Abstract
Induction of alternative mating tactics by surrounding conditions, such as the presence of conspecific males, is observed in many animal species. Satellite behaviour is a remarkable example in which parasitic males exploit the reproductive investment by other males. Despite the abundance of parasitic mating tactics, however, few examples are known in which males alter courtship behaviour as a counter tactic against parasitic rivals. The fruit fly Drosophila prolongata shows prominent sexual dimorphism in the forelegs. When courting females, males of D. prolongata perform ‘leg vibration’, in which a male vibrates the female's body with his enlarged forelegs. In this study, we found that leg vibration increased female receptivity, but it also raised a risk of interception of the female by rival males. Consequently, in the presence of rivals, males of D. prolongata shifted their courtship behaviour from leg vibration to ‘rubbing’, which was less vulnerable to interference by rival males. These results demonstrated that the males of D. prolongata adjust their courtship behaviour to circumvent the social context-dependent risk of leg vibration.
Keywords: alternative mating tactics, social context, courtship behaviour, signal interception, sexual dimorphism
1. Introduction
Conspecific males are potential rivals competing for female mates, and their presence influences male mating behaviour in a range of ways [1,2]. For example, in the presence of rivals, courtship intensity is enhanced in crickets [3] and fighting fish [4], or the duration of copulation is extended in fruit flies [5,6] and soldier flies [7]. In these cases, males increase their investment in reproduction. Conversely, males of stickleback [8] and salamander [9] decrease their investment in the presence of rivals. The most remarkable example is satellite behaviour, in which parasitic males do not perform courtship by themselves but instead intercept females courted by other males (e.g. guppy [10–12] and frog [13]). In this example, parasitic males exploit the investment by other males [2].
Although the presence of parasitic rivals increases the risk of investment loss, it is still better for the courting males to maintain a certain level of effort than discontinuing courtship, which will result in a complete loss of mating opportunity. Under these circumstances, alternative courtship behaviour, which is less vulnerable to interference by rivals, is expected to evolve as a counter tactic against parasitic rivals. However, only a few examples of such alternative courtship tactics have been reported. Male red-spotted newts (Notophthalmus viridescens) shift their mating tactics from ‘hula’ courtship to amplexus courtship at high densities, because ‘hula’ courtship is vulnerable to rival males that mimic female behaviour in an attempt to intercept females [14]. ‘Quiet song’ in birds was suggested to be an alternative courtship tactic to avoid interference by rivals, although this was not verified experimentally [15]. Considering the abundance and importance of parasitic tactics in mating behaviour, we know surprisingly little about the counter tactics against them.
Drosophila prolongata is a fruit fly that shows prominent sexual dimorphism in its forelegs [16–18]. When courting females, males perform ‘leg vibration’, in which a male vibrates the female's abdomen from in front of her using his enlarged forelegs [16]. The functional relationship between leg vibration and the enlarged forelegs suggests that this courtship behaviour has played an important role in the evolution of foreleg morphology. However, males of D. prolongata do not always perform leg vibration, and the proportion of males performing leg vibration varies between strains [19].
In this study, we addressed the following questions through a series of experiments. (1) Do males of D. prolongata have alternative courtship sequences? (2) Is the leg vibration beneficial in increasing female receptivity and required to copulate with reluctant females? (3) Is the leg vibration costly by eliciting interception behaviour of rival males? (4) Do males refrain from leg vibration in the presence of rivals?
2. Material and methods
(a). Fly strains and preparation
Mating behaviour of D. prolongata was shown to be influenced by the genetic background [19]. Therefore, we used multiple strains of known behavioural characteristics to ascertain the robustness of the results. BaVi044 was used in a previous study as the standard strain for the analysis of courtship sequence [16]. BaVi043 and SaPa014 were strains with high and low leg vibration rate, respectively [19]. SaPa010, SaPa002 and BaVi042 were strains with high, medium and low copulation rate, respectively [19]. Flies were reared on ordinary cornmeal medium for Drosophila culture at 20°C in a 12 L : 12 D cycle. Newly eclosed males were maintained individually in single vials (25 mm diameter × 100 mm height, containing cornmeal medium) for 6 days. Prior to the experiment, they were starved for 1 day in single vials containing a piece of wet paper for higher locomotor activity during the observation period. Females were prepared in the same way but in groups of no more than 10 individuals.
