Abstract
Extant panarthropods (euarthropods, onychophorans and tardigrades) are hallmarked by stunning morphological and taxonomic diversity, but their central nervous systems (CNS) are relatively conserved. The timing of divergences of the ground pattern CNS organization of the major panarthropod clades has been poorly constrained because of a scarcity of data from their early fossil record. Although the CNS has been documented in three-dimensional detail in insects from Cenozoic ambers, it is widely assumed that these tissues are too prone to decay to withstand other styles of fossilization or geologically older preservation. However, Cambrian Burgess Shale-type compressions have emerged as sources of fossilized brains and nerve cords. CNS in these Cambrian fossils are preserved as carbon films or as iron oxides/hydroxides after pyrite in association with carbon. Experiments with carcasses compacted in fine-grained sediment depict preservation of neural tissue for a more prolonged temporal window than anticipated by decay experiments in other media. CNS and compound eye characters in exceptionally preserved Cambrian fossils predict divergences of the mandibulate and chelicerate ground patterns by Cambrian Stage 3 (ca 518 Ma), a dating that is compatible with molecular estimates for these splits.
Keywords: Cambrian, brains, Chengjiang, Burgess Shale, Arthropoda
1. Introduction
Panarthropods provide an intriguing test case for exploring the efficacy of neural characters for inferring phylogeny because the group presents the challenges of unrivalled species diversity and a vast spectrum of phenotypic variation. Euarthropoda alone is known from more than 1.2 million extant species and a fossil record spanning more than 520 Myr. This staggering diversity correlates with marked disparity between major groups, a situation that complicates efforts to resolve the arthropod Tree of Life based on morphological data [1].
However, in the face of this variability, neural architecture within major groups is relatively conserved. As a result, neuroanatomical characters have figured prominently in morphology-based phylogenetic analyses of euarthropod phylogeny [2–4], and the topologies so produced show a high degree of congruence with multi-locus molecular estimates of phylogeny [5–7]. Fossils have likewise been afforded an important role in understanding panarthropod evolution in deep time [8,9]. Especially germane to questions of deep phylogeny are non-biomineralized fossils from the Cambrian that record details not only of exoskeletal form but also appendages and internal organs and tissues. The nervous system has until recently been all but undocumented in the compression fossils that dominate discussion of early panarthropods, named Burgess Shale-type preservation [10–12] after the iconic example of the Burgess Shale in Canada. This dearth of neuroanatomical data from Cambrian fossils has meant that neuroanatomy and palaeontology have largely operated exclusively of each other in considerations of panarthropod evolution. A shortcoming of this for neontological approaches is that the timing of character acquisition of extant groups, e.g. when specific ground pattern characters of the chelicerate and mandibulate nervous systems originated, is unconstrained. Fossils are the only direct basis for elucidating ancient morphologies and dating divergences but until recently the fossil record of neuroanatomy has been largely untapped with respect to phylogenetic questions. Herein we review recent findings in panarthropod ‘neuropalaeontology’ with particular reference to its fossilization and significance for systematics.
Systematic nomenclature herein follows recent recommendations [13]. Panarthropoda encompasses Tardigrada, Onychophora and Euarthropoda; crown-group Euarthropoda is composed of Chelicerata and Mandibulata; that clade and those stem-group euarthropod fossils that possess a structurally differentiated deutocerebral appendage are assigned to Deuteropoda.
2. Information loss and retention in the fossil record
The delayed adoption of neuropalaeontology is influenced by a mix of facts and suppositions about decay and its role in information loss in the fossil record. Preservation of internal anatomy in fossils involves stabilization of soft tissue before anatomical detail is lost to decay. Experimental decay series for extant animals are most frequently used to rank tissues into a temporal sequence of their decay proneness, which at one end of the scale are referred to as labile (those lost to decay early) and at the other as recalcitrant (those that withstand considerable decay). The position of particular tissue types in this temporal scale is commonly linked to their fossilization potential [14]. In the extreme case, labile tissues are viewed as unlikely or even impossible to fossilize.
Decay experiments have been conducted on many kinds of invertebrates, the most phylogenetically relevant for this study being those on marine decapod crustaceans [15], branchiopods [16] and on onychophorans [17]. The latter classified the nerve cords as relatively labile and this observation underpinned the authors' scepticism about the preservation potential for neural tissue in panarthropod fossils in general and in Early Palaeozoic Konservat-Lagerstätten in particular.
Some caveats need be acknowledged. The same decay experiments demonstrate that muscle is a labile tissue and so by the same standard we could be led to predict its low fossilization potential. However, diverse taxa from Mesozoic and Cenozoic lithographic limestones, including arthropods, molluscs and fishes, show that muscle can be preserved in fine detail by being replicated by calcium phosphate [18]. Such apatite replication of muscle is represented over a considerable span of the geological record and is not confined to ‘Solnhofen-type’ preservation in platy limestones. Early diagenetic replication of muscle in calcium phosphate is observed in Cambrian Konservat-Lagerstätten, including Sirius Passet in Greenland [19], the Emu Bay Shale in Australia [20] and the Burgess Shale in Canada [21]. Likewise, the onychophoran decay experiments show that the gut is prone to early decay [17], yet the fossil record of the Cambrian is replete with guts [22–24]. In Burgess Shale-type fossils, decay-resistant structures such as the cuticle and the appendages are preserved effectively as two-dimensional carbonaceous compressions; conversely and almost paradoxically, a more labile tissue type, the gut, is frequently preserved in three dimensions [19]. The volatility of the gut serves to localize microbial activity that leads to early diagenetic mineral precipitation [15], morphology again typically being replicated in calcium phosphate [10]. The fidelity of preservation is such that sub-millimetric structures can be resolved in, e.g. the midgut glands of Cambrian arthropods [23,24]. These observations on muscle and the gut of course have no necessary bearing on the fossilization potential of neural tissue (exceptional preservation of muscle and gut could, for example, be dependent on a single taphonomic mode, i.e. phosphatization). They do nonetheless reveal that decay proneness does not in itself equate with non-fossilization. Perhaps, the most stunning example that belies the notion that neural tissue rapidly decays is the discovery of a human brain preserved almost intact over more than 2000 years in the absence of entombment [25].
