Abstract
Neglected tropical diseases (NTDs) have received increasing attention in recent years by the global heath community, as they cumulatively constitute substantial burdens of disease as well as barriers for economic development. A number of common tropical diseases such as malaria, hookworm or schistosomiasis have well-documented economic impacts. However, much less is known about the population-level impacts of diseases that are rare but associated with high disability burden, which represent a great number of tropical diseases. Using an individual-based model of Buruli ulcer (BU), we demonstrate that, through feedbacks between health and economic status, such NTDs can have a significant impact on the economic structure of human populations even at low incidence levels. While average wealth is only marginally affected by BU, the economic conditions of certain subpopulations are impacted sufficiently to create changes in measurable population-level inequality. A reduction of the disability burden caused by BU can thus maximize the economic growth of the poorest subpopulations and reduce significantly the economic inequalities introduced by the disease in endemic regions.
Keywords: neglected tropical diseases, coupled ecological-economic systems, individual-based model
1. Introduction
Long-term economic growth and advances in medicine and technology have contributed to a significant reduction in the burden of human infectious diseases around the world [1]. However, more than 1 billion people, most of them in tropical and subtropical countries, continue to suffer a disproportionate burden of disease and poverty [2,3]. Although the global distribution of communicable diseases can be partly explained by a gradient in climatic and biological factors [4,5], there is a growing focus on potential reinforcing feedback mechanisms between ecology, disease and economics, for explaining the distribution of both poverty and disease [6]. Households and individuals need economic resources to reduce their exposure to infectious diseases and to access quality healthcare [7,8]. Additionally, infectious diseases have short- and long-term impacts on economic growth through reduction of labour productivity, education and investments in order to accumulate economic capital [9–11]. Increasing recognition of the feedbacks between poverty and disease has led to integrative studies on disease-driven poverty traps [12–14].
Feedback mechanisms between poverty and disease ecology are especially important for the study of neglected tropical diseases (NTDs): they are closely associated with particular geographical and environmental conditions in the tropics; they thrive under conditions of poverty, poor sanitation and malnutrition; and they constitute a major barrier for the economic development of the populations affected [15,16]. In sub-Saharan Africa alone, the four most prevalent NTDs are estimated to cause more than 700 million infections per year [3]. But despite their many similarities, NTDs are a very heterogeneous group of diseases with respect to their prevalence, aetiological agents and health consequences. For instance, the four least prevalent NTDs—leprosy, leishmaniasis, dracunculiasis and Buruli ulcer (BU)—are responsible for hardly more than 50 000 infections annually [3], but this group is composed of highly stigmatizing and devastating chronic infections that are responsible for a great burden of disability and important economic impacts on affected households [3]. Although the population-level disability and economic burdens of these diseases is expected to be insignificant, they can constitute important barriers for the economic development and health of specific groups, especially the poorest subpopulations.
An illustrative example is BU, a necrotizing skin disease that caused less than 3000 cases worldwide in 2013, but with more than 90% of the global burden concentrated in sub-Saharan Africa [17]. BU arises in populations living near large bodies of stagnant and slow-flowing waters, where ecological conditions are suitable for Mycobacterium ulcerans, the agent responsible for the disease [18–20]. Despite its low incidence, the focal distribution of the disease makes it particularly devastating in endemic regions, where it contributes to significant disability and causes impoverishment of affected households [21,22]. Most of the morbidity is due to important delays in seeking treatment at the health services, which enables disease progression and leads to the formation of large ulcers. Treatment of these severe stages requires, in addition to the standard eight-week antibiotic therapy [23], surgical procedures involving debridement, skin grafts and much longer hospital stays [24–26]. Contraction of joint movement and functional limitations for life are a common outcome of large ulcers [26,27], which is associated with loss of employment and school abandon [28]. Despite its devastating consequences, measuring the economic impact of such a rare disease in endemic populations is challenging.
There are two common traditions for modelling the economic impacts of disease [13]. Population-level (e.g. ‘macro’) methods focus on aggregated indicators, such as disease prevalence, life-expectancy or per capita income. There are a number of macro-oriented studies that have found substantial population-level economic impacts of disease [6,10,29]. A disadvantage of such approaches is that aggregated indicators necessarily ignore heterogeneity within populations, and they cannot be substantially influenced by rare diseases. By contrast, ‘micro-oriented’ approaches have focused on individual-level characteristics and behaviours, and thus measure either costs of illness or effects of disease on labour productivity or income [11,30,31]. The emphasis here tends to be on diseases with large impacts on the population, allowing for the economic burden to be extrapolated from individual-level effects. Not surprisingly, the rarest diseases on the basis of their insignificant population-level impacts have drawn much less or no attention in the literature of economic burden of disease, which contributes to their neglect in the global health agenda [16]. This paper aims to fill the gap and contribute to the economic analysis of rare-but-devastating diseases through specific models that account for feedbacks between economics and disease from individuals to the populations that they comprise.
Here, we use an individual-based model of BU, which accounts for realistic variability and heterogeneity in individual epidemiological parameters, to analyse the impact of this rare disease on the economic structure of human populations. More specifically, tracking information about each individual allows us to address issues of economic and health inequalities and to focus on worst-off groups within the populations. To construct the model, we first identify the key feedbacks between wealth and Buruli ulcer, which rely mostly on disparities in vulnerability and access to treatment as functions of economic resources. Then, multiple possible scenarios are simulated to study the impact of BU for the whole population and different subpopulations under a gradient of environmental risk. Finally, we test the potential health and economic impact of interventions that mitigate the disability burden of the disease.