(b). Experiment 1: analysis of alternative courtship sequences
Courtship behaviour of D. prolongata consists of tapping, leg display, wing vibration and leg vibration, among which leg vibration was often followed by copulation [16]. However, successful copulations were not always preceded by leg vibration [16,19], suggesting that males of D. prolongata have alternative courtship sequences. As courtship behaviour was observed from above in the previous studies [16,19], some behavioural elements may have been out of view. We re-examined the courtship sequence by closer observations from the side as follows: a male and a female were introduced into a vial (25 mm diameter × 100 mm height) containing cornmeal medium. Then, a cotton plug was pushed into the vial leaving 1 cm of space above the medium. Courtship behaviour was recorded from the side of the vial using a digital camera (HDR-CX720 V; Sony, Tokyo, Japan) for 15 min. All behavioural observations were conducted during the first 4.5 h or the last 2 h of the light phase for higher locomotor activity in both sexes. Movies were played back on a PC and the courtship sequence was inspected visually. Multiple strains were used to ascertain the robustness of the results across various combinations of the genotypes. More than eight successful copulation episodes were analysed for each combination of male (BaVi043 and SaPa014) and female (SaPa010, SaPa002 and BaVi042) strains.
(c). Experiment 2: benefit of leg vibration
Although leg vibration was suggested to stimulate females to accept copulation [16], its function had not been proved experimentally. We examined the effect of leg vibration on female receptivity by the following two observations.
(i). Leg vibration and female receptivity
We presumed that leg vibration is necessary to copulate with reluctant females. To test this hypothesis, male courtship behaviour (BaVi043 and SaPa014) towards females of strains that differed in their copulation rate (SaPa010, SaPa002 and BaVi042) was analysed. The set-up for behaviour observation, ‘arena with a mating stage’, was the same as in a previous study [19]. This set-up enabled simultaneous recordings of many pairs. In brief, a glass chamber (50 mm diameter × 70 mm height; Nichiden-Rika Glass Co., Kobe, Japan), the inner wall of which was treated with silicon polish to prevent the flies from climbing, was used as a mating arena. A disc of wet filter paper was placed on the bottom of the chamber, and at the centre of it was placed a ‘mating stage’, the lid of a 15 ml conical tube (23 mm diameter × 11 mm height) filled with Drosophila instant medium (Formula 4–24 Drosophila Medium, Carolina Biological Supply Co., Burlington, USA). Up to eight chambers were arranged in two rows, isolated from each other by paper partitions, and covered with glass plates. Approximately 5 min after the introduction of a male, a female was introduced into the arena. Mating behaviour was recorded for 1 h using a digital camera installed 80 cm above the chamber. More than 30 pairs were analysed for each combination of male and female strains. Other conditions were the same as experiment 1. The following variables were scored: occurrence of courtship, occurrence of leg vibration, leg vibration duration, occurrence of female wing spreading during leg vibration, copulation success, time to copulation and copulation duration. The effect of female strains was assessed by a likelihood ratio test (reduction in deviance by adding ‘female strain’ as an explanatory variable was examined) using generalized linear models (GLMs) and a Cox proportional hazards model, in which male strain was entered as a covariate. All statistical analyses were performed using R v. 3.1.0 software [20].
(ii). Effect of physical stimulation by leg vibration
Next, we addressed whether physical stimulation by leg vibration is responsible for the enhancement of female receptivity. Performing leg vibration, the male extends both forelegs along the body of the female and vibrates the female's abdomen [16]. We expected that the copulation success rate of males with amputated forelegs would decrease if physical stimulation is responsible for the enhancement. Surgical manipulation of the forelegs was carried out 2 days before the observations. Males were anaesthetized on ice for 30 s and the tarsi of both forelegs were amputated with a surgical knife under a stereomicroscope. Manipulated males were maintained individually in new vials containing cornmeal medium. Strains were the same as those described above (BaVi043 and SaPa014 for males; SaPa010, SaPa002 and BaVi042 for females). Here, multiple strains were used to ascertain the robustness of the effect of the amputation across various genotypes. Mating behaviour was recorded and analysed as described above using the arena with a mating stage. At least 20 pairs were analysed for each combination of strains. The effect of amputation was assessed by a likelihood ratio test (reduction in deviance by adding ‘treatment’ as an explanatory variable was examined) using GLMs and a Cox proportional hazards model, in which male and female strains were entered as covariates.
(d). Experiment 3: cost of leg vibration
We examined how rival males interfere with the courting males, with special reference to the effect of leg vibration.
(i). Interception of females by rival males
First, we addressed whether leg vibration induces interception of females by rival males. In this experiment, the mating stage was removed from the arena (50 mm diameter × 70 mm height) so that all the individuals in the arena were visible to each other. Instead, a piece of yeast paste was placed directly on the wet filter paper on the floor of the arena. Four males and three females were introduced into the arena, and their behaviour was recorded for 1 h from above. The numbers of males and females used in the assay were determined empirically to allow for interactions between individuals to occur within the assay time. At least 13 replications were made for each combination of male (BaVi043 and SaPa014) and female (SaPa010 and BaVi042) strains. Multiple strains were used to ascertain the robustness of the results across various genotypes. Occurrences of leg vibration, and copulation success by courting males and rival males, were scored. Males that courted for more than 20 s before copulation were regarded as courting males, and the other males in the arena were regarded as rival males. The effect of leg vibration was assessed using GLMs, in which male and female strains were entered as covariates.