To explore aspects as to how decay and compaction affect neural tissue preservation, actualistic experiments were conducted on extant proxies, as described in §2a,b, and compared with putative fossilized CNS.
(a). The effect of sediment on neural tissue preservation
Recently, the consequences of differences in sediment mineralogy on the preservation of non-biomineralized recalcitrant tissues in annelids and arthropods have been explored experimentally [26]. In a similar vein, we have conducted experiments with the polychaete Nereis virens to examine the survival of neural tissue in carcasses that have been entombed in sediment and subjected to compaction during the decay process (cf. observations on decay of N. virens in artificial seawater) [27].
In our entombment experiments, living specimens of Nereis virens were kept in seawater at 7°C. Animals were immobilized on ice. Heads with about 1–1.5 cm of the trunk were placed lengthwise on the surface of a 1.0 cm deep layer of fine-grained Carbondale C clay powder soaked in seawater. This was contained in a container approximately 9 cm long, 3.5 cm wide, and 5 cm deep, lined with Teflon film. The polychaete was then covered with a slurry of clay in seawater to a depth of 2.5 cm. The surface was covered with Teflon on which was placed an 8 × 3 cm glass slide. The container and its contents were transferred to the cool room (7°C) and a 2 × 6 × 2 cm brass weight (80 g) was placed on the slide. The container was left in this condition for 14 days, after which the weight was increased to 160 g, and then to 240 g after a further 14 days. This ensemble remained in this condition for one to two months, during which the clay slowly dried. The weights were then removed and the container transferred to room temperature and left for a further 30–40 days. The slide and Teflon were then removed and the hardened clay removed from the container. The clay was cracked open with a chisel until flattened dewatered remains were found as part and counterpart, one of which came away as almost exclusively cuticle; the other contained traces of soft tissue (muscle and ganglia; figure 1a). Excess grains of clay were removed with a fine water-colouring brush prior to photography.
Figure 1.

Entombment experiment with Nereis virens compared with fossilized CNS in the Cambrian euarthropod Chengjiangocaris. (a) N. virens after compression and desiccation in clay. Inset shows ventral nerve cord. (b) Chengjiangocaris kunmingensis [28], showing ventral nerve cord with segmental ganglia in the trunk (image courtesy of J. Yang, J. Ortega-Hernández and X. Zhang). Scale bar, 1 mm.
These experiments reveal that the nerve cord clearly retains its integrity months after death, with discrete segmental ganglia being obvious (figure 1a). Such simulations do not of course reproduce the process of fossilization. Nonetheless, the durability of neural tissue in sediment suggests that decay experiments designed to exclude sediment from the equation are probably ignoring a vital factor in exceptional fossil preservation.
(b). The effects of flattening on brain shape
When interpreting the anteroposterior sequence of neuromeres and segmental tracts in the brains of compression fossils such as the Cambrian examples outlined in §3, there is a need to consider the three-dimensionality of arthropod brains and the potential for topologies to be distorted by compaction.
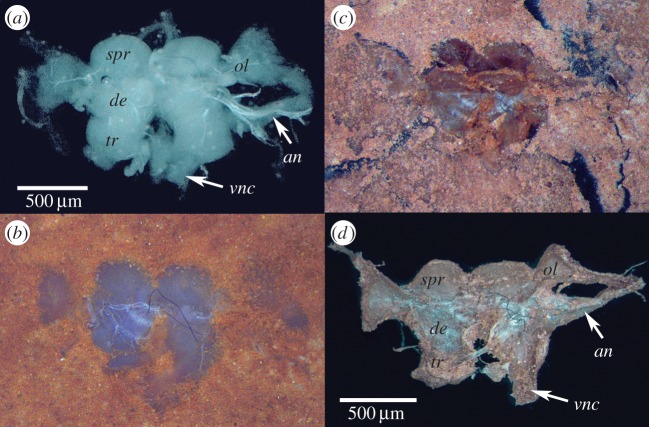
To perform an actualistic experiment, fresh excised brains of the cockroach Periplaneta americana (figure 2a) were placed with antennal nerves downward on a slurry of Carbondale C clay contained in the well of a thick glass slide. Wet clay was then placed over the brain and a heavy glass cover placed on top (figure 2b). The ensemble was left in the cold room for 10 days during which the clay slowly dried. Afterwards, the glass cover was removed and the dried clay carefully brushed away until dewatered tissue was encountered as an extremely thin film of weakly stained material (pinkish blue) not thicker than lens tissue (figure 2d). Control experiments, in which whole heads were embedded, revealed similarly preserved and likewise paper-thin brains.
Figure 2.
Experimental flattening of cockroach brain. (a) Fresh excised brain of Periplaneta americana. (b) Brain embedded in slurry of wet clay. (c) Compacted, drying brain in clay. (d) Flattened brain removed from clay. spr, superior protocerebrum; de, deutocerebrum; tri, tritocerebrum; ol, optic lobe; an, antennal nerve; vnc, ventral nerve connective.
In both experimental conditions, brain profiles did not differ from those of fresh material at the same orientation (figure 2). This suggests that flattening is not likely to lead to erroneous interpretations of the serial register of brain segmentation.
3. The fossil record of panarthropod neural tissue
It is demonstrably incorrect that neural tissue cannot fossilize. The brain is preserved in various kinds of insects from Miocene amber of the Dominican Republic [29,30], including species of Hymenoptera, Diptera and Coleoptera. Although the histological details of neural tissue in these amber fossils are uncertain, brain outlines preserve accurately. Even older ambers, notably the Eocene Baltic amber, likewise preserve the outlines of neural tissue in sufficient fidelity for imaging approaches such as synchrotron microtomography to resolve the brain, optic neuropils, antennal nerves and suboesophageal ganglion in the case of a strepsipteran [31]. Acknowledging that amber is geologically young and possibly the ‘gold standard’ of fossilization (e.g. preserving the mitochondria of flight muscle in insects that also preserve the brain) [30], it is nonetheless special pleading to discard amber as a whole in order to defend the case that neural tissue has no fossil record.
