Abstract
In the past 40 years, comparisons of developmental gene expression and mechanisms of development (evodevo) joined comparative morphology as tools for reconstructing long-extinct ancestral forms. Unfortunately, both approaches typically give congruent answers only with closely related organisms. Chordate nervous systems are good examples. Classical studies alone left open whether the vertebrate brain was a new structure or evolved from the anterior end of an ancestral nerve cord like that of modern amphioxus. Evodevo plus electron microscopy showed that the amphioxus brain has a diencephalic forebrain, small midbrain, hindbrain and spinal cord with parts of the genetic mechanisms for the midbrain/hindbrain boundary, zona limitans intrathalamica and neural crest. Evodevo also showed how extra genes resulting from whole-genome duplications in vertebrates facilitated evolution of new structures like neural crest. Understanding how the chordate central nervous system (CNS) evolved from that of the ancestral deuterostome has been truly challenging. The majority view is that this ancestor had a CNS with a brain that gave rise to the chordate CNS and, with loss of a discrete brain, to one of the two hemichordate nerve cords. The minority view is that this ancestor had no nerve cord; those in chordates and hemichordates evolved independently. New techniques such as phylostratigraphy may help resolve this conundrum.
Keywords: amphioxus, neural crest, nervous system evolution, brain evolution, chordate evolution, phylostratigraphy
1. Introduction
To understand the course of evolution, extinct forms at major nodes of the tree of life are typically reconstructed from commonalities shared by extant forms located on adjacent branches of the tree. This works best within phyla where body plans are similar. Problems arise when body plans are very different, such as between phyla and between rapidly evolving organisms within a phylum. In the past 30 years, phylogenetic analyses with large datasets of nuclear genes have revised many phylogenetic relationships that had been based on mitochondrial genes and/or morphology. In particular, fast-evolving groups such as tunicates and nematodes moved from basal positions to higher levels of the tree, and it has been recognized that their comparatively simple body plans are secondarily reduced.
The classical method for reconstructing ancestral forms has been comparative morphology. It has more recently been joined by comparisons of developmental gene expression and the molecular mechanisms of development (evodevo) as well as sophisticated morphological techniques such as serial transmission electron microscopy (TEM) and confocal microscopy of antibody-labelled specimens. The most recent technique is phylostratigraphy, which examines the evolutionary origins of genes that are expressed in particular structures such as the vertebrate brain [1]. This shows when the genetic framework necessary for building a structure first appeared. When all the methods agree, the hypothetical ancestor has the best chance of approximating the real one. Chordate nervous systems are good examples. All chordates (vertebrates, tunicates and cephalochordates) have dorsal hollow nerve cords. Therefore, the ancestral chordate also most probably had one. However, how much of a brain this organism probably had has been controversial. Vertebrates have a large brain, while the nerve cord in cephalochordates (amphioxus) and tunicates has only a small anterior swelling, the cerebral vesicle or sensory vesicle. Therefore, the question was whether the vertebrate brain was a new structure or had evolved from the anterior end of an ancestral nerve cord like that of modern amphioxus. Several authors thought that the amphioxus cerebral vesicle was equivalent to the entire vertebrate brain [2–7], while Gans & Northcutt [8] argued that the amphioxus cerebral vesicle is homologous only to the vertebrate hindbrain, with the forebrain and midbrain being vertebrate inventions. Others took positions in between these two extremes [9–12]. Answers began to come in the 1990s from two lines of research: comparisons of developmental gene expression (evodevo) and three-dimensional anatomical reconstructions from serial fine sections (TEM). More recently, analyses of genome sequences (phylostratigraphy) and studies of the mechanisms of development have begun to address how and when in evolution vertebrate-specific structures evolved.
Understanding how the chordate central nervous system (CNS) evolved from that of the ancestral deuterostome (i.e. the ancestor of chordates, hemichordates and echinoderms) has been especially challenging. The problems are first, that the morphology of the hemichordates and echinoderms, which form a clade (Ambulacraria) basal to chordates, differs considerably between them and also differs considerably from that of the chordates. Second, the phylogenetic position of a clade uniting the acoel flatworms and xenoturbellids is very uncertain. Phylogenetic analyses with collections of nuclear genes have united acoels, nemertodermatids and xenoturbellids into a single clade, the Xenocoelomorpha, and placed it as sister group of the ambulacraria [13]. However, the acoels are very fast-evolving and, depending on which genes are used for the analyses, skew the tree; sometimes the Xeocoelomorpha are seen as basal bilateria, and sometimes only the nemertodermatids and acoels are placed basally to bilateria with Xenoturbellida as sister group of the Ambulacraria [14]. In any case, it appears that both acoels and xenoturbellids have lost a number of characters [13]. Given these considerations, it is difficult to predict with any degree of certainty the precise structure of the nervous system of the common ancestor of Ambulacraria and Chordata, let alone that of the basal deuterostome. The present review focuses on the evolution of chordate nervous systems and discusses the pros and cons of theories concerning the evolution of the chordate nervous system from that of an ancestral deuterostome.
2. How did the vertebrate brain evolve from that of an invertebrate chordate ancestor?
Although amphioxus and vertebrates split over 550 Ma, both groups are evolving relatively slowly, with the genomes of species of amphioxus evolving even more slowly than that of the slowest-evolving vertebrate known, the elephant shark [15]. The genome of the Florida amphioxus, Branchiostoma floridae, which was the first amphioxus genome to be sequenced, conserves a very high degree of synteny with vertebrate genomes [16]. Comparisons of the B. floridae and vertebrate genomes substantiated the idea first proposed by Ohno [17] that vertebrates had undergone two rounds of whole-genome duplication. Extra copies of many duplicate genes were lost, but those for developmental genes and genes coding for signalling proteins were preferentially retained [16]. It has been postulated that these extra genes gave vertebrates the genetic tool-kit to gain a large, complex brain [18]. The lack of such whole-genome duplications in amphioxus plus this slow rate of evolution support the use of amphioxus as a proxy for the ancestral chordate. Also supporting this use are fossils from the Cambrian such as Haikouella, which resembles modern amphioxus to a large extent but appears to have paired eyes and a larger brain and has, therefore, been proposed as a sister group of vertebrates [19,20]. Of course, modern amphioxus may well have evolved some new characters and changed some old ones over the millennia, but all available evidence indicates that it has changed relatively little.