2. Material and methods
Our modelling framework is based on coupled systems of human disease and economic growth, and stems from a recent body of theory on feedbacks between poverty and disease [12–14]. We adopt an individual-based approach to better capture within-population heterogeneities of BU disease, notably issues such as incubation period, individual delay in seeking medical treatment or treatment duration. A full description of the parameters and their associated values along with the complete algorithm and rationale for the coupled individual-based model is provided in the electronic supplementary material, S1.
(a). Epidemiological model
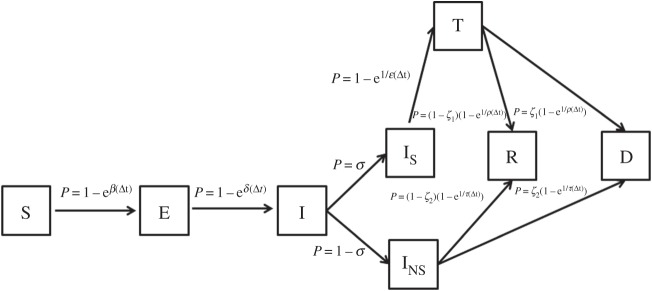
Our disease model is derived from the susceptible-exposed-infected-recovered (SEIR) framework [32] widely used to characterize epidemiological dynamics, and it is adapted to better capture the specificities of BU in rural populations (figure 1). The model takes into consideration susceptible individuals (S) that can become exposed to BU (E) depending on the interplay between an environmental risk and socio-economic susceptibility (β). Exposed individuals develop symptoms and become infected (I) after a certain incubation period (γ). A proportion of infected does not seek treatment at the health services (INS) and the rest (IS) seek treatment (T) after a certain delay (ɛ), which is dependent on socio-economic resources. Finally, non-seekers and individuals under treatment either fully recover (R) or recover with permanent disabilities (D), with probabilities depending on disease progression in each compartment. Importantly, untreated individuals have a smaller probability of full recovery (R) than those treated. We assume that individuals gain permanent immunity to BU after infection and, therefore, neither recovered nor disabled individuals can get re-infected. For the sake of simplicity, we assume that death and birth rates are equal so that the population is stationary [32].
Figure 1.
Model framework for the epidemiological dynamics of Buruli ulcer. The model is based on the susceptible-exposed-infected-recovered (SEIR) framework, where S are susceptible individuals, E are exposed individuals that have not yet developed symptoms and I are infected individuals that can either seek treatment at the health services (IS) or not (INS). Non-seekers and individuals under treatment (T) either fully recover (R) or recover with permanent disabilities (D).
To account for socio-economic differences in exposure, vulnerability and access to treatment for BU, we model the individual transmission rate of BU βi, the probability of seeking treatment σi and the time to seek treatment ɛTi, as linear functions of individual-level physical capital ki,, which is a general proxy for individual wealth (see the electronic supplementary material, S1). Importantly, the model is stochastic and individual-based, so there is considerable heterogeneity in parameter values and outcomes, as with real-world rare events. Therefore, theoretical values for all the epidemiological parameters are assigned to each individual (ɛTi, ρTi, etc.) but the final value and the subsequent links depend on the real value of the parameter for each individual (ɛRi, ρRi, etc.), which are subject to the stochastic process.
(b). Economic growth model
The economic dynamics are based on the simplest standard models of economic growth [13,14,33]. Individual income yi is based on a simple Cobb–Douglas production function and is determined by the level of technology A and a combination of physical capital k and human capital h, which exhibits diminishing returns to investment (0 < α1 + α2 < 1):
| 2.1 |
In the economics literature, human capital typically refers to education and training, but it can also refer to health status as we define it here. In rural areas of sub-Saharan Africa where BU is endemic, individual income is mainly generated through physical labour and thus physical health as human capital, h, is especially important. Equation (2.2) represents h as a function of health status given by an initial human capital for healthy individuals, h0, that is reduced in BU patients by wi:
| 2.2 |
As BU is initially painless and individuals can carry out normal lives until the disease is advanced, we assume for simplicity that infected individuals do not experience a reduction in human capital. For individuals under medical treatment, wi = wT and it is associated with hospitalization or daily visits to the healthcare centre that are responsible for the great indirect costs of the disease [21,22,34]. We attribute disabled individuals a reduced human capital, wi = wD, to account for their reduced ability to work and the impact of functional limitations on school abandon and job loss [27,28], which is reinforced by the strong social stigma and mystical beliefs associated with the disease [35,36]. Finally, physical capital, k, represents individual's wealth, and its accumulation over time results from the investment of a proportion of current income at rate rk, while it depreciates at rate δk:
| 2.3 |
Direct non-medical costs associated with treatment (xi) such as transportation to the health centre and feeding costs are deducted from income for individuals undergoing treatment [21,22,34]. Importantly, when the individuals in the model are renewed (one individual dies and is replaced by a new born), the offspring ‘inherits’ the physical capital of the individual they replace (their ‘parent’).