In recent years, the search for fossilized neural tissue has shifted much deeper in geological time and to a different mode of fossil preservation, specifically to Cambrian fossils of Burgess Shale-type preservation. They are of particular evolutionary importance because they sample animal lineages early in their phylogenetic history.
The Burgess Shale in British Columbia, Canada [32], dates to Cambrian Stage 5, spanning an interval from ca 510–505 Ma. Similar taxa and styles of preservation occur in the broadly coeval units throughout the region, such as the recently documented Marble Canyon assemblage [33]. Neural tissue preservation in the Burgess Shale has until recently been rather sporadic in its documentation, though its history of study extends to the 1970s. Early reports include two specimens of the common priapulid Ottoia prolifica in which a paired reflective strand along the ventral midline was interpreted as the nerve cord [28] (figure 2a), its paired nature possibly representing the margins of a single cord. It is most plausibly situated ventrally, as expected for priapulids. The problematic organism Amiskwia sagittiformis Walcott has likewise had reflective traces in the head and trunk identified as possible cerebral ganglia and the nerve cord, respectively [34].
The other major occurrence of neural tissue preservation in Cambrian fossils is the Chengjiang biota of Yunnan Province, China [35]. This early Cambrian Konservat-Lagerstätten predates the Burgess Shale by some 10 Myr, dating to Cambrian Stage 3. CNS has also been documented in the Xiaoshiba Konservat-Lagerstätten, in the Hongjingshao Formation in Yunnan Province [36]. This biota slightly postdates Chengjiang in Cambrian Stage 3.
The §3a–i summarizes work to date on panarthropod CNS in the Burgess Shale and Chengjiang/Xiaoshiba (figure 3). Representative species are listed following a stemward to crownward sequence with reference to Euarthropoda (following the nomenclature and tree topology of Ortega-Hernández [13]).
Figure 3.
Cambrian Burgess Shale-type fossils preserving traces of the CNS. (a) Ottoia prolifica, USNM 188635, showing ventral nerve cord (vnc) as paired strands. (b) Waptia fieldensis, USNM 83948j, anterior part of head with inverted light. (c) Odaraia expansa, ROM 60746, anterior cephalic structures in cross-polarized light (image from http://burgess-shale.rom.on.ca/en/index.php, courtesy of Jean-Bernard Caron, Royal Ontario Museum). (d,e) Lyrarapax unguispinus, YKLP 13305, anterior part of head: (d) SEM-EDX carbon map; (e) light photograph. (f) Fuxianhuia protensa, YKLP 15006a, anterior cephalic structures, micro X-ray fluorescence iron map (lavender). (g) Alalcomenaeus sp., YKLP 11075, head, neural traces in inverted white coincidence signal of micro-CT (green) and SEM-EDX iron map (magenta). a1, antennal (deutocerebral) tract; a2, tritocerebral tract; an, antenna; br, brain; e, eye; frg, frontal ganglion; lp, lateral protocerebrum; mp, median photoreceptor; of, oesophaegeal foramen; on, optic neuropil; opt, optic tract; pl, protocerebral lobe; pr, protocerebrum. Scale bars, 2 mm except a, 5 mm.
(a). Paucipodia inermis
This lobopodian from Chengjiang includes specimens with paired longitudinal bands spanning several trunk segments. The bands are associated with bilaterally symmetrical rounded swellings preserved with similar violet pigmentation ([37], fig. 4f). These structures were interpreted as the nerve cord and segmental ganglia, respectively [37]. One specimen has paired pigmented lobes in the preoral region ([37], figs 3a and 4g) that were tentatively identified as the brain. However, differences in the style of preservation from CNS in other Chengjiang taxa described in §3b–d,g have prompted questions about the identity of the putative neural tissues in Paucipodia inermis [38].
The most recent phylogenetic analysis of Cambrian lobopodians in the context of panarthropod phylogeny resolves P. inermis (as well as most of the ‘armoured’ lobopodians) as stem-group Onychophora [38]. If the CNS interpretations [37] and the phylogenetic placement are accurate, segmental ganglia in a stem-group onychophoran present a closer similarity to the segmentally ganglionated nerve cords of euarthropods and tardigrades than to extant Onychophora. The onychophoran nerve cord has been viewed as non-ganglionated [39], and the brain is debatably composed of fused ganglia [40].
(b). Lyrarapax unguispinus
Radiodonta, best known from Anomalocaris [21], are large Cambro-Devonian predators that have been placed in various parts of the ecdysozoan tree, ranging from having closest affinities to Cycloneuralia (with convergent similarities to arthropods), being a part of the euarthropod stem group, or belonging to the euarthropod crown group as stem-group chelicerates (reviewed by [8]). Controversy has surrounded the segmental affiliation of their anteriormost appendages and whether they are or are not aligned with those of other fossil groups.
Species of various radiodontans from the Burgess Shale and Chengjiang indicate that these animals have a pair of stalked eyes that project from the dorsolateral part of the head [21,41]. In these biotas, the inferred eyes are preserved as carbon films that lack details of the visual surface. Specimens from Australia clarified the visual surface of Anomalocaris [42]. Lenses are very numerous, more than 16 000 ommatidia per eye, and they have a hexagonal packing arrangement that corresponds closely to various kinds of apposition eyes seen in extant euarthropods. Ommatidial lenses are also known in the Devonian Schinderhannes [43], which has been identified as a radiodontan [13,44]. The discovery that radiodontans have compound eyes strengthens the case for euarthropod affinities and constrains the node at which such organs evolved in the euarthropod stem group.
