DA. Effects of variations in male copulatory behavior on ovulation and implantation in prairie voles, Microtus ochrogaster. Horm Behav. 1974;5:389–396. doi: 10.1016/0018-506x(74)90025-7. [DOI] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 2003;18:1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- Pierce JD, Dewsbury DA. Female preferences for unmated versus mated males in two species of voles (Microtus ochrogaster and Microtus montanus) J Comp Psychol. 1991;105:165–171. [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. κ-opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. J Neurosci. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]