(c). Strategies for prevention of disability
We evaluate the potential impact that disease control strategies aimed at preventing disability in BU cases could have on health and economic outcomes of the population in the long term. We consider a scenario where improved management of BU cases (tertiary prevention) leads to a reduction of 50% in the individual probability of functional limitations, ζ1, once the patient is being treated at the health services [37]. This is mostly achievable through high-quality treatment complemented with intensive rehabilitation and physiotherapy [37,38]. We also estimate additional improvements where efforts are aimed at reducing the time to seek treatment (ɛMax = 30 days) to limit disease progression (secondary prevention) prior to consultation at the health services. This reduction can be achieved in many different ways, such as awareness and education campaigns, active search of BU cases or decentralization of treatment [24,25].
(d). Choice of parameter values
A thorough literature review of the ecology, epidemiology and socio-economics of BU was performed prior to model development in order to identify all relevant parameters in the coupled system and to select appropriate parameter values (table 1). A fully detailed explanation of the choice of each parameter value along with the sources can be found in the electronic supplementary material, S1c. In summary, demographic parameters such as population size and demographic rate were drawn to represent the demographic situation in many BU endemic regions in Central and West Africa. Epidemiological parameters such as incubation period or time to heal with treatment were selected from cross-sectional and longitudinal studies of BU cases in endemic regions. The economic system relies on classic models of economic growth, and we draw the values of many of the economic parameters from this literature [33]. We also used studies on BU socio-economic costs to estimate parameters such as treatment costs and human capital reduction.
Table 1.
List of parameters used in the model.
| symbola | variable | value or rangeb | linksb |
|---|---|---|---|
| demography | |||
| N | total population | 50 000 | |
| D | demographic rate | 24.5 per 1000py | |
| disease | |||
| βMax | environmental risk | 10−5 to 10−1 | |
| β | individual transmission rate | (0.2–1) βMax | dependent on K (see the electronic supplementary material, figure S1) |
| γT | incubation period (days) | 120 | |
| σ | probability of seeking treatment | 0.8–1 | dependent on K (see the electronic supplementary material, figure S1) |
| ɛT | time to seek treatment (days) | 7–365 | dependent on K (see the electronic supplementary material, figure S1) |
| ρT | time to heal with treatment (days) | 60–365 | dependent on ɛR (see the electronic supplementary material, figure S1) |
| ζ1 | probability of disability with treatment | 0.05–0.5 | dependent on ɛR (see the electronic supplementary material, figure S1) |
| τT | time to heal without treatment (days) | 60–365 | |
| ζ2 | probability of disability without treatment | 0.5–1 | dependent on τR (see the electronic supplementary material, figure S1) |
| economics | |||
| k0 | average initial physical capital or wealth ($) | 1750 | |
| α1 | returns to investment for h | 0.5 | |
| α2 | returns to investment for k | 0.33 | |
| h0 | initial human capital | 1 | |
| X | treatment cost ($) | 1 | |
| WT | human capital reduction (under treatment) | 0.8 | |
| WD | human capital reduction (disabled) | 0.5 | |
| A | level of technology | 0.21 | |
| rk | physical capital savings rate | 0.3 | |
| δk | physical capital depreciation rate | 0.1 | |
aThe ‘T’ subscript denotes a theoretical value. The real value (‘R’ subscript) for each individual depends on a stochastic process and is therefore subject to variability.
bThe different parameter values and links are explained in detail in the electronic supplementary material, S1. Simultaneous variations of up to ±25% around these parameter values are considered through Latin-Hypercube sampling.
(e). Model analysis
We run the simulations until equilibrium is reached and characterize important economic and epidemiological outcomes in the population, using the mean value of the last 10 years to obtain robust estimates. We explore model behaviour with a gradient of βMax to analyse all situations that could fit with different epidemiological conditions observed, from low endemic regions to areas located in high-risk clusters, where incidence levels reported for specific populations can be significantly higher. To evaluate the economic impact of strategies for prevention of disability mentioned earlier, variations in economic outcomes were estimated by comparing scenarios with and without interventions, under similar values of βMax. We address the potential uncertainty surrounding the parameter values in our model by accounting for simultaneous variations in multiple epidemiological and economic parameters using the Latin-Hypercube sampling approach [39]. For this, we run 1000 simulations for each βMax with random and simultaneous variations of up to ±25% in parameter values. Finally, we quantify the impact of variations in parameter values on model outcomes through multivariate linear models under a gradient of environmental risks (see the electronic supplementary material, S2). All simulations are done in C++ and the model analysis is done in Matlab, v. 2014b and R statistical software, v. 3.1.2.