(a). What new structures did the vertebrate brain invent?
Comparisons of developmental gene expression together with three-dimensional reconstructions from serial TEM have shown that the amphioxus brain has homologues of most of the features of the vertebrate brain. These include a hindbrain, diencephalic forebrain with a pineal homologue, and perhaps a small midbrain (tectum), which receives input from the frontal eye [21,22]. Clear evidence for a telencephalon is lacking. Although gene markers of the vertebrate telencephalon such as BF1 (FoxG1) are expressed in the anterior tip of the CNS, there is no structure comparable to the olfactory bulbs of the vertebrate telencephalon [21]. As FoxG1 is also expressed in vertebrate diencephalic structures (e.g. the optic stalks), its expression in the amphioxus CNS is not necessarily indicative of a telencephalon. The CNS of amphioxus has no gross anatomical divisions except constriction at the posterior end of the cerebral vesicle; however, as the somites extend to the anterior tip of the animal, they serve as excellent markers of anterior/posterior position. Evidence for a hindbrain comes from expression of Hox genes in nested patterns with the anterior limit of Hox1 at the level of the anterior boundary of somite 2, the Hox2 limit at the level of somite 3, that for Hox3 at the level of somite 4, and that for Hox6 between somites 6 and 7 [23]. In addition, motor neurons, which are located in the vertebrate midbrain and hindbrain, are located at the level of somites 2–6 in the amphioxus nerve cord [24]. They express characteristic motor neuron markers, including the estrogen-related receptor [25]. Evidence that amphioxus has a homologue of the vertebrate diencephalon is strong. Based on fine structure and histology, there is an infundibulum [26,27]. Additional evidence for a diencephalic homologue is the presence of the lamellar body, which has the same fine structure as the pineal in a larval lamprey [28,29]. In addition, consistent with the anterior tip of the amphioxus CNS being diencephalic, in both larvae and adults, the anterior-most part of the CNS includes a photoreceptor or frontal eye, which has been proposed to be homologous to the vertebrate paired eyes [22,29,30].
Evidence from gene expression indicates that amphioxus also has parts of the genetic mechanisms that specify three organizing centres in the vertebrate brain: the anterior neural ridge (ANR) zona limitans intrathalamica (ZLI) and the midbrain/hindbrain boundary (isthmic organizer) (MHB/ISO). Both the anterior tip of the amphioxus CNS and the vertebrate ANR express Dlx5, FoxG1 (BF1) and Fgf8 [21,31–33]. However, in amphioxus, the domain of Fgf8/17/18 extends to the posterior limit of the cerebral vesicle [21,32,33]. In vertebrates, the ZLI is located about midway between the anterior and posterior ends of the diencephalon where a posterior domain of Irx abuts anterior domains of Otx and Fezf [34,35]. Likewise, in amphioxus, a posterior domain of IrxB abuts an anterior domain of Fezf about midway between the anterior and posterior ends of the cerebral vesicle [36]. In both amphioxus and vertebrates, Wnt8 is expressed at or near this boundary as is Fng [37–39]. However, not all genes are identically expressed at this boundary in vertebrates and amphioxus. For example, engrailed is expressed at this boundary in amphioxus [40], but not in vertebrates. Similarly, in both amphioxus and vertebrates, an anterior domain of Otx meets a posterior domain of Gbx. In amphioxus this is at the boundary between the hindbrain and cerebral vesicle and in vertebrates at the MHB. However, while engrailed is expressed at this boundary in vertebrates, it is not in amphioxus [40–42]. It is, therefore, unlikely that the amphioxus MHB and ZLI equivalents are organizers because they lack expression of some genes that are critical for organizer properties. In sum, the data from fine-structural three-dimensional reconstructions and gene expression indicate that the CNS of amphioxus, and, by extension, that of the ancestral vertebrate had a diencephalic forebrain with part of the genetic machinery for the ANR and ZLI, a small midbrain and a hindbrain, with the genetic machinery for positioning the MHB. Thus, the telencephalon is the major brain region that evolved at the base of the vertebrates.
(b). Neural crest
Another structure which the vertebrate brain but has that the amphioxus CNS lacks is neural crest—cells that migrate from the neural plate boundary and give rise to numerous cell types including pigment cells, cells of the adrenal medulla and cartilage and bone [43]. By contrast, in amphioxus, the ectoderm adjacent to the neural plate on either side migrates over it as a sheet, with the leading edge cells displaying lamellipodia (figure 1) [33]. These leading edge cells express Distalless like neural crest cells. In fact, the genes that specify the neural plate and the neural plate border are highly conserved between amphioxus and vertebrates [44]. However, the genes that specify neural crest are not similarly expressed in amphioxus and vertebrates. Notable among them is FoxD3, which is vital for neural crest migration. Of the five vertebrate FoxD genes, only FoxD3 is expressed in neural crest (figure 2). Amphioxus has a single FoxD gene, which is expressed in mesodermal tissues and the anterior neural tube, but not at the edges of the neural plate [47]. Regulatory DNA was identified that directed expression of a reporter gene to all the domains that normally expressed amphioxus FoxD [48]. While this regulatory DNA directed expression to the corresponding domains in the chick, it failed to direct expression to neural crest, demonstrating that after gene duplications in vertebrates, FoxD3 had acquired new regulatory elements [45]. Indeed, the FoxD3 enhancer directing expression to premigratory neural crest [49] has little identity with the amphioxus FoxD3 regulatory region (L. Z. Holland 2015, unpublished data). However, the amino terminal region of the FoxD3 protein also acquired new protein sequences allowing it to induce expression of neural crest genes such as HNK1 [46]. Although this is just one gene, it shows how gene duplication allowed some duplicates to retain old functions while leaving others free to gain new ones. Such acquisition of new gene regulatory elements and new protein sequences has likely occurred also for other duplicate genes during evolution of the vertebrate brain.
