Abstract
Despite increasing concerns about the vulnerability of species' populations to climate change, there has been little overall synthesis of how individual population responses to variation in climate differ between taxa, with trophic level or geographically. To address this, we extracted data from 132 long-term (greater than or equal to 20 years) studies of population responses to temperature and precipitation covering 236 animal and plant species across terrestrial and freshwater habitats. Our results identify likely geographical differences in the effects of climate change on populations and communities in line with macroecological theory. Temperature tended to have a greater overall impact on populations than precipitation, although the effects of increased precipitation varied strongly with latitude, being most positive at low latitudes. Population responses to increased temperature were generally positive, but did not vary significantly with latitude. Studies reporting significant climatic trends through time tended to show more negative effects of temperature and more positive effects of precipitation upon populations than other studies, indicating climate change has already impacted many populations. Most studies of climate change impacts on biodiversity have focused on temperature and are from middle to high northern latitudes. Our results suggest their findings may be less applicable to low latitudes.
Keywords: biodiversity, climatic limiting factors, climate change, energy–water hypothesis, latitudinal variation
1. Background
Wild populations of many species are declining around the world [1,2], ultimately leading to increased rates of extinction [3]. Although habitat loss and degradation, direct exploitation and impacts of invasive alien species have accounted for the majority of these declines so far, climate change is anticipated to become an increasingly important driver of population declines and extinction risk during the course of this century [4–6]. Indeed, climate change has already caused shifts in species' distributions [7,8] and altered ecological communities [9,10]. Although a range of species population declines have been attributed to climate change (e.g. [11]), few climate-mediated extinctions have so far been reported [12].
Gaining a full understanding of the processes by which climate change impacts populations is essential not just to develop improved assessment of risk [13], but also to inform adaptive conservation management [14,15]. Recent evidence has highlighted that climate change will directly drive population change and extinction primarily through altered species interactions [12,16]. However, the precise climatic drivers underpinning such relationships remain unclear and may differ between leading and trailing range margins [17]. There is an urgent need for a robust assessment of the link between climate and species' population changes around the world, to advance significantly the understanding of the relationship between climate change and biodiversity responses. For example, the majority of studies of climate change impacts, whether of phenological (e.g. [7,18]), distributional (e.g. [7,8,19]) or community (e.g. [9,10]) changes, have been in temperate latitudes and have focused on the effects of temperature. Is this focus on temperature appropriate, or are other climatic changes of equal or greater importance?
To address this issue, we examine global patterns in the response of species' populations to climate variables, as a precursor for understanding how populations, communities and ecosystem function may each be affected by climate change. Macroecological theory that species-richness patterns at high latitudes are limited by ambient energy and at low latitudes are limited by moisture availability [20,21], would lead us to predict that geographical variation occurs in the response of species' populations to climate variables, assuming that species-richness represents the summation of individual population responses. This has, to our knowledge, never been tested. In addition, we test whether population responses to climate variables differ between taxa, in order to test their generality, and by trophic level, to assess the extent to which changes in climate may have different impacts on different trophic levels, as a much predicted sign of potential ecosystem disruption in response to climate change [16,22]. This is achieved through a systematic review of the literature documenting variation in demographic responses (specifically population trends, survival and birth rates) of terrestrial and freshwater vertebrates, invertebrates and plants to climatic variation, followed by meta-analysis of the extracted data.