3. Results
(a). Population-level impact of Buruli ulcer
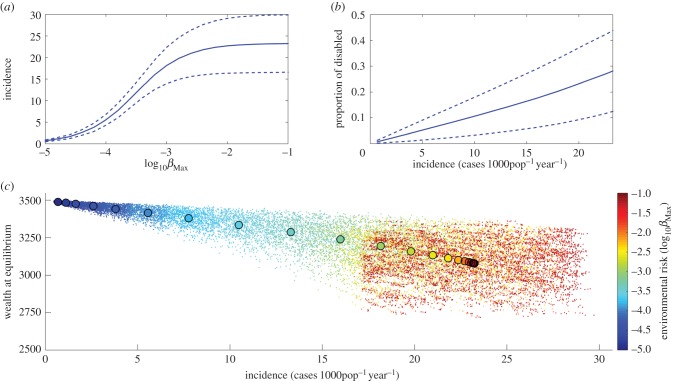
By simultaneously accounting for feedbacks between poverty and disease at the individual level, we show in figure 2 how the disease and economic dynamics interact within the population. An increase in the background environmental risk, represented by βMax, results in a higher number of BU cases, with a limit at an incidence of approximately 24 cases 1000py−1 (figure 2a), which represents the influx of individual renewal by demography. This increase in incidence has an impact on the proportion of disabled in the population. Because individuals experience disabilities for life, the number of disabled in the population is considerably higher than the annual incidence of newly infected individuals (figure 2b). Figure 2c illustrates the relationship between BU incidence and the average physical capital (or wealth) of the population at equilibrium for a wide range of initial disease transmission rates. We observe a decrease in the average wealth of the population at higher incidence levels, which are determined by the environmental risk. However, because the average loss in per capita wealth is less than 10% at the highest environmental risk, such a relatively rare NTD cannot create population-level poverty traps (i.e. self-reinforcing mechanisms that result in the persistence of poverty at the population level).
Figure 2.
Mean effect of Buruli ulcer on the population. (a) Impact of environmental risk on Buruli ulcer incidence. The solid line represents the mean incidence for each environmental risk and dashed lines are the 95% confidence intervals. (b) Impact of Buruli ulcer incidence on the proportion of disabled people in the population. (c) Link between incidence and wealth at equilibrium for a gradient in environmental risk. The colour changes with the environmental risk, from dark blue at the lowest risk towards dark red at the highest risk. Each dot represents one simulation with parameter values selected through Latin Hypercube sampling, and the larger circles represent the mean value of all 1000 simulations for each βMax.
(b). Population-level inequalities
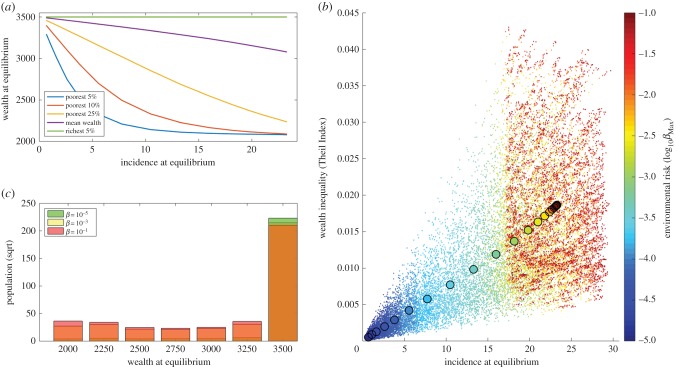
The individual-based approach allows us to evaluate not only the average dynamics of the population but also the impact of BU in creating inequalities in health and wealth (figure 3). Although the model shows a marginal impact on the average wealth of the wealthiest subgroups, there are substantial economic impacts on the poorest subgroups of the population (figure 3a). At very low incidence levels all subgroups maximize economic growth, but as BU incidence increases, the economic impact of the disease results in a great divergence in the wealth of the richest and poorest subgroups. This is owing to a progressive concentration of the population in the extremes of the wealth distribution leading to a polarization of the wealth and health outcomes in the population (figure 3b). Although most of the population enjoys economic growth and good health, a small proportion suffers a disproportionate burden of disability and poverty, losing up to 40% of their wealth. Figure 3c illustrates the relationship between BU incidence and economic inequalities of the population, as described by the Theil Index, for a wide range of disease transmission rates. We observe that an increase in BU incidence results in a linear increase in the average Theil Index in the population.
Figure 3.
Impact of Buruli ulcer on economic inequalities. (a) Impact of Buruli ulcer incidence on the mean wealth of different population subgroups. (b) Distribution of wealth at equilibrium in populations under different environmental risks. (c) Link between incidence and wealth inequality at equilibrium for a gradient in environmental risk. The colour changes with the environmental risk, from dark blue at the lowest risk towards dark red at the highest risk. Each dot represents one simulation with parameter values selected through Latin Hypercube sampling, and the larger circles represent the mean value of all 1000 simulations for each βMax.
(c). Impact of strategies for prevention of disability
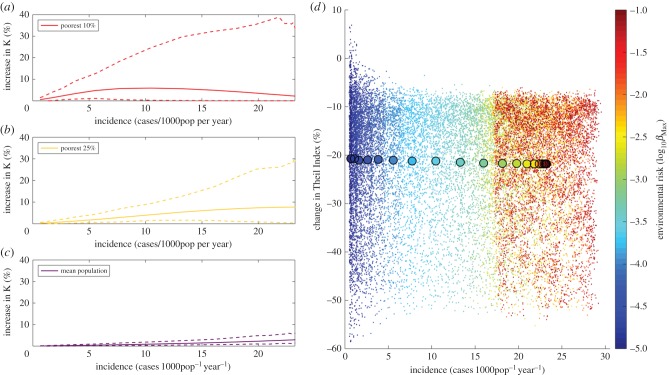
We show in figure 4 how public health strategies that improve management of clinical cases with the aim of reducing disabilities can improve economic equity in populations suffering from BU. Although the impact of these strategies on the average wealth of the population is negligible, their impact is significantly higher in the worse-off groups. In our simulations, improved management increases the wealth of the poorest subgroups by about 10% on average (solid lines), compared with a situation where there is no such strategy. Furthermore, the impact of these strategies could be maximized in populations that have the highest incidence levels of BU, where the maximum wealth increase could be up to 40% in the best scenarios (dashed lines). The differential impact they have on best- and worst-off groups contributes to the depolarization of the distribution of wealth and disabilities in the population, which results in a great reduction of wealth inequalities of approximately 20% on average, and up to 50% in the best scenarios (figure 4). Additional improvements can be achieved in both the wealth of worse-off groups and wealth inequalities when combining these strategies with those aiming at reducing the time to seek treatment and hence disease progression in BU patients (see the electronic supplementary material, S4).