Figure 1.
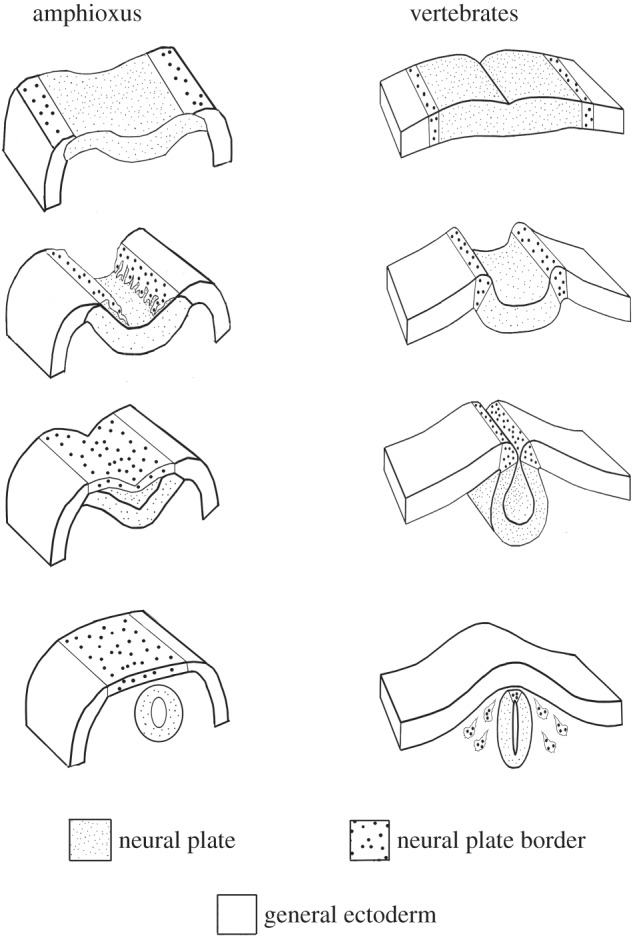
Neurulation in amphioxus and vertebrates. Top: at the late gastrula stage both amphioxus and vertebrates have a neural plate with a neural plate border region. Second from top: at the early neurula stage, in amphioxus, the neural plate border region detaches from the edges of the neural plate and moves over it by lamellipodia. By contrast, in vertebrates, the neural plate border region remains attached to the neural plate as it rounds up. Third from top: at the late neurula stage, in amphioxus, the free edges of the neural plate border region fuse in the dorsal midline, and the neural plate begins to round up underneath the dorsal ectoderm. In vertebrates at a comparable stage, the neural tube has completed rounding up. Bottom: In amphioxus, the neural plate rounds up completely and detaches from the ectoderm. In vertebrates, the neural tube detaches from the ectoderm, and the neural plate border region gives rise to neural crest cells that migrate below the ectoderm and give rise to such structures as pigment cells, cells of the adrenal medulla, parts of cranial ganglia.
Figure 2.
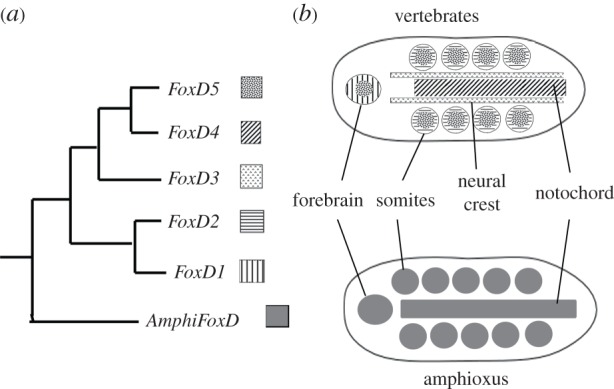
(a) Phylogenetic relations of the amphioxus FoxD gene and the five vertebrate FoxD genes that arose from whole-genome and gene duplications. (b) AmphiFoxD is expressed in the forebrain, somites and notochord. In vertebrates, the ancestral FoxD expression domains have been partitioned among four of the five duplicates, FoxD1, FoxD2, FoxD4 and FoxD5. FoxD3 has acquired a new domain in neural crest. Experimental evidence has shown that FoxD3 acquired both new regulatory elements and a new amino terminal sequence, both of which are essential for its role in neural crest [45,46].
It has been argued that ascidian tunicate larvae may have some cells related to neural crest, but this is far from certain. Some migratory cells in the vicinity of the nerve cord were shown to migrate and develop into pigment cells, but these were not migrating from edges of the neural plate [50]. In addition, it was found that ectopic expression of Twist could induce some cells to migrate away from the Ciona neural tube [51]. However, tunicates are evolving rapidly. Their genomes are very reduced with loss of several developmental genes, and the larvae have relatively few cells. Therefore, even though tunicates are the sister group of vertebrates, it is impossible to reconstruct their common ancestor to obtain a clear idea of how many vertebrate features this ancestor had before the whole-genome duplications.