2. Material and methods
(a). Review
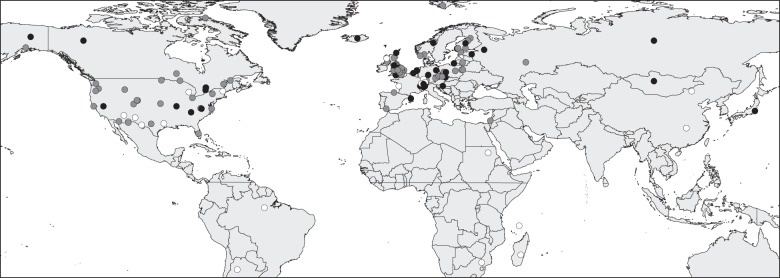
A literature search was conducted using ISI Web of Knowledge on 14 November 2011 for the purpose of identifying the full range of studies with potential to identify mechanisms of climate change impacts upon natural populations [16]. For this reason, the search used key words to identify studies relating demographic changes (Population*, Demograph*, Reproduct*, Decline*, Abundance, Breeding, Survival, Mortality, Fecundity, Density, Productivity) to potential climate change impacts (Climat*, Global warming, Sea-level rise, Elevated CO2, Elevated carbon dioxide, Global environmental change) that clearly related changes to specific environmental drivers (Temperature*, Fire*, Glaci*, Snow pack, O2, Oxygen, Flood*, Drought*, Ground-water levels, Precipitation, Thermal stratification, Sea-level rise, Cloud cover, Humidity, CO2 or Carbon dioxide, UV, Ultra violet, Water current, Salinity, Nutrient, Erosi*, Wind, Rainfall, Storm, Hurricane, Cyclone, Typhoon). This generated 30 880 hits, which were filtered by title, abstract and content to produce a final list of studies that correlated annual variation in demographic metrics with temperature and precipitation variables, over a time-scale (minimum 20 years) sufficient to detect effects of climate variation above other processes affecting abundance (electronic supplementary material, table S1). Very few studies examined mechanisms other than temperature or precipitation, hence the focus on those two variables. Impacts on species within the marine environment were excluded. This final list comprised 132 studies from across the globe (figure 1), which covered 101 bird, 37 mammal, four amphibian, 13 fish, 49 invertebrate and 32 plant species. No studies on reptiles were found that matched our criteria.
Figure 1.
Location of studies of at least 20 years duration that related population time series to either temperature (black circles) or precipitation (white circles) variables, or to both (grey circles).
(b). Analysis
Studies contained analyses of one or more time-series of a range of population metrics, from estimates of birth and survival rates to changes in abundance. They also covered taxa with widely varying life-histories. It was therefore not possible to produce a standardized metric of the magnitude of demographic responses to climatic variables. Instead, for each combination of time-series parameters and climatic variables, we separately estimated the overall impact of temperature and/or precipitation upon changes in species' populations, using a single metric (e) to describe a gradient from negative through equivocal to positive effects. This was calculated from the number of statistically significant positive tests (where a positive relationship is indicative of increased birth, survival or population growth rates with increases in the relevant climate variable), as a function of the total number of tests (t). Where studies contained multiple time-series for different species or sites, or considered different demographic parameters, climatic parameters (i.e. temperature or precipitation) or different tests with different analytical methodologies (i.e. univariate or multivariate) for the same population, these were treated as separate rows in the dataset.
In order to test our hypotheses, we separated relationships between demographic responses and climate into significantly positive (p), significantly negative and non-significant (n) effects. Following [16], these were used to calculate an effect score (e) amenable to empirical modelling using standard approaches:
| 2.1 |
Thus, in a study which included three tests of the effects of different aspects of a particular variable (e.g. from different time periods or impacting different demographic metrics) upon a single species, e = 1 if all three effects were significantly positive, e = 0 if all were significantly negative, e = 0.5 if all were non-significant and e = 0.66 if two effects were significantly positive and one was significantly negative. Values of e = 0.5 therefore indicate that either no cases were significantly affected by that variable, or there was an equal balance of positive and negative correlations. Values from 0.5 to 1 show an increasing tendency towards a greater proportion of significantly positive effects, while values from 0.5 to 0 show an increasing tendency towards significant negative effects. This approach therefore converts the categorical scores of significant positive, significant negative or non-significant effects, into a proportion (e).