Lyrarapax unguispinus, a radiodontan from Chengjiang with an enlarged, paddle-like anterior trunk flap, also preserves the eyes, and the carbon signal associated with them shows relationships that indicate the preservation of neural tissue [45]. A transverse tract extending from the eye represents the optic nerve (figure 3d,e). Assuming the same segmental relationships as eyes have in all panarthropods, this constrains the interpretation of the protocerebral part of the brain. A pair of carbon spots preserved similarly to the retinae is located in the anteromedian part of the head (figure 3d). Based on a common mode of preservation and their connection to the protocerebrum, these paired structures are taken to be parts of the nervous system. Their pre-ocular position corresponds to the origination of a pre-ocular appendage pair, the robust, spinose frontal appendages. The reflective patches are thus interpreted as a pair of frontal ganglia associated with these appendages [45].
Extant euarthropods lack frontal appendages and frontal ganglia, but it has been suggested that onychophorans possess a segmentally homologous appendage pair, the so-called antennae or frontal appendages [46], and neuropil areas associated with these appendages have been identified as ganglia [47].
(c). Fuxianhuia protensa
Fuxianhuia protensa is a member of a clade from the early Cambrian of South China collectively known as Fuxianhuiida. The group has been resolved in the euarthropod stem group in numerous phylogenetic analyses [44,48,49]. Bergström et al. [49] documented two specimens of F. protensa from the Chengjiang biota that they suggested preserve possible traces of CNS. One of these was drawn upon to interpret brain morphology in this species [50]. The structure in question is situated medially in the head (figure 3f). Its anteromedial outline is a pair of symmetrical lobes, interpreted as protocerebral. It throws symmetrical tracts into paired appendages, specifically the antennae and a specialized post-antennal appendage pair [36]. The same style of preservation is seen in tracts that extend through the optic lobes, interpreted as the optic nerve. In comparison to small brains in extant crustaceans such as shrimps, F. protensa is interpreted as having proto-, deuto- and tritocerebral neuromeres in its pre-stomodaeal brain.
Putative neural tissue in the original F. protensa specimen shows pronounced enrichment in iron [50] (figure 3f). Scanning electron microscopy revealed that the iron in the brain and optic lobes is in the form of framboids of pyrite crystals (figure 4b). As in Chengjiang fossils generally, iron oxide and iron hydroxide pseudomorphs follow pyrite [51,52], sulfur having been depleted in the weathering process. The framboidal, crystalline morphology has been interpreted as early diagenetic pyrite [51], suggestive of neural tissue having been replicated by pyrite mineralization. The counterpart, however, shows carbon between the pyrite framboids (figure 4a), as would be consistent with an original carbon template and subsequent tissue-specific localization of pyrite. A role for carbon in preservation of neural tissue in F. protensa is also seen in other specimens, in which energy dispersive X-ray spectroscopy (SEM-EDX) depicts a strong carbon signal in the inferred brain and nerve tracts. In these cases, iron is instead preferentially concentrated in recalcitrant extracellular tissues like cuticle, as is commonly the case in Chengjiang fossils [51,52]. It has been proposed that relocalization of iron in Chengjiang fossils occurred late in diagenesis [53], but the diagnostic pseudomorphs in the brain of F. protensa argue against pyrite not having played a role in fixation of these tissues. The interplay between carbon and pyrite in the preservation of the same kind of soft tissue has come to be recognized as a pervasive pattern in Ediacaran–Cambrian Konservat-Lagerstätten, the dynamic between the two taphonomic modes sensitive to variation in bacteria over minute spatial and temporal scales [54].
Figure 4.
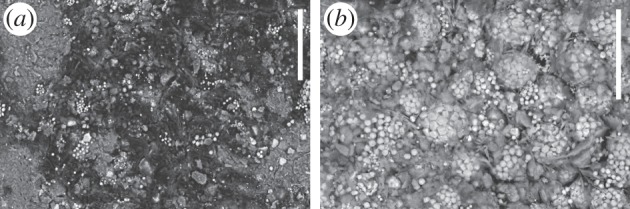
Neural tissue preservation in Fuxianhuia protensa. (a) Carbon on nerve track from deutocerebral region (YKLP 15006b, counterpart); (b) pyrite framboids and crystals from eye region (YKLP 15006a, part). Scale bars, 20 µm.
(d). Chengjiangocaris kunmingensis
In addition to the brain of F. protensa, parts of the nervous system have been documented in another fuxianhuiid taxon, Chengjiangocaris kunmingensis, from the early Cambrian Xiaoshiba site. This material [36] shows the ventral nerve cord as the expected arthropod rope-ladder nerve cord with a pair of ganglia in each segment (figure 1b).
(e). Odaraia alata
The bivalved Burgess Shale arthropod Odaraia alata is currently considered to be a member of the euarthropod stem group, closely allied to a number of other bivalved Cambrian taxa that are resolved as a paraphyletic assemblage stemward of fuxianhuiids [44]. Neuroanatomy has been documented in the head of O. alata [55], with traces of the brain represented in multiple specimens. A pair of large lateral eyes is preserved as highly reflective carbon films, and reflective tracts extending from the eyes (the optic nerves) converge medially on a bilateral symmetrical bilobate field that has the same style of preservation (figure 3c). The confluence with the eyes, bilateral symmetry and correspondence in position and relationships to a protocerebrum underpin the interpretation of the medial reflective area as the brain [55]. Three reflective spots are bilaterally symmetrically positioned anterior to the inferred protocerebrum and are connected to it. They are evidently associated with a small triangular sclerite (‘anterior sclerite’ sensu [56]) that lies between the anteromedial notch between the carapace valves. The trio of spots has been interpreted as medially positioned photoreceptors [55].
(f). Mollisonia symmetrica
Optic neuropils have been reported in Mollisonia symmetrica, a Burgess Shale arthropod of undetermined affinities from the Marble Canyon locality ([33], figure 3p,q).