(ii). Response of rival males to leg vibration
Next, we examined the relationship between leg vibration and the response of rival males. We expected that rival males would respond to the courting male immediately after the occurrence of leg vibration if they use it as a cue. We also aimed to determine the distance in which leg vibration elicits the response of rival males. Accordingly, larger glass chambers (90 mm diameter, 90 mm height; Sansyo, Tokyo, Japan) were used in this experiment. Two pieces of yeast paste were placed on the chamber floor, and seven males and five females, both from BaVi044, were introduced in it. The numbers of males and females used in the assay were determined empirically to allow for interactions between individuals to occur within the assay time. Behaviour was recorded from above for 3 h, and nine replications were made. Response of rival males to leg vibration (response time after the occurrence of leg vibration, and the distance from the courting male) was measured. The following were considered as responses of rival males: turning their body to the location of leg vibration, approaching females, attempting copulation and copulation.
(iii). Sounds associated with leg vibration
We addressed whether sounds were generated in association with leg vibration. A male and a female (BaVi044) were introduced into a plastic chamber (13 mm diameter × 6 mm height) and then a stainless mesh cover (# 0.5 mm) was placed on the top of it. Courtship sounds were recorded using a particle velocity sensor (PU-regular probe; Microflown Technologies, Arnhem, The Netherlands) installed over the chamber at a distance of 1 mm from the mesh cover. The sound signals were amplified (MFSC-2 2ch signal conditioner; Microflown Technologies), digitized at a sampling rate of 25.6 kHz and 0.7 Hz high-pass filtered (PULSE type 3560-B analogue-to-digital converter; Brüel and Kjær, Nærum, Denmark), and stored as WAV format files (software PULSE LabShop v. 15.1.0; Brüel and Kjær). Sounds were recorded from eight different pairs. The contrast of root mean square amplitude between wing vibration and leg vibration within a same sound file was measured using the software Audacity v. 2.0.3.0 (http://audacity.sourceforge.net).
(iv). Role of auditory organs in responding to leg vibration
The most distal part of the antennae (i.e. the third segment including an arista) is the major auditory organ in Drosophila, and amputation of the third segments or aristae is known to impair the fly's response to acoustic signals [21–23]. We expected that amputation of the antennae of males would diminish their response to leg vibration performed by other males if they use airborne sounds of leg vibration as a cue. Manipulation of auditory organs was conducted on the next day of eclosion. After being anaesthetized on ice, the antennal third segment (funicle including arista) or arista alone (less than one-quarter of the arista was left) was unilaterally or bilaterally amputated using forceps. Control (intact) males underwent anaesthetization in the same way. Four males and three females (BaVi044) were observed for 2 h in a small arena without a mating stage. Occurrence of leg vibration, response of rival males to leg vibration and copulation success by courting males and rival males were scored. At least 17 replications were made for each treatment.
(e). Experiment 4: effect of rivals on courtship behaviour
We examined whether courting males alter their courtship sequence in the presence of rival males. We predicted that the number of males performing leg vibration would decrease in the presence of rival males to avoid the risk of female interception. One or two males and a female were introduced into the arena with a mating stage, and behaviour was recorded for 1 h. Occurrence of leg vibration, copulation success and interaction between males were observed. Multiple strains were used to ascertain the robustness of the results across various genotypes. The effect of rival males was assessed using GLMs, in which male and female strains were entered as covariates.
3. Results
(a). Experiment 1: alternative courtship sequences in Drosophila prolongata
We reanalysed the sequence of courtship behaviour in D. prolongata (electronic supplementary material, video S1). Analysis of 54 successful copulation episodes revealed a new behavioural element that had been overlooked in the previous study. This element was named ‘rubbing’, in which the male gently rubs the female's abdomen with his forelegs from behind, immediately before attempting copulation (electronic supplementary material, figure S1 and video S1). Rubbing was observed before all successful copulations, whereas leg vibration was not always observed. When leg vibration occurred, it was always followed by rubbing (electronic supplementary material, figure S1). Therefore, males of D. prolongata have two alternative courtship sequences: one was rubbing preceded by leg vibration, and the other was rubbing without leg vibration.
Individual males were observed to exhibit both types of courtship sequence. Among 26 males that copulated successfully after rubbing with leg vibration, nine individuals had performed rubbing without leg vibration prior to the failed attempt. Conversely, among 28 males that copulated by rubbing without leg vibration, two individuals had performed leg vibration more than 1 min before the successful copulation. Thus, both of the alternative courtship sequences were plastically expressed by the same individuals.