Taking a worked example, Jovani & Tella [23] consider the impacts of mean maximum temperature and total rainfall between 1 April and 15 May upon nestling survival and the proportion of successful nests of white stork Ciconia ciconia. When analysed in isolation, temperature has significant positive impacts upon both measures of breeding success (p = 2, n = 0, t = 2, e = 1), but when analysed in combination with precipitation, these significant relationships were lost (p = 0, n = 2, t = 2, e = 0.5), and replaced by two negative relationships with precipitation (p = 0, n = 0, t = 2, e = 0). This was represented by three separate rows of data describing univariate relationships with temperature, and multivariate relationships with both temperature and precipitation.
Climate variables were classified into those that describe the effects of temperature and those relating to precipitation. Variables unrelated to this were not considered further, as there were insufficient cases for analysis. Our analysis was based upon whether statistically significant relationships were identified. However, the probability of a statistically significant relationship occurring may vary with a range of additional study-specific parameters describing the methodological approaches used. To account for this, we collected information about a number of such parameters that were available and comparable across studies: the duration of a study, the number of statistical tests performed, the type of test performed (univariate or multivariate) and the type of demographic variable considered (survival, reproductive success or abundance). To quantify the importance of these additional parameters we modelled the proportion of statistically significant tests (variable importance) as a function of these study-specific parameters, plus a two-level factor separating studies of temperature from precipitation. This was simplified using backwards deletion of non-significant (p > 0.05) terms, and the remaining study-specific terms included in all subsequent models to check for any potential resulting bias.
Effect score (e) was modelled separately as either a function of taxonomic identity (bird, mammal, amphibian, fish, invertebrate, plant), or trophic level (producer, primary consumer, secondary consumer or higher), to test for consistency in population responses between taxa and across food-webs. Latitudinal patterns in relationships with temperature or precipitation were investigated separately by regressing e against study latitude (degrees north or south from the equator). We also tested whether the patterns for temperature and precipitation differed from each other, using the interaction between latitude and climatic variable-type as a two-level factor in a model containing data for both variables. In order to check the consistency of these patterns between taxa, we repeated this analysis of latitudinal gradients separately for temperature and precipitation, but including the additional effect of the interaction between a categorical variable describing taxonomic identity (bird, mammal, amphibian, fish, invertebrate, plant) and latitude.
Studies varied in the extent to which they examined population responses to variation in temperature and precipitation in the context of climate change, or simply tracked responses to fluctuations in climatic variables with no long-term increase or decrease. As the mechanism linking climatic variation to population change may change in the context of climate change [16], we additionally tested the effect of whether a climate variable exhibited a long-term trend or not, as a two-level factor, upon e, and whether any spatial patterns in the relationships between climatic variables and population responses were robust to the impacts of climate change. This was achieved by repeating the regression of e against latitude separately for temperature and precipitation, but including the interaction between latitude and a two-level factor separating climatic variables with a long-term trend from those with no significant trend. This was possible for a subset of 56 studies that reported trends in climatic variables, of which 40 included at least one climatic variable with a significant temporal trend, and 26 included at least one variable with no temporal trend.
All models of variation in e with respect to the predictor variables were examined within a generalized linear mixed modelling (GLMM) framework using a binomial error distribution and logit link function. The number of positive and non-significant cases (2p + n) were modelled as a function of twice the number of tests (2t) to reflect the fact that each test was scored with a maximum of 2 if significantly positive (following equation (2.1)). Study identity was included as a random effect to account for the potential risk of pseudoreplication at the study level. We also considered the inclusion of additional taxonomic levels (species, genus, family, order and higher) as random effects, but for most analyses, either in isolation or combination, they did not account for any additional covariance. The exception was the inclusion of genus in the analysis of study-specific parameters, but, as this did not alter our final model and had only a small impact on the parameter estimates, it is not reported. We did not pursue an analysis that fully accounted for taxonomic non-independence using genetic relatedness further, as this analysis indicated it would not improve model performance and parameter estimation, and given the difficulties of resolving this across such divergent taxa [24]. To ensure model convergence, models were fitted using the Laplace method. Analyses were conducted in SAS v. 9.2.