(g). Alalcomenaeus sp.
Alalcomenaeus and the well-known Leanchoilia [57] belong to a controversial group of Cambrian arthropods known as Megacheira, or great-appendage arthropods. The colloquial name is derived from their anteriormost appendage pair, which has three spine-like podomeres that in the relevant species each bear a flagellum [57]. Partly depending on the segment with which the great appendage is affiliated, megacheirans are variably identified as stem-group euarthropods [44] or as crown-group euarthropods related to chelicerates. The latter scheme homologizes the structure of a chelicera and the great appendage. This is based on shared possession of an elbow joint that separates a basal pedunculate part and a distal part with extensions that contribute to a chela composed of a fixed and a movable finger [58,59].
Neuroanatomical interpretations are based on a specimen of the Chengjiang megacheiran Alalcomenaeus sp. [60]. It is preserved in dorsal view, with internal and external structures both subjected to pyritization. Overlaying the iron signal from the pyrite with the density difference detected by micro-CT permits a profile identifiable as trace nervous system to be visualized (figure 3g). It depicts continuity between the eyes (paired on each side of the head) and a swelling that corresponds to the position and morphology of an optic neuropil. The latter is connected to a region bordering an elongate oesophageal foramen that has the expected relationships of the protocerebrum of chelicerates. This is then followed caudally by more diffuse neural tissue that includes ganglia associated with the segmental appendages of the head and trunk.
A single optic neuropil separated from the protocerebrum and serving paired eyes is shared with Chelicerata among living arthropods. A concentration of neural tissue in the post-ocular region is associated with the attachment site of the great appendage. This alignment of head segments is consistent with a homology of chelicerae and great appendages as deutocerebral appendages.
(h). Helmetia expansa
Helmetia expansa from the Burgess Shale belongs to Conciliterga, a Cambrian clade within a group of Palaeozoic taxa that includes trilobites. Depending on its exact composition, the names Lamellipedia [61] or Artiopoda [62] are applied to this ‘trilobitomorph’ assemblage, and their affinities to Mandibulata or Chelicerata remain contested.
As described above for O. alata, H. expansa has an anterior sclerite positioned anteromedial to the cephalic shield. This sclerite is associated with a pair of carbonaceous reflective spots or ‘frontal organs’ that are, as for O. alata, interpreted as photoreceptors [55]. Also as for O. alata, the eyes share this same style of preservation as highly reflective organs and the optic nerve is traceable medial to the eye. The common pattern of the anterior sclerite in Odaraia and Helmetia being associated with frontal organs that are, at least in the case of O. alata, demonstrably connected to the protocerebrum, underpins a homology of the sclerite itself between these stem- and crown-group euarthropods [55].
(i). Waptia fieldensis
Neural tissue preservation has been identified in a crustacean-like arthropod from the Burgess Shale, Waptia fieldensis [63,64]. The species has not been comprehensively revised in modern times and whether its similarities to crustaceans are indicative of close affinities in the euarthropod crown group is unresolved. Nonetheless, the fossils preserve mechanosensory and chemosensory sensilla on their appendages that can be interpreted both structurally and functionally in comparison to malacostracan crustaceans. In some specimens, the optic nerve can be traced through the eye stalk [63] to a comparably pigmented median region that corresponds to the position and expected morphology of a brain [64] (figure 3b).
4. Conclusion
The interpretations of Cambrian fossils summarized herein set constraints on the timing of nervous system evolution in euarthropods. If, for example, Alalcomenaeus and other Megacheira are total-group chelicerates, then the chelicerate and by extension mandibulate ground patterns are at least as old as it, or some 518 Myr. The standard placement of fuxianhuiids in the euarthropod stem group [44,48,49] suggests that mandibulate-like characters of F. protensa, such as three neuromeres in the pre-stomodaeal brain and nested optic neuropils [50], may resolve more deeply in the euarthropod total-group than conventionally assumed. Placing L. unguispinus and fellow radiodontans more basally in the euarthropod stem, pre-ocular frontal appendages and frontal ganglia emerge as characters that were probably present in the deepest parts of the euarthropod stem lineage.
Molecular dating interprets the deep nodes of crown-group lineages of Euarthropoda as having evolved by the Cambrian [7,65,66]. This draws on calibrations that identify particular early Cambrian fossils as crown-group euarthropods, such as the crown-group pancrustaceans Yicaris [67] and Wujicaris [68] from Cambrian Stage 3, and total-group branchiopods known from small carbonaceous fossils [69] as early as Cambrian Stage 4. The findings from neuropalaeontology are likewise consistent with clades such as total-group Chelicerata and Mandibulata being present in the main burst of the Cambrian explosion and having already evolved diagnostic characters of brain organization.
Acknowledgement
Images of Chengjiangocaris kunmingensis and Odaraia alata were kindly provided by J. Yang, J. Ortega-Hernández, X. Zhang and J.-B. Caron.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by a Leverhulme Trust Research Project grant no. (F/00 696/T) to G.D.E.; a Natural Environment Research Council (NERC) Independent Research Fellowship (NE/L011751/1) and by the National Natural Science Foundation of China (U1302232) to X.M.; the University of Arizona's Center for Insect Science and a University of Arizona Regents' fund award to N.J.S.