(b). Experiment 2: benefit of leg vibration—increasing female receptivity
(i). Leg vibration was necessary to copulate with reluctant females
To obtain an insight into the effect of leg vibration on copulation success, we used females of strains that differed in their copulation rate [19]. The copulation rate was dependent on female strains (coefficient = −1.84 and −5.03, z = −1.66 and −4.86, p = 0.097 and <0.001 for SaPa002 and BaVi043, respectively), but not on male strains (coefficient = −0.23, z = −0.58, p = 0.565), confirming a difference in female receptivity (electronic supplementary material, table S1). In particular, females of BaVi042 copulated at low rates and required longer courtship before acceptance of copulation, suggesting that they are reluctant to copulate (figure 1a; electronic supplementary material, figure S2a). The leg vibration rate (proportion of males that performed leg vibration immediately before copulation) was negatively associated with female receptivity across the strains. Leg vibration rates were higher with BaVi042 females than with females of SaPa010 or SaPa002 (electronic supplementary material, table S1; figure 1b) in both male strains. In fact, more than 90% of males that copulated with BaVi042 females performed leg vibration, whereas only 40% of unsuccessful males did, suggesting that leg vibration is required to copulate with unreceptive females. An association between female receptivity and leg vibration was noted when the occurrence of leg vibration was plotted against the time elapsed from the initiation of courtship (electronic supplementary material, figure S3). The probability of males performing leg vibration increased as time elapsed from the initiation of courtship (25.5% performed leg vibration in 0–100 s, while 79.0% did in 100–2600 s), suggesting that males did not perform leg vibration on receptive females (accepted copulation immediately) but did with unreceptive females (required longer courtship).
Figure 1.
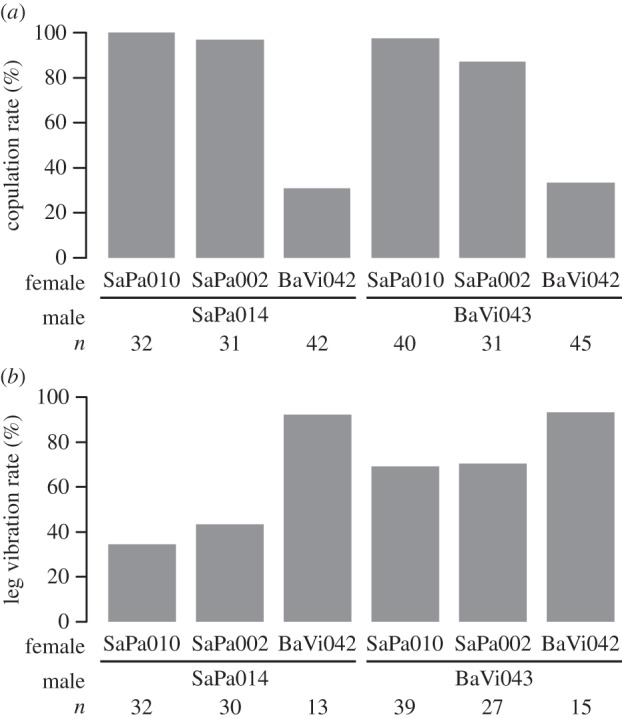
Experiment 2(i)—Female receptivity and occurrence of leg vibration. The effect of female strains was examined by a likelihood ratio test (GLM with binomial distribution). (a) Proportion of pairs that copulated within the observation period (χ2 = 113.41, d.f. = 1, p < 0.001). (b) Proportion of pairs in which males performed leg vibration within 1 min before copulation among the successfully copulating pairs (χ2 = 18.217, d.f. = 1, p < 0.001).
(ii). Physical stimulation by leg vibration increased female receptivity
To further examine the effect of leg vibration on female receptivity, we observed the copulation success rate of males with amputated forelegs. When the tips of the forelegs (tarsal segments) were amputated, males still courted females and performed leg vibration (electronic supplementary material, table S1 and figures S4a,b,c), but the forelegs did not reach the female's abdomen. As a result, copulation rate was decreased and time to copulation was increased (electronic supplementary material, table S1 and figures S4d and S5a). More specifically, copulation success after leg vibration was significantly decreased by foreleg amputation (figure 2; χ2 = 43.654, d.f. = 1, p < 0.001), suggesting that physical stimulation of the females' abdomen is necessary to increase their receptivity. Females spread their wings before successful copulation, and thus wing spreading was considered to be a sign of the female's acceptance of copulation (electronic supplementary material, tables S2 and S3, and supplementary document). The occurrence of wing spreading was also decreased by foreleg amputation, supporting the notion that the decrease in copulation success rate was not due to the male's inability to complete copulation, but due to a failure to enhance female receptivity (electronic supplementary material, table S1 and figure S4e). The effect of foreleg amputation should be least in the pairings between SaPa010 females (highly receptive and tending to copulate without leg vibration) and SaPa014 males (performing less leg vibration). In fact, the number of copulated pairs without leg vibration (electronic supplementary material, table S1, ‘leg vibration immediately before copulation’ = no) among observed pairs was not different between intact and foreleg-amputated males (21/32 and 12/24, respectively; χ2 = 0.1439, d.f. = 1, p = 0.705), suggesting that females were not avoiding damaged males. Nevertheless, a possibility that foreleg amputation affected other aspects of courtship behaviour cannot be completely excluded, because significant differences in copulation duration, leg vibration duration and proportion of males that performed leg vibration were observed between intact and foreleg-amputated males (electronic supplementary material, table S1).