3. Results
There was no significant effect of type of statistical test (F1,1024 = 1.38, p = 0.24) or demographic variable (F2,1025 = 2.18, p = 0.12) upon the probability of a study returning a significant effect of a climate variable. However, study duration (F1,1027 = 15.56, p < 0.0001, β = 0.013 ± 0.0032), the number of tests (F1,1027 = 6.68, p = 0.0099, β = –0.047 ± 0.018) and climate variable identity (F1,1027 = 3.90, p = 0.049; β for precipitation relative to temperature = −0.24 ± 0.12) remained in the final model after backwards deletion of non-significant terms. Studies of longer duration and with fewer tests were most likely to return statistically significant effects of weather variables. These terms were retained in all subsequent models as additional study-specific parameters.
On average, effect scores (e) were more positive for increases in temperature than precipitation (F1,1027 = 4.10, p = 0.043; mean temperature effect, β = 0.17 ± 0.079; precipitation, β = 0.065 ± 0.079). This was because precipitation variables were associated with a lower probability of being statistically significant than temperature variables, as shown by the negative term for precipitation in the previous model of probability of significance.
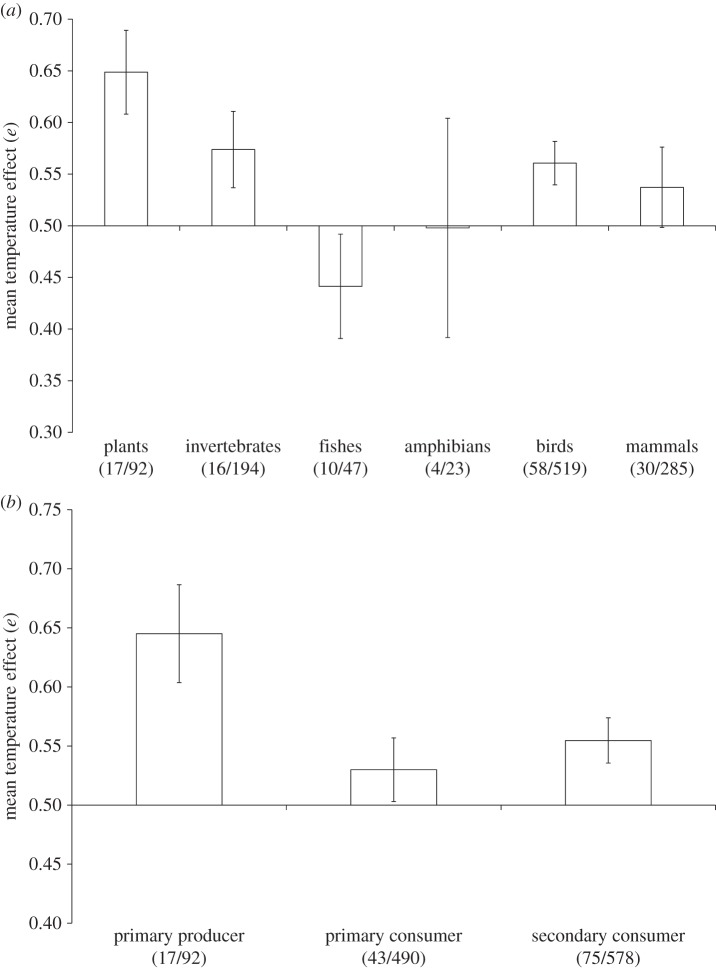
The mean effect (e) of temperature did not differ significantly between taxonomic groups (F5,528 = 2.06, p = 0.069) or trophic level (F2,528 = 2.69, p = 0.069), although only birds and plants, and producers and secondary consumers showed significant positive responses to temperature (figure 2). There was even less variation in the mean effect of precipitation (taxa, F5,426 = 0.69, p = 0.63; trophic level, F2,426 = 1.17, p = 0.31), and no taxa or trophic levels showed consistent significant effects of precipitation upon their populations.