(c). Phylostratigraphy
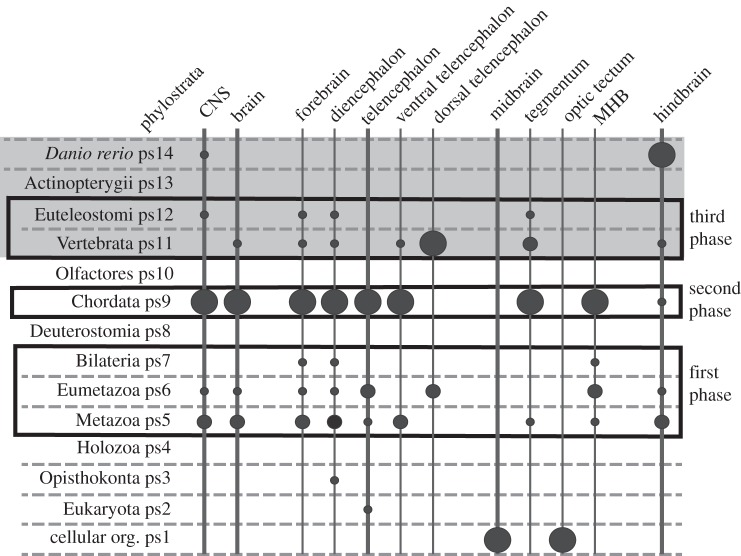
Phylostratigraphy is a relatively new approach to investigate when particular structures evolved. This method uses comparative genomics to determine when genes expressed in a particular anatomical structure evolved (figure 3) [52]. This is not to say when the structure itself evolved, but only to predict when the genetic framework for a structure such as the brain evolved. For example, an analysis of genes involved in development of sensory structures in vertebrates showed that genes for the eyes, including the lens evolved first, with peaks for the number of new genes for the retina and eye evolving in deuterostomes and for the lens in cephalochordates [53]. By contrast, the peak appearances of new genes for the olfactory system, ear and lateral line as well as that for cranial placodes occur in tunicates while those for neural crest, adenohypophysis and trigeminal placode and ganglion are in vertebrates; however, a minor peak for the adenohypophysis occurs with the chordates [53]. When this type of analysis was applied to the brain regions, genes for the whole brain, forebrain (including diencephalon and telencephalon), midbrain and hindbrain made their peak appearance in amphioxus, although there were minor peaks for all but the midbrain genes at the base of the metazoan and in the vertebrates (figure 3) [1]. However, when the telecephalon was divided into dorsal and ventral regions, a peak for the genes of the ventral telencephalon occurred in amphioxus, but the peak for the dorsal telencephalon was in the vertebrates. There were less pronounced peaks for the ventral telencephalon in agnathans and euteoleosts. When the midbrain was subdivided, there was a striking peak for the MHB in amphioxus and a minor peak in agnathans, while the tegmentum had dual peaks in amphioxus and agnathans. In addition, there are peaks of new gene appearance for the MHB and the tegmentum at the base of the Metazoa [1]. These results indicate that most of the genes involved in patterning the vertebrate brain arose in the cephalochordate ancestor. Exceptions are that most of those for the dorsal telencephalon arose in vertebrates, while those for the midbrain and optic tectum arose before evolution of eukaryotes [52]. This sheds doubt on the proposal that the larval ectoderm of direct-developing hemichordates had the genetic mechanisms for patterning the forebrain, diencephalon (including the zonal limitans intrathalamica) and midbrain/hindbrain boundary and that cephalochordates have lost the MHB and ZLI [54].
Figure 3.
Summary of a phylostratographic analysis of the zebrafish (Danio rerio) CNS. The phylostrata are at the left. The vertebrate section is shaded grey. The size of the circles is proportional to the number of genes expressed in a given region of the vertebrate brain that first appeared in that phylostratum. Phylostratum 8 (ps8) includes echinoderms and hemichordates. Phylostratum 9 includes cephalochordates (amphioxus), while phylostratum 10 includes tunicates. The largest number of genes expressed in the zebrafish brain first appeared in cephalochordates. Adapted from [1].
3. Can scenarios for evolution of the chordate nervous system from that of an ancestral deuterostomes be reconciled?
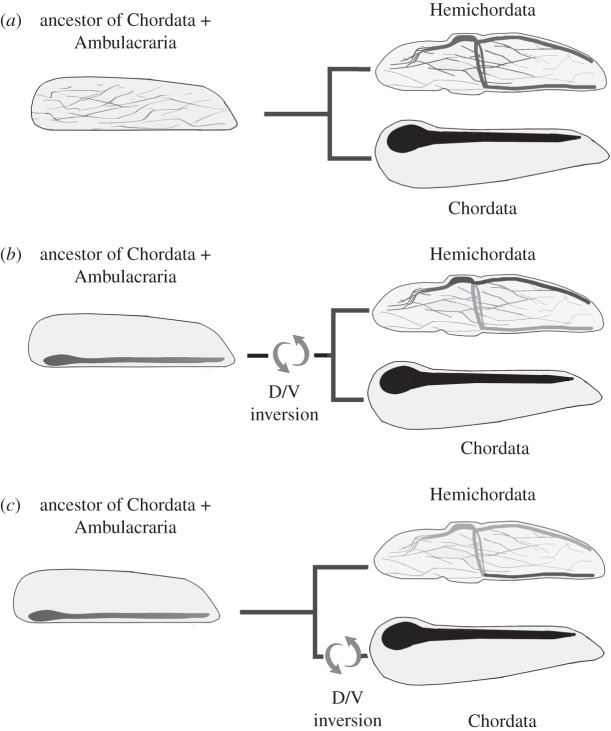
If reconstructing the common ancestor of tunicates and vertebrates is problematical, reconstructing the common ancestor of Ambulacraria (echinoderms and hemichordates) and chordates may be impossible. It could be chordate-like, hemichordate-like, echinoderm-like or something else (figure 4). It is generally agreed that the adult echinoderm nerve cords are not related to chordate nerve cords. The chief evidence is that during development, the echinoderm nerve cords do not express Hox genes [56]. Whether either of the hemichordate nerve cords is homologous to the chordate nerve cord is controversial [57]. There is no evident brain in enteropneust hemichordates, although the proboscis ectoderm contains many neurons. The dorsal nerve cord does undergo a sort of neurulation in the region of the collar and has most often been proposed as homologous to the chordate nerve cord [58,59]. However, some authors could not decide which of the two nerve cords was homologous to the chordate one [60]. Relevant to this argument is that in neither indirect, nor direct-developing hemichordates does the larval nervous system contribute substantially to the adult nervous system, although in the direct-developing hemichordate, Saccoglossus kowalevskii, ectodermal neurons in the larval proboscis may carry over to the ectoderm of the adult [59,61,62]. Although expression of some nerve cell markers has been studied during hemichordate metamorphosis [59,61,62], a thorough analysis of developmental gene expression in the hemichordate nerve cord has not been done and is sorely needed. One possibility is that a nerve cord in the common ancestor of the Ambulacraria and Chordata was more like that in a modern cephalochordate than like either of those in a modern enteropneust hemichordate and that it became secondarily reduced in hemichordates. Perhaps as the brain ceased to neurulate, it became spread out in the ectoderm of the proboscis. This would explain expression of genes such as Otx in the forebrain of chordates and in the proboscis ectoderm of hemichordates.