References
- 1.Giribet G, Edgecombe GD. 2012. Reevaluating the arthropod tree of life. Ann. Rev. Entomol. 57, 167–186. ( 10.1146/annurev-ento-120710-100659) [DOI] [PubMed] [Google Scholar]
- 2.Strausfeld NJ. 1995. Crustacean–insect relationships: the use of brain characters to derive phylogeny amongst segmented invertebrates. Brain Behav. Evol. 52, 186–206. ( 10.1159/000006563) [DOI] [PubMed] [Google Scholar]
- 3.Harzsch S. 2006. Neurophylogeny: architecture of the nervous system and a fresh view on arthropod phylogeny. Integr. Comp. Biol. 46, 162–194. ( 10.1093/icb/icj011) [DOI] [PubMed] [Google Scholar]
- 4.Strausfeld NJ, Andrew DR. 2011. A new view of insect-crustacean relationships I. Inferences from neural cladistics and comparative neuroanatomy. Arthropod. Struct. Dev. 40, 276–288. ( 10.1016/j.asd.2011.02.002) [DOI] [PubMed] [Google Scholar]
- 5.Regier JC, Shultz JW, Zwick A, Hussey A, Ball B, Wetzer R, Martin JW, Cunningham CW. 2010. Arthropod relationships revealed by phylogenomic analysis of nuclear protein-coding sequences. Nature 463, 1079–1083. ( 10.1038/nature08742) [DOI] [PubMed] [Google Scholar]
- 6.Rota-Stabelli O, Campbell L, Brinkmann H, Edgecombe GD, Longhorn SJ, Peterson KJ, Pisani D, Philippe H, Telford MJ. 2011. A congruent solution to arthropod phylogeny: phylogenomics, microRNAs and morphology support monophyletic Mandibulata. Proc. R. Soc. B 278, 298–306. ( 10.1098/rspb.2010.0590) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rehm P, Meusemann K, Borner J, Misof B, Burmester T. 2014. Phylogenetic position of Myriapoda revealed by 454 transcriptome sequencing. Mol. Phylogenet. Evol. 77, 25–33. ( 10.1016/j.ympev.2014.04.007) [DOI] [PubMed] [Google Scholar]
- 8.Edgecombe GD, Legg DA. 2013. The arthropod fossil record. In Arthropod biology and evolution—molecules, development, morphology (eds Minelli A, Boxshall G, Fusco G), pp. 393–425. Berlin, Germany: Springer. [Google Scholar]
- 9.Edgecombe GD, Legg DA. 2014. Origins and early evolution of arthropods. Palaeontology 57, 457–468. ( 10.1111/pala.12105) [DOI] [Google Scholar]
- 10.Butterfield NJ. 2003. Exceptional fossil preservation and the Cambrian Explosion. Integr. Comp. Biol. 43, 166–177. ( 10.1093/icb/43.1.166) [DOI] [PubMed] [Google Scholar]
- 11.Gaines RR, Briggs DEG, Zhao Y. 2008. Cambrian Burgess Shale-type deposits share a common mode of fossilization. Geology 36, 755–758. ( 10.1130/G24961A.1) [DOI] [Google Scholar]
- 12.Gaines RR, Hammarlund EU, Hou X, Qi C, Gabbott SE, Zhao Y, Peng J, Canfield DE. 2012. Mechanism for Burgess Shale-type preservation. Proc. Natl Acad. Sci. USA 109, 5180–5184. ( 10.1073/pnas.1111784109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ortega-Hernández J. In press. Making sense of ‘lower’ and ‘upper’ stem-group Euarthropoda, with comments on the strict use of Arthropoda von Siebold, 1848. Biol. Rev. ( 10.1111/brv.12168) [DOI] [PubMed] [Google Scholar]
- 14.Sansom RS, Gabbott SE, Purnell MA. 2011. Decay of vertebrate characters in hagfish and lamprey (Cyclostomata) and the implications for the vertebrate fossil record. Proc. R. Soc. B 278, 1150–1157. ( 10.1098/rspb.2010.1641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Briggs DEG, Kear AJ. 1994. Decay and mineralization of shrimps. Palaios 9, 431–456. ( 10.2307/3515135) [DOI] [Google Scholar]
- 16.Butler AD, Cunningham JA, Budd GE, Donoghue PCJ. 2015. Experimental taphonomy of Artemia reveals the role of endogenous microbes in mediating decay and fossilization. Proc. R. Soc. B 282, 20150476 ( 10.1098/rspb.2015.0476) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murdock DJE, Gabbott SE, Mayer G, Purnell MA. 2014. Decay of velvet worms (Onychophora), and bias in the fossil record of lobopodians. BMC Evol. Biol. 14, 222 ( 10.1186/s12862-014-0222-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilby PR, Briggs DEG. 1997. Taxonomic trends in the resolution of detail preserved in fossil phosphatized soft tissues. Geobios 20, 493–502. ( 10.1016/S0016-6995(97)80056-3) [DOI] [Google Scholar]
- 19.Budd GE. 1998. Arthropod body-plan evolution in the Cambrian with an example from anomalocaridid muscle. Lethaia 31, 197–210. ( 10.1111/j.1502-3931.1998.tb00508.x) [DOI] [Google Scholar]
- 20.Briggs DEG, Nedin C. 1997. The taphonomy and affinities of the problematic fossil Myoscolex from the Lower Cambrian Emu Bay Shale of South Australia. J. Paleontol. 71, 22–32. [Google Scholar]
- 21.Daley AC, Edgecombe GD. 2014. Morphology of Anomalocaris canadensis from the Burgess Shale. J. Paleontol. 88, 68–91. ( 10.1666/13-067) [DOI] [Google Scholar]
- 22.Lerosey-Aubril R, Hegna TA, Kier C, Bonino E, Habersetzer J, Carre M. 2012. Controls on gut phosphatisation: the trilobites from the Weeks Formation Lagerstätte (Cambrian; Utah). PLoS ONE 7, e32934 ( 10.1371/journal.pone.0032934) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butterfield NJ. 2002. Leanchoilia guts and the interpretation of three-dimensional structures in Burgess Shale-type fossils. Paleobiology 28, 155–171. () [DOI] [Google Scholar]