Figure 2.

Experiment 2(ii)—Amputation of foreleg tarsi decreased the copulation success rate after leg vibration. Proportions of pairs that copulated within 1 min after the first bout of leg vibration are shown. The effect of amputation was significant by a likelihood ratio test (GLM with binomial distribution; χ2 = 43.654, d.f. = 1, p < 0.001).
(c). Experiment 3: cost of leg vibration—eliciting parasitic behaviour of rival males
(i). Leg vibration and interception of females
We found that the interception of courted females by rival males occurred frequently in groups consisting of more than 1 male (electronic supplementary material, video S2). The rival males copulated with the courted females without performing courtship by themselves. Mate interceptions occurred only when leg vibration was performed by the courting males (table 1). Most of the rivals responded within 1 s from the initiation of leg vibration (figure 3a). The proportion of responding males decreased as the distance from the courting male increased, and no males responded from a distance of greater than 5 cm (figure 3b).
Table 1.
Experiment 3(i)—Association between leg vibration and interception of females. The number of copulations achieved by courting and rival males was counted with reference to the occurrence of preceding leg vibration. The effect of leg vibration on the occurrence of interception was significant by a likelihood ratio test (GLM with binomial distribution; χ2 = 27.062, d.f. = 1, p < 0.001).
| strain |
with leg vibration |
without leg vibration |
|||
|---|---|---|---|---|---|
| male | female | courting male | rival male | courting male | rival male |
| SaPa014 | SaPa010 | 5 | 1 | 34 | 0 |
| BaVi042 | 7 | 1 | 9 | 0 | |
| BaVi043 | SaPa010 | 2 | 2 | 38 | 0 |
| BaVi042 | 5 | 7 | 9 | 0 | |
Figure 3.
Experiment 3(ii,iii)—Response of rival males to the sound of leg vibration. (a) Distribution of the response timing of rival males relative to the initiation of leg vibration. (b) Proportion of rivals responding to leg vibration at each distance. Responses of rival males consisted of the following: turning their body to the direction of leg vibration, approaching females, attempting copulation and copulation. BaVi044 was used for both sexes. (c) Oscillogram of sounds associated with a series of wing vibrations and leg vibrations. On average, the amplitude of leg vibration was 9.31 times larger than that of wing vibration (n = 8).
(ii). Sounds associated with leg vibration
Considering the quick response to the initiation of leg vibration and its attenuation within several centimetres, airborne sounds generated by leg vibration were suggested to be the cue for the rival males. In fact, the amplitude of the sound recorded during leg vibration was larger than that of wing vibration (figure 3c), supporting the possibility that the rival males detect the sound associated with leg vibration.
(iii). Rival males responded to airborne sounds associated with leg vibration
The most distal part of the antennae (i.e. the third segment including an arista) is the major auditory organ in Drosophila, and amputation of the third segment or aristae is known to impair the fly's response to acoustic signals [21–23]. We examined whether amputation of the antennae of males diminished their response to leg vibration performed by other males. Males with amputated antennae courted females and performed leg vibration normally. When both of the antennae were amputated, however, the proportion of males that responded to leg vibration performed by other males decreased drastically, supporting the notion that leg vibration was detected by rival males as airborne sounds (table 2). Accordingly, the interception of courted females was not observed when both of the antennae were amputated (electronic supplementary material, table S4).
Table 2.
Experiment 3(iv)—Amputation of auditory organs of males diminished their response to leg vibration performed by other males.
| organ amputated | no. rival malesa |
proportion of responding males (%) | significanceb | |
|---|---|---|---|---|
| responded | not responded | |||
| intact | 278 | 173 | 61.6 | — |
| third segmentc (bilateral) | 0 | 104 | 0 | * |
| third segment (unilateral) | 79 | 73 | 52.0 | n.s. |
| arista (bilateral) | 12 | 330 | 3.5 | * |
| arista (unilateral) | 140 | 251 | 36.0 | * |
aMales engaged in copulation were not counted.
bAsterisks indicate significant difference from the control (intact males) by Fisher's exact test with Dunnett's correction at p < 0.001 level.
cAntennal third segment including an arista was amputated.