Figure 4.
Three schemes for evolution of the chordate and hemichordate nerve cords. (a) The ancestor of chordates and ambulacrarians had a nerve net. Nerve cords in hemichordates and chordates evolved independently [54]. (b) The ancestor of the Ambulacraria plus Chordata clade had a ventral nerve cord. A dorsal/ventral inversion occurred at the base of the ambulacraria plus Chordata. Thus, the dorsal nerve cord of hemichordates is homologous to the chordate nerve cord. (c) The ancestor of the Ambulacraria plus Chordata had a ventral nerve cord. A dorsal/ventral inversion occurred in the lineage leading to chordates. Thus, the ventral nerve cord of hemichordates is homologous to the dorsal nerve cord of chordates. Adapted from [55].
An alternative view is that neither hemichordate nerve cord is homologous to the chordate nerve cord; the nerve cords in the two groups evolved independently [54,63]. These authors (Pani et al. and Lowe et al.) have shown that some of the genes mediating A/P patterning of the larval ectoderm of S. kowalevskii are expressed in similar patterns in the vertebrate CNS. These include Otx, which is expressed in the proboscis ectoderm of S. kowalevskii and in the chordate forebrain. As Otx is not expressed in the ectoderm outside the CNS in chordates, its expression in the hemichordate may indicate an evolutionary relationship between the chordate forebrain and the proboscis ectoderm. However, some other genes expressed in the larval S. kowalevskii ectoderm, such as Hox genes, are expressed in both the CNS and in the ectoderm generally in chordates, making them poor indicators of homology between the chordate nerve cord and larval ectoderm in hemichordates. In addition, the domains of genes such as Fezf and Irx and Otx and Gbx do not abut in the hemichordate ectoderm as they do in the chordate nerve cord. Even more complicating is that the genes expressed in medio-lateral patterns in the vertebrate and amphioxus CNS are not similarly expressed in the S. kowalevskii larval ectoderm. Moreover, in amphioxus and vertebrates, opposition between posterioventral bone morphogenic protein (BMP) and dorsalanterior nodal/vg1 signalling segregates the neuroectoderm from the remainder of the ectoderm, but this is apparently not the case in S. kowalevskii. These considerations make it all the more important for a thorough study of gene expression in the hemichordate nerve cords.
A third view is that the chordate nerve cord evolved from the ciliated bands of an auricularia-larval like adult similar to larvae of holothurians. This idea was postulated by Garstang [64] and modified by Romer [65]. Although Garstang later recanted [66], it is still current [67]. In this scheme, the common ancestor of chordates, hemichordates and echinoderms had an adoral ciliary band as well as one that extended around the mouth and anus. There was a nerve ring underlying the circumoral ciliated band. This ring evolved into the circumoral nerve ring of echcinoderms, while in enteropneusts, the posterior part of the ciliated band evolved into the collar nerve cord. In chordates, the circumoral nerve ring moved dorsally to become the nerve cord. These ideas, however, do not seem viable in the light of the studies showing that except for neurons in the proboscis, little, if any, of the larval nervous system carries over into the adult in indirect developing ambulacrarians [59,62]. In sum, while it is unlikely that the chordate nerve cord evolved from the larval nervous system of a hemichordate-like ancestral deuterostome, other schemes for evolving the chordate nerve cord from either the dorsal nerve cord or the ventral nerve cord are more reasonable. While the scheme for evolving the chordate nerve cord from the hemichordate ectoderm is less likely, it cannot at present be ruled out.
4. Conclusion
Evodevo studies plus modern microscopy methods have been highly successful tools for addressing the question of how the vertebrate brain evolved from the brain of an invertebrate chordate ancestor. This ancestor probably had a nerve cord with a hindbrain, diencephalic forebrain and perhaps a small midbrain. While it had the genetic scaffolds for the major organizing centres of the vertebrate brain—the ANR, ZLI and MHB—vertebrates added a number of genes to these scaffolds. The increased complexity of the gene networks in these regions of the vertebrate brain is probably correlated with the acquisition of their organizer properties. The increased complexity of the gene network operating at the edges of the chordate neural plate is due at least in part to the retention of duplicate genes deriving from the two rounds of whole-genome duplication at the base of the vertebrates. An example is FoxD3, one of the five vertebrate duplicates of a single ancestral chordate FoxD gene. Experiments showed that vertebrate FoxD3 acquired both new regulatory elements and new protein sequences allowing it to be expressed in neural crest and to induce expression of other neural crest genes. This phenomenon may explain why many duplicate genes for transcription factors and signalling pathways were retained in vertebrates and how they facilitated the evolution of new structures.
It has been far more problematic to understand where the chordate nerve cord came from. The body plans of hemichordates and echinoderms (Ambulacraria) differ both from one another and from those of vertebrates. Schemes for deriving the chordate nerve cord from the ciliated bands of larval ambulacrarians do not seem credible as their adult nervous systems develop largely independently of their larval ones. Similarly, scenarios deriving the chordate nerve cord from an adult echinoderm nerve cord are also not viable as genes are expressed very differently in nerve cords from the two groups. Opinions of the relationship between the chordate and the two hemichordate nerve cords are mixed, ranging from no relationship at all, chordate and hemichordate nerve cords evolving independently, to the collar nerve cord being homologous to the chordate nerve cord or either of the two hemichordate nerve cords being homologous to the chordate nerve cord. Without intermediate forms, it will be difficult or impossible to decide among these scenarios. The goldilocks principle—that to infer homologies, organisms must be similar, but not identical—continues to hold for evolution of the chordate nervous system.