Figure 4.
Economic impact of strategies aiming at prevention of disabilities. (a–c) Impact on the wealth of different subgroups of the population. The solid lines represent the average wealth increase in subgroups and the dashed lines show minimum and maximum increases at different incidence levels. In all cases, the wealth in the population at equilibrium for each simulation in the model with intervention is compared with the wealth at equilibrium for a simulation with the same parameter values in the model without intervention. (d) Reduction of wealth inequality for a gradient in environmental risk. The colour changes with the environmental risk, from dark blue at the lowest risk towards dark red at the highest risk. Each dot represents the decrease in Theil Index for one pair of simulations with parameter values selected through Latin Hypercube sampling, and the larger circles represent the mean decrease in all 1000 simulations for each βMax.
4. Discussion and conclusion
Neglected tropical diseases (NTDs) have received increasing attention in recent years by international organizations and funding agencies, as they are both a major cause of morbidity and a barrier for economic development of the poor [15,16]. The obvious interconnection between poverty and disease is beginning to be formalized in coupled models of infectious diseases and economics, showing that populations with high prevalence of diseases and low economic resources can theoretically be trapped in a ‘vicious cycle’ of long-term poverty and high disease burdens [12–14]. While such models may apply to diseases like malaria, hookworm or schistosomiasis, they do not provide insights on the impact of diseases with low prevalence but great disability burden. Our results for BU show how even low incidence levels of disease can have a significant impact on the population through feedbacks that drive inequalities. A high environmental risk can lead to long-term clusters of disease and poverty in subgroups of the population and this effect is stronger when coupled to poor economic conditions.
Despite research progress in areas like ecology, epidemiology and social sciences, many aspects surrounding BU epidemiology and socio-economic feedbacks are still poorly understood and can be highly context specific. For this reason, the aim of this study was to present a general modelling framework for understanding the feedbacks between the environment, BU and poverty that can be tailored to local populations or adapted to other rare tropical diseases. We rely on conservative assumptions for many of the parameters (electronic supplementary material, S1c), especially for those with important impacts on model outcomes in the sensitivity analysis (electronic supplementary material, S2). We take parameter uncertainty into account in the presentation of model results by including multiple simulations with random variations of up to ±25% in our parameter estimates, in order to show the range of potential economic outcomes. This necessarily implies that economic estimates in this study are subject to considerable uncertainty, especially at higher incidence levels. An important assumption of this study is that individuals get permanent immunity after recovery from BU infection. In the absence of immunity, the potential outcomes of the disease could be even more devastating (electronic supplementary material, S3).
The scarcity of data on the relationship between poverty and key epidemiological parameters, such as disease risk or health seeking behaviours in tropical regions, makes it challenging to realistically model socio-economic feedbacks for NTDs. As a conservative choice, we consider linear functional relationships between epidemiological parameters and socio-economic status. However, the gap between wealthier and poorer populations may be even greater. For instance, the probability of seeking treatment can be very low in poor populations until a certain economic threshold is attained, or it may exhibit diminishing returns after that and be invariably high for wealthier subgroups. The use of less conservative, nonlinear functional forms (logarithmic, exponential or sigmoidal) may therefore be more appropriate in some cases, which would result in more inherent nonlinearities in the system with stronger feedbacks. This would potentially increase not only the gap between wealthier and poorer populations as a result of the disease, but also the economic benefits of interventions. Future studies could greatly benefit from a better understanding of the socio-economic determinants of NTDs, through systematic field data collection.
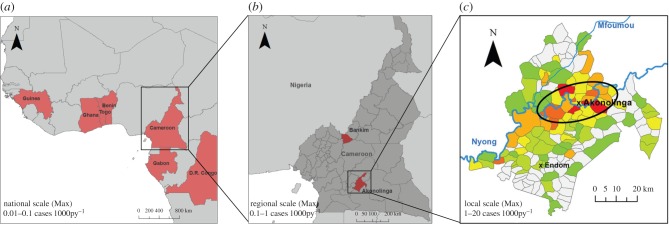
Owing to their tendency to cause significant life-long disability, the population-level effect of BU and other rare tropical diseases is high even at their characteristic low incidences. As opposed to malaria or hookworm, which have a small but cumulative effect on a great proportion of the population, BU is devastating for the very few who are affected. Our results demonstrate that at these low incidence levels, the inequalities created in the population as a result of highly debilitating diseases can be significant. A sustained long-term annual incidence of 5 to 20 cases 1000py−1 can leave a significant proportion of the population with permanent disabilities. In the context of low-resource settings, where no subsidies exist for disabled individuals and most economic activities rely heavily on physical labour, such a disability burden results in a major economic burden. As a result, Buruli ulcer can create a polarization in the distribution of wealth in the population, dragging part of it into a spiral of poverty and disease while the aggregate economy is generally not affected. Although BU incidence in affected regions within endemic countries rarely exceeds 1 case 1000py−1 [18,40–42], the highly focal distribution of the disease results in clusters within endemic regions, with some populations having up to 5–20 cases 1000−1 inhabitants−1 [18,20,41,43,44]. Therefore, while the economic outcomes shown in this study might not be appropriate for reported BU incidence levels at national or regional scales, they can reflect the situation of many high-risk populations within endemic regions (figure 5), and can also correspond to incidence levels observed for other rare tropical diseases [3].