(d). Experiment 4: the presence of rivals affected courtship behaviour
Discovery of social context-dependent cost of leg vibration prompted us to examine whether D. prolongata males change their courtship sequence in the presence of other males. The leg vibration rate decreased in the presence of a rival male, suggesting that males shifted their courtship sequence to ‘rubbing without leg vibration’ (figure 4). Except in one case, in which the males fought heavily before copulation, intense physical contact between males was not observed, indicating that the behavioural shift was an active response of courting males rather than due to them being passively prevented from performing leg vibration. Nevertheless, the proximate interaction between males attenuated the occurrence of leg vibration. When the interaction between males (low intensity contact by appendage or threatening) occurred immediately before copulation, leg vibration was less frequently observed (electronic supplementary material, table S5), suggesting that the concurrent presence of rivals is required for the behavioural shift. In this regard, the observed behavioural plasticity may not be a case of ‘audience effect’ in a strict sense, which is defined as a behavioural shift induced by the presence of an audience without any interaction with the performer [24].
Figure 4.

Experiment 4—Leg vibration rate is dependent on social context. Proportions of pairs in which males performed leg vibration within 1 min before copulation are shown. Grey bars indicate results of experiments using one male and one female, and white bars indicate those using two males and one female. The effect of social context was significant by a likelihood ratio test (GLM with binomial distribution; χ2 = 19.212, d.f. = 1, p < 0.001).
4. Discussion
(a). Biological function of leg vibration
In this study, we confirmed that leg vibration has a pre-copulatory function of enhancing the female receptivity. An insight into the mechanism underlying this function may be gained from the analysis of a newly identified courtship behaviour, rubbing. Rubbing comprises a gentle stimulation of the female's abdomen. Similar behaviour (physical stimulation of the female's abdomen by the forelegs prior to copulation) has been reported in other Drosophila species (‘touching’ in virilis group [25]; ‘touch’, ‘rubbing’, ‘strike’, ‘vibrating’ and ‘thrust’ in Hawaiian picture-winged species [26,27]). A common sensory bias—physical stimulation of the abdomen induces the acceptance of copulation—may be shared by females of Drosophila species. If this is the case, males of D. prolongata may have exploited the female's sensory bias by acquiring leg vibration, in which the enlarged forelegs efficiently stimulate the female's abdomen.
In addition to the pre-copulatory function, leg vibration may also have post-copulatory function. Although the occurrence of leg vibration was not correlated with copulation duration (electronic supplementary material, figure S6), it should be further investigated from other aspects such as fertility, fecundity, sperm competition and rejection of remating.
(b). Signal interception by rival males
Rival males responded to the leg vibration of courting males, which was considered a modified version of signal interception (i.e. vibratory signals to females are intercepted as auditory signals by unintended receivers) [28]. Although D. prolongata courtship behaviour consists of multiple elements involving visual and auditory signalling [16], only leg vibration was the target of signal interception. Such a preference for a particular step of courtship in signal interception was also reported in damselfish. Males of damselfish are more attracted to courtship sounds generated immediately before copulation than those generated in the initial stages of courtship [29]. In D. prolongata, leg vibration may be an indicator of the presence of a female that will accept copulation with a high probability.
The interception of females by responding to leg vibration was considered a parasitic mating tactic, because the rival males copulated with the courted females without performing courtship by themselves, exploiting the investment by other males. What kind of males adopt this parasitic tactic remains to be investigated. Males of D. prolongata compete for food territories [19]. Both in the laboratory and field observations, subordinate males tended to stay the side of the food source, hiding from the dominating male, which occupied the top surface of the food source. In the field observation, these subordinate males frequently joined in the courtship when a female appeared, suggesting the existence of alternative mating tactics under the natural condition [19]. The parasitic mating tactic might be a choice of subordinate males.
(c). Alternative courtship tactics depending on social context
In the extreme cases, more than half of the courted females were intercepted by rival males when courting males performed leg vibration (table 1; electronic supplementary material, table S4). In many Drosophila species, females do not accept a second mating for several days (reviewed in [30,31]). Although there was variation in the remating tendency among strains of D. prolongata [19], courting males are not likely to have a chance to mate with the intercepted females. Therefore, leg vibration bears a social context-dependent risk of losing invested time and energy in vain. According to the changing cost–benefit balance of performing leg vibration, males of D. prolongata modify their courtship sequence; they refrain from leg vibration and perform more rubbing in the presence of rivals, which is a less effective tactic to increase female receptivity, but not least adaptive because it is less vulnerable to rivals. Therefore, the two courtship sequences could be considered as alternative courtship tactics.
Changes in mating behaviour induced by the presence of rivals often involve reduction of courtship effort itself (leafhopper [32,33]; stickleback [8]; newt and salamander [29,34,35]). On the other hand, examples involving an alternative courtship tactic that is less vulnerable to rivals are limited (e.g. red-spotted newt [14]). Such alternative courtship tactics may be cryptic, like the rubbing in D. prolongata, and may have been overlooked. Because it must be better to keep courting than quitting courtship, which results in a loss of mating opportunity, this type of alternative mating tactic might be found in a wide range of animal species.