- 24.Vannier J, Liu J, Lerosey-Aubril R, Vinther J, Daley AC. 2014. Sophisticated digestive systems in early arthropods. Nat. Comm. 5, 3641 ( 10.1038/ncomms4641) [DOI] [PubMed] [Google Scholar]
- 25.O'Connor S, Ali A, Al-Sabah S, Anwar D, Bergström E et al. 2011. Exceptional preservation of a prehistoric human brain from Heslington, Yorkshire, UK. J. Arch. Sci. 38, 1641–1654. ( 10.1016/j.jas.2011.02.030) [DOI] [Google Scholar]
- 26.Wilson LA, Butterfield NJ. 2014. Sediment effects on the preservation of Burgess Shale-type compression fossils. Palaios 29, 145–154. ( 10.2110/palo.2013.075) [DOI] [Google Scholar]
- 27.Briggs DEG, Kear AJ. 1993. Decay and preservation of polychaetes: taphonomic thresholds in soft-bodied organisms. Palaeobiology 19, 107–135. [Google Scholar]
- 28.Conway Morris S. 1977. Fossil priapulid worms. Spec. Papers Palaeontol. 20, 1–155. [Google Scholar]
- 29.Henwood AH. 1992. Soft-part preservation of beetles in Tertiary amber from the Dominican Republic. Palaeontology 35, 901–912. [Google Scholar]
- 30.Grimaldi D, Bonwich E, Delannoy M, Doberstein S. 1994. Electron microscopic studies of mummified tissues in amber fossils. Am. Mus. Novitates 3097, 1–31. [Google Scholar]
- 31.Pohl H, Wipfler B, Grimaldi D, Beckmann F, Beutel RG. 2010. Reconstructing the anatomy of the 42-million-year-old fossil Mengea tertiaria (Insecta, Strepsiptera). Naturwissenschaften 97, 855–859. ( 10.1007/s00114-010-0703-x) [DOI] [PubMed] [Google Scholar]
- 32.Briggs DEG, Erwin DH, Collier FJ. 1994. The fossils of the Burgess Shale. Washington, DC: Smithsonian Institution Press. [Google Scholar]
- 33.Caron J-B, Gaines RR, Aria C, Mángano MG, Streng M. 2014. A new phyllopod bed-like assemblage from the Burgess Shale of the Canadian Rockies. Nat. Comm. 5, 3210 ( 10.1038/ncomms4210) [DOI] [PubMed] [Google Scholar]
- 34.Conway Morris S. 1977. A redescription of the Middle Cambrian worm Amiskwia sagittiformis Walcott from the Burgess Shale of British Columbia. Paläontol. Z. 51, 271–287. ( 10.1007/BF02986576) [DOI] [Google Scholar]
- 35.Hou X, Aldridge RJ, Bergström J, Siveter DJ, Siveter DJ, Feng X. 2004. The Cambrian fossils of Chengjiang, China: the flowering of early animal life. Oxford, UK: Blackwell. [Google Scholar]
- 36.Yang J, Ortega-Hernández J, Butterfield NJ, Zhang X. 2013. Specialized appendages in fuxianhuiids and the head organization of early euarthropods. Nature 494, 468–471. ( 10.1038/nature11874) [DOI] [PubMed] [Google Scholar]
- 37.Hou X, Ma X, Zhao J, Bergström J. 2004. The lobopodian Paucipodia inermis from the Lower Cambrain Chengjiang fauna, Yunnan, China. Lethaia 37, 235–244. ( 10.1080/00241160410006555) [DOI] [Google Scholar]
- 38.Smith MR, Ortega-Hernández J. 2014. Hallucigenia’s onychophoran-like claws and the case for Tactopoda. Nature 514, 363–366. ( 10.1038/nature13576) [DOI] [PubMed] [Google Scholar]
- 39.Mayer G, Harzsch S. 2007. Immunolocalization of serotonin in Onychophora argues against segmental ganglia being an ancestral feature of arthropods. BMC Evol. Biol. 7, 118 ( 10.1186/1471-2148-7-118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin C, Mayer G. 2015. Insights into the segmental identity of post-oral commissures and pharyngeal nerves in Onychophora based on retrograde fills. BMC Neurosci. 16, 53 ( 10.1186/s12868-015-0191-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen JY, Ramsköld L, Zhou GQ. 1994. Evidence for monophyly and arthropod affinity of Cambrian giant predators. Science 264, 1304–1308. ( 10.1126/science.264.5163.1304) [DOI] [PubMed] [Google Scholar]
- 42.Paterson JR, García-Bellido DC, Lee MSY, Brock GA, Jago JB, Edgecombe GD. 2011. Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature 480, 237–240. ( 10.1038/nature10689) [DOI] [PubMed] [Google Scholar]
- 43.Kühl G, Briggs DEG, Rust J. 2009. A great-appendage arthropod with a radial mouth from the Lower Devonian Hunsrück Slate, Germany. Science 323, 771–773. ( 10.1126/science.1166586) [DOI] [PubMed] [Google Scholar]
- 44.Legg DA, Sutton MD, Edgecombe GD. 2013. Arthropod fossil data increase congruence of morphological and molecular phylogenies. Nat. Comm. 4, 2485 ( 10.1038/ncomms3485) [DOI] [PubMed] [Google Scholar]
- 45.Cong P, Ma X, Hou X, Edgecombe GD, Strausfeld NJ. 2014. Brain structure resolves the segmental affinity of anomalocaridid appendages. Nature 513, 538–542. ( 10.1038/nature13486) [DOI] [PubMed] [Google Scholar]
- 46.Scholtz G, Edgecombe GD. 2006. The evolution of arthropod heads: reconciling morphological, developmental and palaeontological evidence. Dev. Genes Evol. 216, 395–415. ( 10.1007/s00427-006-0085-4) [DOI] [PubMed] [Google Scholar]