Competing interests
I have no competing interests.
Funding
The research is supported by NSF grant no. IOS-1353688.
References
- 1.Šestak MS, Domazet-Lošo T. 2015. Phylostratigraphic profiles in zebrafish uncover chordate origins of the vertebrate brain. Mol. Biol. Evol. 32, 299–312. ( 10.1093/molbev/msu319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steida L. 1873. Studien über den Amphioxus lanceolatus. Mem. Acad. Imp. Sci. St. Petersbourg ser. VII 19, 1–70 + pl.I-IV. [Google Scholar]
- 3.Huxley TH. 1875. Preliminary note upon the brain and skull of Amphioxus lanceolatus. Proc. R. Soc. Lond. 23, 127–132. ( 10.1098/rspl.1874.0017) [DOI] [Google Scholar]
- 4.Ayers H. 1890. Contribution to the morphology of the vertebrate head. Zool. Anzeiger 13, 504–507. [Google Scholar]
- 5.Ayers H. 1907. Vertebrate cephalogenesis. III. Amphioxus and Bdellostoma. Apparently privately printed, pp. 1–40.
- 6.Guthrie DM. 1975. The physiology and structure of the nervous system of amphioxus (the lancelet), Branchiostoma lanceolataum Pallas. Symp. Zool. Soc. Lond. 36, 43–80. [Google Scholar]
- 7.Delsman HC. 1913. Ist das Hirnbläschen des Amphioxus dem Gehirn der Kranioten homolog? Anatomischer Anz. 44, 481–497. [Google Scholar]
- 8.Gans C, Northcutt RG. 1983. Neural crest and the origin of vertebrates: a new head. Science 220, 268–274. ( 10.1126/science.220.4594.268) [DOI] [PubMed] [Google Scholar]
- 9.Ariëns Kappers CU. 1929. The evolution of the nervous system in invertebrates and man. Haarlem, The Netherlands: Erven Bohn. [Google Scholar]
- 10.Hatschek B. 1892. Die Metamerie des Amphioxus und des Ammocoetes. Verh. Anat. Ges. 6, 136–161. [Google Scholar]
- 11.Balfour FM. 1885. A treatise on comparative embryology, 2nd edn London, UK: MacMillan. [Google Scholar]
- 12.Delsman HC. 1922. The ancestry of vertebrates. Amersfoort, The Netherlands: Valkoff. [Google Scholar]
- 13.Philippe H, Brinkmann H, Copley RR, Moroz LL, Nakano H, Poustka AJ, Wallberg A, Peterson KJ, Telford MJ. 2011. Acoelomorph flatworms are deuterostomes related to Xenoturbella. Nature 470, 255–258. ( 10.1038/nature09676) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Achatz J, Chiodin M, Salvenmoser W, Tyler S, Martinez P. 2013. The Acoela: on their kind and kinships, especially with nemertodermatids and xenoturbellids (Bilateria incertae sedis). Org. Divers. Evol. 13, 267–286. ( 10.1007/s13127-012-0112-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue J-X, Yu J-K, Putnam NH, Holland LZ. 2014. The transcriptome of an amphioxus, Asymmetron lucayanum, from the Bahamas: a window into chordate evolution. Genome Biol. Evol. 6, 2681–2696. ( 10.1093/gbe/evu212) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putnam NH, et al. 2008. The amphioxus genome and the evolution of the chordate karyotype. Nature 453, 1064–1071. ( 10.1038/nature06967) [DOI] [PubMed] [Google Scholar]
- 17.Ohno S. 1970. Evolution by gene duplication. Berlin, Germany: Springer. [Google Scholar]
- 18.Holland PWH, Garcia-Fernandez J, Williams NA, Sidow A. 1994. Gene duplications and the origins of vertebrate development. Development 1994 (suppl.), 125–133. [PubMed] [Google Scholar]
- 19.Mallatt J, Chen J-Y. 2003. Fossil sister group of craniates: predicted and found. J. Morphol. 258, 1–31. ( 10.1002/jmor.10081) [DOI] [PubMed] [Google Scholar]
- 20.Morris SC, Caron J-B. 2014. A primitive fish from the Cambrian of North America. Nature 512, 419–422. ( 10.1038/nature13414) [DOI] [PubMed] [Google Scholar]
- 21.Toresson H, Maritnez-Barbera JP, Beardsley A, Caubit X, Krauss S. 1998. Conservation of BF-1 expression in amphioxus and zebrafish suggests evolutionary ancestry of anterior cell types that contribute to the vertebrate telencephalon. Dev. Genes Evol. 208, 431–439. ( 10.1007/s004270050200) [DOI] [PubMed] [Google Scholar]
- 22.Lacalli TC. 1996. Frontal eye circuitry, rostral sensory pathways and brain organization in amphioxus larvae: evidence from 3D reconstructions. Phil. Trans. R. Soc. Lond B 351, 243–263. ( 10.1098/rstb.1996.0022) [DOI] [Google Scholar]
- 23.Schubert M, Holland ND, Laudet V, Holland LZ. 2006. A retinoic acid-Hox hierarchy controls both anterior/posterior patterning and neuronal specification in the developing central nervous system of the cephalochordate amphioxus. Dev. Biol. 296, 190–202. ( 10.1016/j.ydbio.2006.04.457) [DOI] [PubMed] [Google Scholar]
- 24.Lacalli TC, Kelly SJ. 1999. Somatic motoneurones in amphioxus larvae: cell types, cell position and innervation patterns. Acta Zool. Stockholm 80, 113–124. ( 10.1046/j.1463-6395.1999.80220004.x) [DOI] [Google Scholar]
- 25.Bardet P-L, Schubert M, Horard B, Holland LZ, Laudet V, Holland ND, Vanacker J-M. 2005. Expression of estrogen-receptor related receptors in amphioxus and zebrafish: implications for the evolution of posterior brain segmentation at the invertebrate-to-vertebrate transition. Evol. Dev. 7, 223–233. ( 10.1111/j.1525-142X.2005.05025.x) [DOI] [PubMed] [Google Scholar]