Figure 2.
Variation in the mean effect (e) of temperature (±s.e.) upon populations with (a) taxa and (b) trophic level. Values more than 0.5 describe increasingly positive relationships between the climate variable and demographic responses to a maximum of 1, and values less than 0.5 represent increasingly negative relationships. Values in parentheses denote the number of studies/the number of independent rows of data contributing to each analysis.
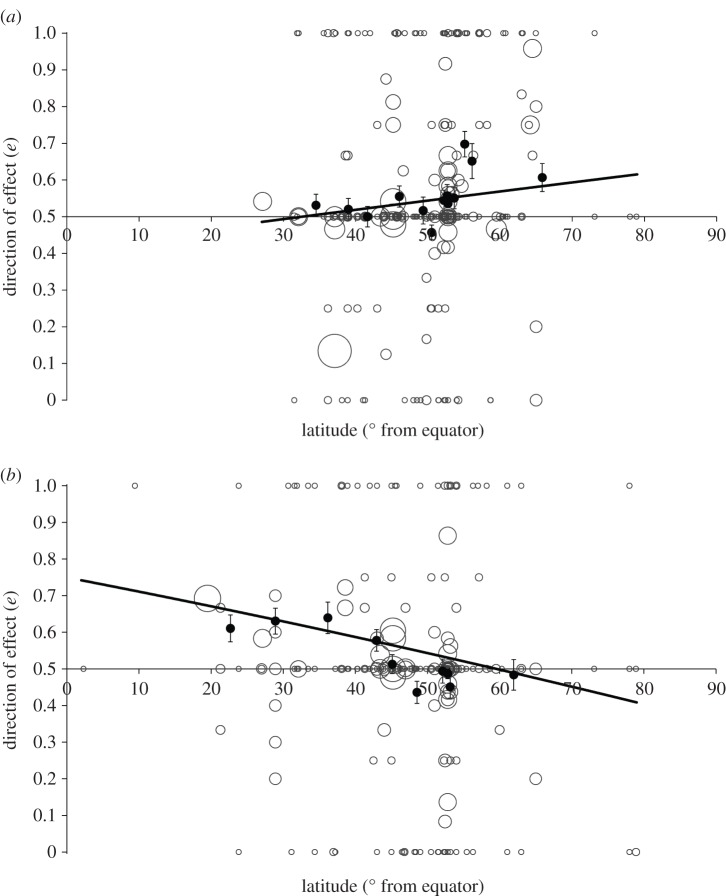
The effects of variation in temperature and precipitation on e showed contrasting latitudinal patterns (climate variable × latitude interaction; F1,1025 = 16.86, p < 0.0001). Increased precipitation had its largest and most positive impact upon demographic responses (greater population growth, survival and birth rates with more precipitation) at low latitudes, but tended towards a weak negative effect at high latitudes. This trend of precipitation effect with latitude was highly significant (β = −0.018 ± 0.0046, F1,426 = 15.65, p < 0.001; figure 3b). By contrast, there was no significant effect of latitude upon the relationship between temperature and e (β = 0.0084 ± 0.0070, F1,528 = 1.45, p = 0.23; figure 3a). This pattern resulted from variation in the direction of population responses to precipitation with latitude (electronic supplementary material, figure S1), and was not caused by any latitudinal variation in the probability of a study returning a significant effect of precipitation (F1,426 = 0.49, p = 0.48), as tested by a GLMM of the proportion of tests which produced statistically significant results, as a function of latitude. Neither was there any effect of latitude upon the probability of a significant effect of temperature (F1,528 = 0.26, p = 0.61).
Figure 3.