- 47.Cong P, Ma X, Hou X, Edgecombe GD, Strausfeld NJ. 2014. Reply to ‘Latest anomalocaridid affinities challenged’. Nature 516, E3–E4. ( 10.1038/nature13861) [DOI] [PubMed] [Google Scholar]
- 48.Budd GE. 2002. A palaeontological solution to the arthropod head problem. Nature 417, 271–275. ( 10.1038/417271a) [DOI] [PubMed] [Google Scholar]
- 49.Bergström J, Hou X, Zhang X, Clausen S. 2008. A new view of the Cambrian arthropod Fuxianhuia. GFF 130, 189–201. ( 10.1080/11035890809452772) [DOI] [Google Scholar]
- 50.Ma X, Hou X, Edgecombe GD, Strausfeld NJ. 2012. Complex brain and optic lobes in an early Cambrian arthropod. Nature 490, 258–261. ( 10.1038/nature11495) [DOI] [PubMed] [Google Scholar]
- 51.Gabbott SE, Hou X, Norry MJ, Siveter DJ. 2004. Preservation of Early Cambrian animals of the Chengjiang biota. Geology 32, 901–904. ( 10.1130/G20640.1) [DOI] [Google Scholar]
- 52.Zhu M, Babcock LE, Steiner M. 2005. Fossilization modes in the Chengjaing Lagerstätte (Cambrian of China): testing the roles of organic preservation and diagenetic alteration in exceptional preservation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 220, 31–46. ( 10.1016/j.palaeo.2003.03.001) [DOI] [Google Scholar]
- 53.Forchielli A, Steiner M, Kashbohm J, Hu S, Keupp H. 2014. Taphonomic traits of clay-hosted early Cambrian Burgess Shale-type fossil Lagerstätten in South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 398, 59–85. ( 10.1016/j.palaeo.2013.08.001) [DOI] [Google Scholar]
- 54.Schiffbauer JD, Xiao S, Cai Y, Wallace AF, Hua H, Hunter J, Xu H, Peng Y, Kaufman AJ. 2014. A unifying model for Neoproterozoic-Palaeozoic exceptional fossil preservation through pyritization and carbonaceous compression. Nat. Comm. 5, 5754 ( 10.1038/ncomms6754) [DOI] [PubMed] [Google Scholar]
- 55.Ortega-Hernández J. 2015. Homology of head sclerites in Burgess Shale euarthropods. Curr. Biol. 25, 1625–1631. ( 10.1016/j.cub.2015.04.034) [DOI] [PubMed] [Google Scholar]
- 56.Budd GE. 2008. Head structure in upper stem-group arthropods. Palaeontology 51, 561–573. ( 10.1111/j.1475-4983.2008.00752.x) [DOI] [Google Scholar]
- 57.Haug JT, Briggs DEG, Haug C. 2012. Morphology and function in the Cambrian Burgess Shale megacheiran arthropod Leanchoilia superlata and the application of a descriptive matrix. BMC Evol. Biol. 12, 162 ( 10.1186/1471-2148-12-162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cotton TJ, Braddy SJ. 2004. The phylogeny of arachnomorph arthropods and the origin of the Chelicerata. Trans. R. Soc. Edinb. Earth Sci. 94, 169–193. [Google Scholar]
- 59.Haug JT, Waloszek D, Maas A, Liu Y, Haug C. 2012. Functional morphology, ontogeny and evolution of mantis shrimp-like predators in the Cambrian. Palaeontology 55, 369–399. ( 10.1111/j.1475-4983.2011.01124.x) [DOI] [Google Scholar]
- 60.Tanaka G, Hou X, Ma X, Edgecombe GD, Strausfeld NJ. 2013. Chelicerate neural ground pattern in a Cambrian great appendage arthropod. Nature 502, 364–367. ( 10.1038/nature12520) [DOI] [PubMed] [Google Scholar]
- 61.Stein M, Budd GE, Peel JS, Harper DAT. 2013. Arthroaspis n. gen., a common element of the Sirius Passet Lagerstätte (Cambrian, North Greenland), sheds light on trilobite ancestry. BMC Evol. Biol. 13, 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortega-Hernández J, Legg DA, Braddy SJ. 2013. The phylogeny of aglaspidid arthropods and the internal relationships within Artiopoda. Cladistics 29, 15–45. ( 10.1111/j.1096-0031.2012.00413.x) [DOI] [PubMed] [Google Scholar]
- 63.Strausfeld NJ. 2005. The evolution of crustacean and insect optic lobes and the origins of chiasmata. Arth. Struct. Dev. 34, 235–256. ( 10.1016/j.asd.2005.04.001) [DOI] [Google Scholar]
- 64.Strausfeld NJ. 2011. Some observations on the sensory organization of the crustaceamorph Waptia fieldensis Walcott. Palaeontograph. Can. 31, 157–169. [Google Scholar]
- 65.Lee MSY, Soubrier J, Edgecombe GD. 2013. Rates of phenotypic and genomic evolution during the Cambrian explosion. Curr. Biol. 23, 1–7. ( 10.1016/j.cub.2012.10.044) [DOI] [PubMed] [Google Scholar]
- 66.Rota-Stabelli O, Daley AC, Pisani D. 2013. Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Curr. Biol. 23, 392–398. ( 10.1016/j.cub.2013.01.026) [DOI] [PubMed] [Google Scholar]
- 67.Zhang X-G, Siveter DJ, Waloszek D, Maas A. 2007. An epipodite-bearing crown-group crustacean from the Lower Cambrian. Nature 449, 595–598. ( 10.1038/nature06138) [DOI] [PubMed] [Google Scholar]
- 68.Zhang X-G, Maas A, Haug JT, Siveter DJ, Waloszek D. 2010. A eucrustacean metanauplius from the lower Cambrian. Curr. Biol. 20, 1075–1079. ( 10.1016/j.cub.2010.04.026) [DOI] [PubMed] [Google Scholar]
- 69.Harvey THP, Vélez MI, Butterfield NJ. 2012. Exceptionally preserved crustaceans from western Canada reveal a cryptic Cambrian radiation. Proc. Natl Acad. Sci. USA 109, 1589–1594. ( 10.1073/pnas.1115244109) [DOI] [PMC free article] [PubMed] [Google Scholar]