- 26.Lacalli TC, Kelly SJ. 2000. The infundibular balance organ in amphioxus larvae and related aspects of cerebral vesicle organization. Acta Zool. Stockholm 81, 37–47. ( 10.1046/j.1463-6395.2000.00036.x) [DOI] [Google Scholar]
- 27.Olsson R, Yulis R, Rodriguez EM. 1994. The infundibular organ of the lancelet (Branchiostoma lanceolatum, Acrania): an immunocytochemnical study. Cell Tissue Res. 277, 107–114. ( 10.1007/BF00303086) [DOI] [Google Scholar]
- 28.Pu GA, Dowling JE. 1981. Anatomical and physiological characteristics of pineal photoreceptor cell in the larval lamprey, Petromyzon marinus. J. Neurophysiol. 46, 1018–1038. [DOI] [PubMed] [Google Scholar]
- 29.Lacalli TC. 2008. Basic features of the ancestral chordate brain: a protochordate perspective. Brain Res. Bull. 75, 319–323. ( 10.1016/j.brainresbull.2007.10.038) [DOI] [PubMed] [Google Scholar]
- 30.Vopalensky P, Pergner J, Liegertova M, Benito-Gutiérrez E, Arend D, Kozmika Z. 2012. Molecular analysis of the amphioxus frontal eye unravels the evolutionary origin of the retina and pigment cells of the vertebrate eye. Proc. Natl Acad. Sci. USA 109, 15 383–15 388. ( 10.1073/pnas.1207580109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang L, Zhang H, Hu G, Wang H, Abate-Shen C, Shen MM. 1998. An early phase of embryonic Dlx5 expression defines the rostral boundary of the neural plate. J. Neurosci. 18, 8322–8330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertrand S, Camasses A, Somorjai I, Belgacem MR, Chabrol O, Escande M-L, Pontarotti P, Escriva H. 2011. Amphioxus FGF signaling predicts the acquisition of vertebrate morphological traits. Proc. Natl Acad. Sci. USA 108, 9160–9165. ( 10.1073/pnas.1014235108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland ND, Panganiban G, Henyey EL, Holland LZ. 1996. Sequence and developmental expression of AmphiDll, an amphioxus Distal-less gene transcribed in the ectoderm, epidermis and nervous system: insights into evolution of craniate forebrain and neural crest. Development 122, 2911–2920. [DOI] [PubMed] [Google Scholar]
- 34.Jeong J-Y, Einhorn Z, Mathur P, Chen L, Lee S, Kawakami K, Guo S. 2007. Patterning the zebrafish diencephalon by the conserved zinc-finger protein Fezl. Development 134, 127–136. ( 10.1242/dev.02705) [DOI] [PubMed] [Google Scholar]
- 35.Scholpp S, Foucher I, Staudt N, Peukert D, Lumsden A, Houart C. 2007. Otx1 l, Otx2 and Irx1b establish and position the ZLI in the diencephalon. Development 134, 3167–3176. ( 10.1242/dev.001461) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Irimia M, Piñeiro C, Maeso I, Gómez-Skarmeta JL, Casares F, Garcia-Fernàndez J. 2010. Conserved developmental expression of Fezf in chordates and Drosophila and the origin of the zona limitans intrathalamica (ZLI) brain organizer. EvoDevo 1, 7 ( 10.1186/2041-9139-1-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schubert M, Holland LZ, Panopoulou GD, Lehrach H, Holland ND. 2000. Characterization of amphioxus AmphiWnt8: insights into the evolution of patterning of the embryonic dorsoventral axis. Evol. Dev. 2, 85–92. ( 10.1046/j.1525-142x.2000.00047.x) [DOI] [PubMed] [Google Scholar]
- 38.Mazet F, Shimeld S. 2003. Characterisation of an amphioxus Fringe gene and the evolution of the vertebrate segmentation clock. Dev. Genes Evol. 213, 505–509. ( 10.1007/s00427-003-0351-7) [DOI] [PubMed] [Google Scholar]
- 39.Martinez-Ferre A, Navarro-Garberi M, Bueno C, Martinez S. 2013. Wnt signal specifies the intrathalamic limit and its organizer properties by regulating Shh induction in the alar plate. J. Neurosci. 33, 3967–3980. ( 10.1523/jneurosci.0726-12.2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Holland LZ, Kene M, Williams NA, Holland ND. 1997. Sequence and embryonic expression of the amphioxus engrailed gene (AmphiEn): the metameric pattern of transcription resembles that of its segment-polarity homolog in Drosophila. Development 124, 1723–1732. [DOI] [PubMed] [Google Scholar]
- 41.Castro LFC, Rasmussen SLK, Holland PWH, Holland ND, Holland LZ. 2006. A Gbx homeobox gene in amphioxus: insights into ancestry of the ANTP class and evolution of the midbrain/hindbrain boundary. Dev. Biol. 295, 40–51. ( 10.1016/j.ydbio.2006.03.003) [DOI] [PubMed] [Google Scholar]
- 42.Erickson T, Scholpp S, Brand M, Moens CB, Jan Waskiewicz A. 2007. Pbx proteins cooperate with engrailed to pattern the midbrain–hindbrain and diencephalic–mesencephalic boundaries. Dev. Biol. 301, 504–517. ( 10.1016/j.ydbio.2006.08.022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knecht AK, Bronner-Fraser M. 2002. Induction of the neural crest: a multigene process. Nat. Rev. Genet. 3, 453–461. ( 10.1038/nrm832) [DOI] [PubMed] [Google Scholar]
- 44.Meulemans D, Bronner-Fraser M. 2004. Gene-regulatory interactions in neural crest evolution and development. Dev. Cell 7, 291–299. ( 10.1016/j.devcel.2004.08.007) [DOI] [PubMed] [Google Scholar]