Latitudinal variation in the impact of (a) temperature and (b) precipitation upon species' populations. Direction of effect values more than 0.5 describe increasingly positive relationships between the climate variable and demographic responses to a maximum of 1, and values less than 0.5 represent increasingly negative relationships to a minimum of 0. Values of 0.5 indicate either no significant impacts or an equal mix of positive and negative effects. Points represent mean values (±s.e.) from bins of equal-sized categories of approximately 50 cases. Circles show the spread of raw data from each study and are sized according to the number of cases they contribute. Fitted lines cover the latitudinal range of the studies analysed.
Relationships between climate effect and latitude were largely consistent across taxa for precipitation (taxa × latitude interaction; F5,424 = 1.37, p = 0.24; electronic supplementary material, figure S2b) and temperature (taxa × latitude interaction; F5,526 = 2.07, p = 0.068; electronic supplementary material, figure S2a), although there was a significant negative relationship between temperature effect (e) and latitude for fishes, and a significant positive relationship for birds (electronic supplementary material, figure S2). The effect of latitude upon population responses to temperature and precipitation was unaffected by trophic level (F2,525 = 0.02, p = 0.98 and F2,423 = 0.16, p = 0.85, respectively).
Across the subset of studies which tested for potential trends in climatic variables, there were significant differences in the effect on populations (e) of both temperature (F1,117 = 5.85, p = 0.017) and precipitation (F1,95 = 8.22, p = 0.0051), between those that reported a significant trend in climate variable and those that reported no trend (figure 4). In cases where there was a trend in climate variables, there were fewer positive relationships with temperature, but more positive relationships with precipitation. This was owing to a doubling of the proportion of studies exhibiting a significant negative effect of temperature upon populations (23 out of 209 cases in studies reporting a temperature trend compared to 5 out of 102 cases with no temperature trend) and a reduction in the proportion reporting positive temperature effects (45 out of 209 cases in studies with a temperature trend compared to 33 out of 102 cases without). The pattern for precipitation was driven by a decline in the number of studies reporting negative impacts upon populations where a significant precipitation trend was reported (2 out of 34 cases) compared to studies with no precipitation trend (12 out of 102 cases), but an increase in the proportion of studies reporting positive impacts (19 out of 34 cases and 27 out of 102 cases, respectively). After accounting for these effects by including climatic trend as a two-level factor, there was no difference in the relationships between e and latitude between variables exhibiting a significant long-term trend through time and those with no such trend, for either precipitation (F1,94 = 0.43, p = 0.51) or temperature (F1,116 = 2.43, p = 0.12), as tested by the interaction between latitude and climatic trend. Thus, the latitudinal patterns described across all studies did not vary with respect to whether there was evidence of climatic change or not.
Figure 4.
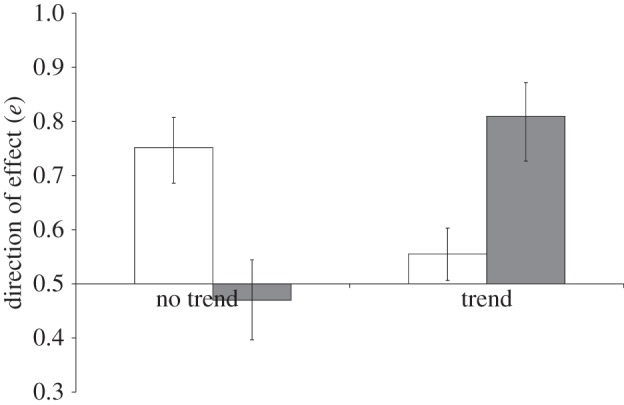
Variation in the mean impact (±s.e.) of temperature (white bars) and precipitation (grey bars) upon species' demographic responses with climate change. Studies with evidence of statistically significant trends in the climate variables contribute to the trend bar, while studies which reported no evidence for a trend contribute to the no trend bar.