Figure 5.
Bringing results to scale. (a) Most Buruli ulcer cases concentrate in Central and Western African countries, where annual incidence generally ranges from 0.01 to 0.1 cases 1000py−1 [45]. (b) Within endemic countries, disease cases accumulate in specific regions with favourable environmental traits such as slow-flowing rivers or flooded areas, where incidence typically ranges from 0.1 to 1 cases 1000py−1 [18,40–42]. (c) Within endemic regions, spatial clusters with higher incidence of 1–5 cases 1000py−1 can be observed (ellipse) and villages with the highest reported incidence in these clusters can have up to 5–20 cases 1000py−1 [18,20,41,43,44]. (a,b) were produced with ArcMap v. 10.2.2 to illustrate countries and regions with high long-term incidence, and (c) was adapted from Landier et al. [18] to show distribution of spatial clusters at local scales.
In our analysis, the background environmental risk was the main determinant of the long-term clusters of poverty and disease in our model. Pathogens responsible for NTDs like BU are highly embedded in their local environment and their risk is highly sensitive to environmental changes. Exploitation of natural resources or improvement in infrastructure is necessary for economic growth in tropical countries, but this often comes at the expense of rapid environmental change that can increase the prevalence of local infectious diseases [46]. For instance, changes in agricultural practices, deforestation and the construction of roads and dams have contributed to the emergence of BU endemic areas [18–20]. Given the potential that some NTDs have to drag part of the population into poverty, the negative consequences of environmental degradation could fall disproportionally on few individuals, while most of the population enjoys the economic benefits. Investment in the public health system can help alleviate these indirect negative consequences in subgroups of the population and contribute to health equity.
This study illustrates how a reduction of the disability burden caused by BU, which could be extended to other devastating NTDs, is not only important on public health grounds but is also justified as a social justice intervention. Prevention of disability, one of the main goals of the World Health Organization strategy for BU [37], can maximize the economic growth of the least well-off and reduce significantly the economic inequity generated by the disease. A combination of early detection of cases with improved case management has the greatest potential to achieve this. The benefits of awareness, education campaigns and active search of BU cases have been previously reported [24,25], but research on BU treatment and management lags way behind most of the other NTDs [38]. Many of the financial and geographical barriers associated with late presentation of cases [35,47] could be overcome with a change to a decentralized all-oral antibiotherapy, given in an outpatient basis [48]. In addition, implementation of simplified wounds dressing and rehabilitation programmes, among others, could greatly improve case management [38]. Investing on research and action against highly debilitating diseases like BU should be on the agenda of international organizations and funding agencies.
In conclusion, we provide a general framework to study feedbacks between environmental risks, BU and economics and show that even rare diseases can be a source of health and economic inequalities in endemic populations. Although highly prevalent diseases such as malaria or hookworm have provided important cases for studying economic feedbacks because the links are evident at the population level, a number of neglected diseases may have a profound impact on the economic structure of human populations even at low prevalence levels. Understanding the feedbacks between the environment, NTDs and poverty at both individual and population levels is thus key to finding new ways of fighting these dreadful threats.
Supplementary Material
Acknowledgements
We are thankful to the members of the consortium ANR-EXTRA MU (Laurent Marsollier, Sara Eyangoh, Arnaud Fontanet, Philippe Le Gal, Kevin Carolan, Danny Lo Seen) and the staff at MSF in Akonolinga (Cameroun) for insightful discussions. Special thanks to Estelle Marion, Jordi Landier and Marie Thérèse Ngo Nsoga for sharing information that helped in the development of model hypotheses.
Authors' contributions
A.G., C.N.N., J.F.G., M.B. and B.R. conceived and designed the study. A.G. and B.R. performed the modelling and analyses. A.G., C.N.N., G.T., M.Be., J.F.G., M.B. and B.R. wrote the paper. All authors gave final approval for the publication.
Competing interests
The authors declare that they have no competing financial or non-financial interests.
Funding
This research was supported by the French Public Health Doctoral Network and the French School of Public Health (EHESP), through a mobility grant, a PhD doctoral fellowship and supplementary research funding. Additional funding came from the French National Research Agency (ANR 11 CEPL 00704 EXTRA-MU).