- 45.Yu J-K, Meulemans D, McKeown SJ, Bronner-Fraser M. 2008. Insights from the amphioxus genome on the origin of vertebrate neural crest. Genome Res. 18, 1127–1132. ( 10.1101/gr.076208.108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ono H, Kozmik Z, Yu J-K, Wada H. 2014. A novel N-terminal motif is responsible for the evolution of neural crest-specific gene-regulatory activity in vertebrate FoxD3. Dev. Biol. 385, 396–404. ( 10.1016/j.ydbio.2013.11.010) [DOI] [PubMed] [Google Scholar]
- 47.Yu JK, Holland ND, Holland LZ. 2002. An amphioxus winged helix/forkhead gene, AmphiFoxD: insights into vertebrate neural crest evolution. Dev. Dyn. 225, 289–297. ( 10.1002/dvdy.10173) [DOI] [PubMed] [Google Scholar]
- 48.Yu JK, Holland ND, Holland LZ. 2004. Tissue-specific expression of FoxD reporter constructs in amphioxus embryos. Dev. Biol. 274, 452–461. ( 10.1016/j.ydbio.2004.07.010) [DOI] [PubMed] [Google Scholar]
- 49.Simões-Costa MS, McKeown SJ, Tan-Cabugao J, Sauka-Spengler T, Bronner ME. 2012. Dynamic and differential regulation of stem cell factor FoxD3 in the neural crest Is encrypted in the genome. PLoS Genet. 8, e1003142 ( 10.1371/journal.pgen.1003142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeffery WR, Strickler AG, Yamamoto Y. 2004. Migratory neural crest-like cells form body pigmentation in a urochordate embryo. Nature 43, 696–699. ( 10.1038/nature02975) [DOI] [PubMed] [Google Scholar]
- 51.Abitua PB, Wagner E, Navarrete IA, Levine M. 2012. Identification of a rudimentary neural crest in a non-vertebrate chordate. Nature 492, 104–107. ( 10.1038/nature11589) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Domazet-Lošo T, Brajković J, Tautz D. 2007. A phylostratigraphy approach to uncover the genomic history of major adaptations in metazoan lineages. Trends Genet. 23, 533–539. ( 10.1016/j.tig.2007.08.014) [DOI] [PubMed] [Google Scholar]
- 53.Sestak MS, Bozicevic V, Bakaric R, Dunjko V, Domazet-Loso T. 2013. Phylostratigraphic profiles reveal a deep evolutionary history of the vertebrate head sensory systems. Front. Zool. 10, 18 ( 10.1186/1742-9994-10-18) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pani AM, Mullarkey EE, Aronowicz J, Assimacopoulos S, Grove EA, Lowe CJ. 2012. Ancient deuterostome origins of vertebrate brain signalling centres. Nature 483, 289–294. ( 10.1038/nature10838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Holland LZ, Carvalho JE, Escriva H, Laudet V, Schubert M, Shimeld SM, Yu JK. 2013. Evolution of bilaterian central nervous systems: a single origin? Evodevo 4, 27 ( 10.1186/2041-9139-4-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arenas-Mena C, Cameron AR, Davidson EH. 2000. Spatial expression of Hox cluster genes in the ontogeny of a sea urchin. Development 127, 4631–4643. [DOI] [PubMed] [Google Scholar]
- 57.Holland ND. In press. Nervous systems and scenarios for the invertebrate-to-invertebrate transition. Phil. Trans. R. Soc. B 371, 20150047 (doi:10.1098.rstb.2015.0047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaul S, Stach T. 2010. Ontogeny of the collar cord: neurulation in the hemichordate Saccoglossus kowalevskii. J. Morphol. 271, 1240–1259. ( 10.1002/jmor.10868) [DOI] [PubMed] [Google Scholar]
- 59.Miyamoto N, Nakajima Y, Wada H, Saito Y. 2010. Development of the nervous system in the acorn worm Balanoglossus simodensis: insights into nervous system evolution. Evol. Dev. 12, 416–424. ( 10.1111/j.1525-142X.2010.00428.x) [DOI] [PubMed] [Google Scholar]
- 60.Nomaksteinsky M, Röttinger E, Dufour HD, Chettouh Z, Lowe CJ, Martindale MQ, Brunet J-F. 2009. Centralization of the deuterostome nervous system predates chordates. Curr. Biol. 19, 1264–1269. ( 10.1016/j.cub.2009.05.063) [DOI] [PubMed] [Google Scholar]
- 61.Cunningham D, Casey ES. 2014. Spatiotemporal development of the embryonic nervous system of Saccoglossus kowalevskii. Dev. Biol. 386, 252–263. ( 10.1016/j.ydbio.2013.12.001) [DOI] [PubMed] [Google Scholar]
- 62.Kaul-Strehlow S, Urata M, Minokawa T, Stach T, Wanninger A. 2015. Neurogenesis in directly and indirectly developing enteropneusts: of nets and cords. Org. Divers. Evol. 15, 405–422. ( 10.1007/s13127-015-0201-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lowe CJ, Wu M, Salic A, Evans L, Lander E, Stange-Thomann N, Gruber CE, Gerhart J, Kirschner M. 2003. Anteroposterior patterning in hemichordates and the origins of the chordate nervous system. Cell 113, 853–865. ( 10.1016/s0092-8674(03)00469-0) [DOI] [PubMed] [Google Scholar]
- 64.Garstang S. 1894. On some bipinnariae from the English Channel. Q. J. Microsc. Sci. 35, 451–460, +pl. XXVIII. [Google Scholar]
- 65.Romer AS. 1972. The vertebrate as a dual animal—somatic and visceral. Evol. Biol. 6, 121–156. ( 10.1007/978-1-4684-9063-3_5) [DOI] [Google Scholar]
- 66.Garstang W. 1929. The origin and evolution of larval forms. Rep. Br. Ass. Adv. Sci. 1928, 77–98. [Google Scholar]
- 67.Davidson E, Peterson KJ, Cameron RA. 1995. Origin of bilaterian body plans: evolution of developmental regulatory mechanisms. Science 270, 1319–1325. ( 10.1126/science.270.5240.1319) [DOI] [PubMed] [Google Scholar]