4. Discussion
We have identified strong spatial patterns in the response of species' populations to climate across the globe. Species' demographic responses at low latitudes (around less than 45°) were positively related to precipitation, suggesting that in low latitude biomes, drought is the most commonly limiting factor of populations across a range of taxa (e.g. [25,26]). Conversely, at medium to high latitudes (around more than 50°), there was no overall effect of precipitation upon populations, but owing to a generally positive effect of temperature upon many taxa, populations here were more likely to be cold- than water-limited. This suggests that warming in temperate latitudes may generally increase population abundance, leading to range expansion, as recently observed in a number of taxa (e.g. [27,28]).
Crucially, this emphasizes strong geographical differences in the potential for effects of climate change on populations and communities. Many studies at medium and high latitudes have focused on the effects of warming upon species and communities (e.g. [9,10]), where poleward shifts in species' distributions are widely reported [7,8,29]. However, our results suggest that such a focus on the effects of temperature at lower latitudes (e.g. [30,31]) may underestimate the sensitivity of species, communities and systems to climate change by failing to consider potential effects of precipitation and drought. In such regions, we would expect species' distribution shifts to be less-strongly poleward than at higher latitudes, but driven more by changes in precipitation patterns. This probably accounts for the limited poleward shift in the climate niche of tropical and subtropical bird species [32], notwithstanding recent observations of significant upwards elevation change [33]. Indeed, the evidence for a significant positive latitudinal gradient in the effect of temperature upon bird populations alone (electronic supplementary material, figure S2) supports this assertion. The converse relationship for fishes suggests that population responses to temperature may be more negative in freshwater, particularly at higher latitudes, potentially because water temperature is negatively confounded with other environmental parameters, such as oxygen saturation, also important for many fish species [34].
Importantly, our results account for two apparent biases in the published literature. Firstly, it is clear that studies of longer duration were most likely to have produced statistically significant results. Although intuitive, the fact that this effect was apparent across analyses of time-series each of which was at least 20 years in duration is noteworthy, and emphasizes the value of long-term studies for detecting the ecological impacts of climate change. Secondly, we found that studies presenting only one or a small number of variables were more likely to be associated with significant relationships. This finding is suggestive of a bias in the published literature, where studies of a small number of climatic variables that fail to identify statistically significant relationships are less likely to be published. This means that the absolute values presented in this paper may be subject to bias, and potentially inflated towards extremes if non-significant results are under-reported. However, for this to have affected our conclusions, any publication bias would have to vary systematically with taxonomic group or geographical location, which seems unlikely.
Only 3% of species used in this analysis were classified as of conservation concern (threatened or near threatened) on the International Union for Conservation of Nature Red List (www.iucnredlist.org), suggesting that most of the species studied were relatively common and widespread, and hence more amenable to long-term research. Our results should therefore not be taken to mean that warming will be beneficial to all species. Many other studies indicate that species with narrow temperature and habitat niches, particularly those that are cold-associated, typically decline in response to warming [9,10]. Instead, our results are consistent with increasing evidence that warming has resulted in an increase in widespread generalist species at the cost of such specialists [9,35].
The importance of water availability at low latitudes and greater relative importance of temperature at high latitudes is consistent with the processes previously identified as driving spatial variation in species richness around the world, and described by the energy–water hypothesis [20,36]. Because of the importance of plant productivity in driving richness patterns in consumers, these same climatic influences have been detected in a range of other taxa [21,37]. This was not simply because studies from low latitudes (less than 25° latitude) were restricted to desert environments (one of seven studies only), as savannah (four studies), coastal (one study) and rainforest (one study) habitats were also represented. To the best of our knowledge, our study is the first to suggest that the established macroecological pattern of the energy–water hypothesis may be related to variation in the relationship between climate and changes in individual species' populations around the world, potentially indicating a close link between species population size, species distribution and species richness. If these ecological parameters are indeed determined by the same underlying process, we would expect climatic changes that lead to population increases to cause species' range expansions which, when replicated across multiple species, will then alter species-richness patterns. Matching this expectation, recent warming in the UK has led to increases in the abundance of common and widespread resident breeding birds [35], which have expanded their distributions [38], leading to an increase in species' richness [9], while UK butterflies that have shown positive population increases have also tended to expand their distributions [39].