Acknowledgement
We thank Dr Aki Ejima for technical advice on surgical manipulation of auditory organs.
Ethics
Laboratory-maintained insects were used in all experiments. No licences or permits were required for this research, and no ethical approval was required.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
S.S. designed the study, carried out all the experiments and data analysis, and drafted the manuscript; A.K. participated in the observation of behaviour; T.T. participated in the sound recording; Y.I. drafted the manuscript; T.M. conceived of the study, designed the study and drafted the manuscript. All authors gave final approval for publication.
Competing interests
The authors have no competing interests.
Funding
This study was supported by Japan Society for the Promotion of Science grant nos. 23128511, 25128702 and 26660263 to T.M.
References
- 1.Bretman A, Gage MJG, Chapman T. 2011. Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol. Evol. 26, 467–473. ( 10.1016/j.tree.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 2.Oliveira RF, Taborsky M, Brockmann HJ. 2008. Alternative reproductive tactics: an integrative approach. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Callander S, Kahn AT, Hunt J, Backwell PRY, Jennions MD. 2013. The effect of competitors on calling effort and life span in male field crickets. Behav. Ecol. 24, 1251–1259. ( 10.1093/beheco/art059) [DOI] [Google Scholar]
- 4.Dzieweczynski TL, Lyman S, Poor EA. 2009. Male Siamese fighting fish, Betta splendens, increase rather than conceal courtship behavior when a rival is present. Ethology 115, 186–195. ( 10.1111/j.1439-0310.2008.01602.x) [DOI] [Google Scholar]
- 5.Bretman A, Fricke C, Chapman T. 2009. Plastic responses of male Drosophila melanogaster to the level of sperm competition increase male reproductive fitness. Proc. R. Soc. B 276, 1705–1711. ( 10.1098/rspb.2008.1878) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bretman A, Westmancoat JD, Gage MJG, Chapman T. 2012. Individual plastic responses by males to rivals reveal mismatches between behaviour and fitness outcomes. Proc. R. Soc. B 279, 2868–2876. ( 10.1098/rspb.2012.0235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barbosa F. 2012. Males responding to sperm competition cues have higher fertilization success in a soldier fly. Behav. Ecol. 23, 815–819. ( 10.1093/beheco/ars035) [DOI] [Google Scholar]
- 8.Le Comber S, Faulkes C, Formosinho J, Smith C. 2003. Response of territorial males to the threat of sneaking in the three-spined stickleback (Gasterosteus aculeatus): a field study. J. Zool. 261, 15–20. ( 10.1017/S0925836903003911) [DOI] [Google Scholar]
- 9.Uzendoski K, Maksymovitch E, Verrell P. 1993. Do the risks of predation and intermale competition affect courtship behavior in the salamander Desmognathus ochrophaeus. Behav. Ecol. Sociobiol. 32, 421–427. ( 10.1007/BF00168826) [DOI] [Google Scholar]
- 10.Evans J, Magurran A. 1999. Male mating behaviour and sperm production characteristics under varying sperm competition risk in guppies. Anim. Behav. 58, 1001–1006. ( 10.1006/anbe.1999.1212) [DOI] [PubMed] [Google Scholar]
- 11.Fraser BA, Janowitz I, Thairu M, Travis J, Hughes KA. 2014. Phenotypic and genomic plasticity of alternative male reproductive tactics in sailfin mollies. Proc. R. Soc. B 281, 20132310 ( 10.1098/rspb.2013.2310) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rezucha R, Reichard M. 2014. The effect of social environment on alternative mating tactics in male Endler's guppy, Poecilia wingei. Anim. Behav. 88, 195–202. ( 10.1016/j.anbehav.2013.12.010) [DOI] [Google Scholar]
- 13.Zamudio KR, Chan LM. 2008. Alternative reproductive tactics in amphibians. In Alternative reproductive tactics: an integrative approach (eds Oliveira RF, Taborsky M, Brockmann HJ), pp. 300–331. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 14.Verrell P. 1983. The influence of the ambient sex-ratio and intermale competition on the sexual behavior of the red-spotted newt, Notophthalmus viridescens (Amphibia, Urodela, Salamandridae). Behav. Ecol. Sociobiol. 13, 307–313. ( 10.1007/BF00299678) [DOI] [Google Scholar]
- 15.Dabelsteen T, McGregor PK, Lampe HM, Langmore NE, Holland J. 1998. Quiet song in song birds: an overlooked phenomenon. Bioacoustics 9, 89–105. ( 10.1080/09524622.1998.9753385) [DOI] [Google Scholar]