References
- 1.Omran AR. 1971. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Mem. Fund Q. 29, 509–538. ( 10.2307/3349375) [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Ezzati M, Lopez AD. 2007. Measuring the burden of neglected tropical diseases: the global burden of disease framework. PLoS Negl. Trop. Dis. 1, e114 ( 10.1371/journal.pntd.0000114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hotez PJ, Kamath A. 2009. Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl. Trop. Dis. 3, e412 ( 10.1371/journal.pntd.0000412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guernier V, Hochberg ME, Guégan J-F. 2004. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2, e141 ( 10.1371/journal.pbio.0020141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn RR, Davies TJ, Harris NC, Gavin MC. 2010. Global drivers of human pathogen richness and prevalence. Proc. R. Soc. B 277, 2587–2595. ( 10.1098/rspb.2010.0340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonds MH, Dobson AP, Keenan DC. 2012. Disease ecology, biodiversity, and the latitudinal gradient in income. PLoS Biol. 10, e1001456 ( 10.1371/journal.pbio.1001456) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates I, Fenton C, Gruber J, Lalloo D, Lara AM, Squire SB, Theobald S, Thomson R, Tolhurst R. 2004. Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect. Dis. 4, 267–277. ( 10.1016/S1473-3099(04)01002-3) [DOI] [PubMed] [Google Scholar]
- 8.CSDH. 2008. Closing the gap in a generation. Health equity through action on the social determinants of health. Final Report of the Commission on Social Determinants of Health.
- 9.Bloom DE, Canning D. 2012. The health and wealth of nations. Science 287, 1207–1209. ( 10.1126/science.287.5456.1207) [DOI] [PubMed] [Google Scholar]
- 10.Gallup J, Sachs J. 2001. The economic burden of malaria. Am. J. Trop. Med. Hyg. 64, 85–96. [DOI] [PubMed] [Google Scholar]
- 11.Bleakley H. 2010. Health, human capital, and development. Annu. Rev. Econ. 2, 283–310. ( 10.1146/annurev.economics.102308.124436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonds MH, Keenan DC, Rohani P, Sachs JD. 2010. Poverty trap formed by the ecology of infectious diseases. Proc. R. Soc. B 277, 1185–1192. ( 10.1098/rspb.2009.1778) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ngonghala CN, Pluciński MM, Murray MB, Farmer PE, Barrett CB, Keenan DC, Bonds MH. 2014. Poverty, disease, and the ecology of complex systems. PLoS Biol. 12, e1001827 ( 10.1371/journal.pbio.1001827) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pluciński MM, Ngonghala CN, Getz WM, Bonds MH. 2013. Clusters of poverty and disease emerge from feedbacks on an epidemiological network. J. R. Soc. Interface 10, 20120656 ( 10.1098/rsif.2012.0656) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. 2010. Working to overcome the global impact of neglected tropical diseases First WHO report on neglected tropical diseases.
- 16.Hotez PJ, Fenwick A, Savioli L, Molyneux DH. 2009. Rescuing the bottom billion through control of neglected tropical diseases. Lancet 373, 1570–1575. ( 10.1016/S0140-6736(09)60233-6) [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 2014. World Health Observatory.
- 18.Landier J, Gaudart J, Carolan K, Lo Seen D, Guégan J-F, Eyangoh S, Fontanet A, Texier G. 2014. Spatio-temporal patterns and landscape-associated risk of Buruli ulcer in Akonolinga, Cameroon. PLoS Negl. Trop. Dis. 8, e3123 ( 10.1371/journal.pntd.0003123) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marion E, et al. 2011. Geographic expansion of Buruli ulcer disease, Cameroon. Emerg. Infect. Dis. 17, 551–553. ( 10.3201/eid1703.091859) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brou T, Broutin H, Elguero E, Asse H, Guegan J-F. 2008. Landscape diversity related to Buruli ulcer disease in Côte d'Ivoire. PLoS Negl. Trop. Dis. 2, e271 ( 10.1371/journal.pntd.0000271) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grietens KP, Boock AU, Peeters H, Hausmann-Muela S, Toomer E, Ribera JM. 2008. ‘It is me who endures but my family that suffers’: social isolation as a consequence of the household cost burden of Buruli ulcer free of charge hospital treatment. PLoS Negl. Trop. Dis. 2, e321 ( 10.1371/journal.pntd.0000321) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown Amoakoh H, Aikins M. 2013. Household cost of out-patient treatment of Buruli ulcer in Ghana: a case study of Obom in Ga South Municipality. BMC Health Serv. Res. 13, 507 ( 10.1186/1472-6963-13-507) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nienhuis WA, et al. 2010. Antimicrobial treatment for early, limited Mycobacterium ulcerans infection: a randomised controlled trial. Lancet 375, 664–672. ( 10.1016/S0140-6736(09)61962-0) [DOI] [PubMed] [Google Scholar]
- 24.Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Guédénon A, Scott JT, Dramaix M, Portaels F. 2004. Mycobacterium ulcerans disease (Buruli ulcer) in rural hospital, Southern Benin, 1997–2001. Emerg. Infect. Dis. 10, 1391–1398. ( 10.3201/eid1008.030886) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agbenorku P, Agbenorku M, Amankwa A, Tuuli L, Saunderson P. 2011. Factors enhancing the control of Buruli ulcer in the Bomfa communities, Ghana. Trans. R. Soc. Trop. Med. Hyg. 105, 459–465. ( 10.1016/j.trstmh.2011.05.003) [DOI] [PubMed] [Google Scholar]