- 16.Setoguchi S, Takamori H, Aotsuka T, Sese J, Ishikawa Y, Matsuo T. 2014. Sexual dimorphism and courtship behavior in Drosophila prolongata. J. Ethol. 32, 91–102. ( 10.1007/s10164-014-0399-z) [DOI] [Google Scholar]
- 17.Singh BK, Gupta JP. 1977. Two new and two unrecorded species of the genus Drosophila Fallen (Diptera: Drosophilidae) from Shillong, Meghalaya, India. Proc. Zool. Soc. 30, 31–38. [Google Scholar]
- 18.Toda M. 1991. Drosophilidae (Diptera) in Myanmar (Burma) VII. the Drosophila melanogaster species-group, excepting the D. montium species-subgroup. Orient. Insects 25, 69–94. ( 10.1080/00305316.1991.10432216) [DOI] [Google Scholar]
- 19.Kudo A, Takamori H, Watabe H, Ishikawa Y, Matsuo T. 2015. Variation in morphological and behavioral traits among isofemale strains of Drosophila prolongata (Diptera: Drosophilidae). Entomol. Sci. 18, 221–229. ( 10.1111/ens.12116) [DOI] [Google Scholar]
- 20.R Core Team. 2014. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 21.Ejima A, Griffith LC. 2008. Courtship initiation is stimulated by acoustic signals in Drosophila melanogaster. PLoS ONE 3, e3246 ( 10.1371/journal.pone.0003246) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gleason JM, Pierce AA, Vezeau AL, Goodman SF. 2012. Different sensory modalities are required for successful courtship in two species of the Drosophila willistoni group. Anim. Behav. 83, 217–227. ( 10.1016/j.anbehav.2011.10.029) [DOI] [Google Scholar]
- 23.Gopfert M, Robert D. 2001. Turning the key on Drosophila audition. Nature 411, 908 ( 10.1038/35082144) [DOI] [PubMed] [Google Scholar]
- 24.Matos RJ, Schlupp I. 2005. Performing in front of an audience: signalers and the social environment. In Animal communication networks (ed. McGregor PK.), pp. 68–83. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 25.Hoikkala A, Hoy R, Kaneshiro K. 1989. High-frequency clicks of Hawaiian picture-winged Drosophila species. Anim. Behav. 37, 927–934. ( 10.1016/0003-3472(89)90137-1) [DOI] [Google Scholar]
- 26.Spieth H. 1978. Courtship patterns and evolution of Drosophila adiastola and Drosophila planitibia species subgroups. Evolution 32, 435–451. ( 10.2307/2407610) [DOI] [PubMed] [Google Scholar]
- 27.Vedenina VY, Ivanova TI, Lazebny OE. 2013. Analysis of courtship behavior in closely related species of Drosophila virilis group: a new approach arises new questions. J. Insect Behav. 26, 402–415. ( 10.1007/s10905-012-9359-z) [DOI] [Google Scholar]
- 28.Searcy WA, Nowicki S. 2005. The evolution of animal communication: reliability and deception in signaling systems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 29.Kenyon T. 1994. The significance of sound interception to males of the bicolor damselfish, Pomacentrus partitus, during courtship. Environ. Biol. Fishes 40, 391–405. ( 10.1007/BF00005282) [DOI] [Google Scholar]
- 30.Markow T. 2002. Perspective: female remating, operational sex ratio, and the arena of sexual selection in Drosophila species. Evolution 56, 1725–1734. ( 10.1111/j.0014-3820.2002.tb00186.x) [DOI] [PubMed] [Google Scholar]
- 31.Markow T, O'Grady P. 2005. Evolutionary genetics of reproductive behavior in Drosophila: connecting the dots. Annu. Rev. Genet. 39, 263–291. ( 10.1146/annurev.genet.39.073003.112454) [DOI] [PubMed] [Google Scholar]
- 32.Mazzoni V, Lucchi A, Cokl A, Presern J, Virant-Doberlet M. 2009. Disruption of the reproductive behaviour of Scaphoideus titanus by playback of vibrational signals. Entomol. Exp. Appl. 133, 174–185. ( 10.1111/j.1570-7458.2009.00911.x) [DOI] [Google Scholar]
- 33.Mazzoni V, Presern J, Lucchi A, Virant-Doberlet M. 2009. Reproductive strategy of the Nearctic leafhopper Scaphoideus titanus Ball (Hemiptera: Cicadellidae). Bull. Entomol. Res. 99, 401–413. ( 10.1017/S0007485308006408) [DOI] [PubMed] [Google Scholar]
- 34.Aragon P. 2009. Conspecific male chemical cues influence courtship behaviour in the male newt Lissotriton boscai. Behaviour 146, 1137–1151. ( 10.1163/156853909X413097) [DOI] [Google Scholar]
- 35.Verrell P, Krenz J. 1998. Competition for mates in the mole salamander, Ambystoma talpoideum: Tactics that may maximize male mating success. Behaviour 135, 121–138. ( 10.1163/156853998793066357) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.