- 26.Vincent QB, et al. 2014. Clinical epidemiology of laboratory-confirmed Buruli ulcer in Benin: a cohort study. Lancet Glob. Health 2, e422–e430. ( 10.1016/S2214-109X(14)70223-2) [DOI] [PubMed] [Google Scholar]
- 27.Ellen DE, Stienstra Y, Teelken MA, Dijkstra PU, van der Graaf WTA, van der Werf TS. 2003. Assessment of functional limitations caused by Mycobacterium ulcerans infection: towards a Buruli ulcer functional limitation score. Trop. Med. Int. Health 8, 90–96. ( 10.1046/j.1365-3156.2003.00976.x) [DOI] [PubMed] [Google Scholar]
- 28.Stienstra Y, et al. 2005. Factors associated with functional limitations and subsequent employment or schooling in Buruli ulcer patients. Trop. Med. Int. Health 10, 1251–1257. ( 10.1111/j.1365-3156.2005.01519.x) [DOI] [PubMed] [Google Scholar]
- 29.Bloom DE, Canning D, Sevilla J. 2004. The effect of health on economic growth: a production function approach. World Dev. 32, 1–13. ( 10.1016/j.worlddev.2003.07.002) [DOI] [Google Scholar]
- 30.Miguel E, Kremer M. 2004. Worms: identifying impacts on education and health in the presence of treatment externalities. Econometrica 72, 159–217. ( 10.1111/j.1468-0262.2004.00481.x) [DOI] [Google Scholar]
- 31.Lucas A. 2010. Malaria eradication and educational attainment: evidence from Paraguay and Sri Lanka. Am. Econ. J. Appl. Econ. 2, 46–71. ( 10.1257/app.2.2.46) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson RM, May RM. 1991. Infectious diseases of humans. Dynamics and control. Oxford, UK: Oxford University Press; [cited 2014 September 10]. [Google Scholar]
- 33.Aghion P, Durlauf SN. 2005. Handbook of economic growth. Amsterdam, The Netherlands: Elsevier B. V. [Google Scholar]
- 34.Asiedu K, Etuaful S. 1998. Socioeconomic implications of Buruli ulcer in Ghana: a three-year review. Am. J. Trop. Med. Hyg. 59, 1015–1022. [DOI] [PubMed] [Google Scholar]
- 35.Ackumey MM, Gyapong M, Pappoe M, Kwakye-Maclean C, Weiss MG. 2012. Illness meanings and experiences for pre-ulcer and ulcer conditions of Buruli ulcer in the Ga-West and Ga-South municipalities of Ghana. BMC Public Health 12, 264 ( 10.1186/1471-2458-12-264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stienstra Y, van der Graaf WTA, Asamoa K, van der Werf TS. 2002. Beliefs and attitudes toward Buruli ulcer in Ghana. Am. J. Trop. Med. Hyg. 67, 207–213. [DOI] [PubMed] [Google Scholar]
- 37.Lehman L, Simonet L, Saunderson P, Agbenorku P. 2006. Buruli ulcer: prevention of disability (POD). Geneva, Switzerland: World Health Organization. [Google Scholar]
- 38.O'Brien DP, Comte E, Serafini M, Ehounou G, Antierens A, Vuagnat H, Christinet V, Hamani MD, du Cros P. 2014. The urgent need for clinical, diagnostic, and operational research for management of Buruli ulcer in Africa. Lancet Infect. Dis. 14, 435–440. ( 10.1016/S1473-3099(13)70201-9) [DOI] [PubMed] [Google Scholar]
- 39.Iman RL, Davenport JM, Zeigler DK. 1980. Latin hypercube sampling (program user's guide).
- 40.Debacker M, Aguiar J, Steunou C, Zinsou C, Meyers WM, Scott JT, Dramaix M, Portaels F. 2004. Mycobacterium ulcerans disease: role of age and gender in incidence and morbidity. Trop. Med. Int. Health 9, 1297–1304. ( 10.1111/j.1365-3156.2004.01339.x) [DOI] [PubMed] [Google Scholar]
- 41.Bratschi MW, et al. 2013. Geographic distribution, age pattern and sites of lesions in a cohort of Buruli ulcer patients from the Mapé Basin of Cameroon. PLoS Negl. Trop. Dis. 7, e2252 ( 10.1371/journal.pntd.0002252) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amofah G, Bonsu F, Tetteh C, Okrah J, Asamoa K, Asiedu K, Addy J. 2002. Buruli ulcer in Ghana: results of a national case search. Emerg. Infect. Dis. 8, 167–170. ( 10.3201/eid0802.010119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porten K, et al. 2009. Prevalence of Buruli ulcer in Akonolinga health district, Cameroon: results of a cross sectional survey. PLoS Negl. Trop. Dis. 3, e466 ( 10.1371/journal.pntd.0000466) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner T, Benbow ME, Brenden TO, Qi J, Johnson RC. 2008. Buruli ulcer disease prevalence in Benin, West Africa: associations with land use/cover and the identification of disease clusters. Int. J. Health Geogr. 7, 25 ( 10.1186/1476-072X-7-25) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. 2015. Global Health Observatory Data Repository.
- 46.Aguirre AA, Ostfeld RS, Tabor GM, House C, Pearl MC. 2002. Conservation medicine. Ecological health in practice. Oxford, UK: Oxford University Press. [Google Scholar]
- 47.Ackumey MM, Gyapong M, Pappoe M, Maclean CK, Weiss MG. 2012. Socio-cultural determinants of timely and delayed treatment of Buruli ulcer: implications for disease control. Infect. Dis. Poverty 1, 6 ( 10.1186/2049-9957-1-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chauty A, et al. 2011. Oral treatment for Mycobacterium ulcerans infection: results from a pilot study in Benin. Clin. Infect. Dis. 52, 94–96. ( 10.1093/cid/ciq072) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.