The majority of studies reviewed did not report whether there had been changes in the temperature and precipitation variables examined during the study. Many of these studies may therefore be of population responses to fluctuations in these variables, rather than an examination of the long-term responses to climate change. However, within the subset of studies that did report trends in the climatic variables considered, we found significant effects of those trends upon the relationships between climatic variables and population parameters. In the context of such climate change, the effects of temperature were more negative, and those of precipitation more positive, suggesting that climate change may alter the pattern of population responses to climate variables, for example, through disrupting biotic relationships between species [16]. Importantly, our finding suggests that climate change has already impacted species' populations globally. However, the latitudinal gradients in species' responses to precipitation did not vary with this wider climate change context, suggesting that the patterns identified will continue to hold in an altered climate, even though the mechanisms linking populations to climate variables, and the absolute magnitude of population responses to those variables, may vary. In other words, water availability in the tropics and temperature more widely, are likely to continue to be the most important climatic drivers of population change, even in a changed climate.
5. Conclusion
The strong latitudinal gradients in population responses to precipitation, and generally positive effects of temperature upon plants and birds have important implications for assessing and predicting the impacts of climate change on global biodiversity. Our results emphasize that water availability is likely to limit populations at low latitudes and therefore the vulnerability of species in these regions to climate change is at least as likely to be related to changes in precipitation as to warming. Conservation management for climate change adaptation in these areas should therefore consider the management of water resources. Critically, given the greater uncertainty in future projections of precipitation and drought compared with temperature [40,41], estimated impacts on tropical species are likely to be less certain than those of species at higher latitudes, whose populations are more likely to be temperature-dependent. This is a particular problem given that the majority of all species, and also of currently threatened species, are concentrated in the tropics [42,43] and are relatively poorly monitored [44]. Further, as the majority of studies of climate change impacts on biodiversity are from medium and high northern latitudes where temperature changes are likely to be the most important drivers of change, their findings may be less applicable to poorly studied tropical areas. This uncertainty in future exposure and sensitivity of tropical species is sobering given the urgent policy needs for robust assessments of climate change risk for species and communities.
Supplementary Material
Supplementary Material
Data accessibility
Full details of the references identified by the literature search, and from which information was extracted, are listed in the electronic supplementary material, table S1. The extracted dataset used in the analysis is available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.3p93t.
Authors' contributions
J.W.P.-H. conceived the study in consultation with all other authors. The literature review was conducted by N.O., D.J.B., J.C. and E.W., with assistance from J.W.P.-H. and C.B. The data were analysed by N.O. and J.W.P.-H. with input from W.J.S., T.A., E.W., C.B., S.H.M.B., D.J.C.G. and J.C. The article was written by J.W.P-H. and N.O., with edits and revisions provided by all authors.
Competing interests
We have no competing interests.
Funding
This research was funded by the Cambridge Conservation Initiative (http://www.conservation.cam.ac.uk/), a strategic collaboration between the University of Cambridge and nine leading conservation organizations, thanks to the generosity of the Arcadia Fund.
References
- 1.Loh J, Green RE, Ricketts T, Lamoreux J, Jenkins M, Kapos V, Randers J. 2005. The Living Planet Index: using species population time series to track trends in biodiversity. Phil. Trans. R. Soc. B. 260, 289–295. ( 10.1098/rstb.2004.1584) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Butchart SHM, et al. 2010. Global biodiversity: indicators of recent declines. Science 328, 1164–1168. ( 10.1126/science.1187512) [DOI] [PubMed] [Google Scholar]
- 3.Barnosky AD, et al. 2011. Has the Earth's sixth mass extinction already arrived? Nature 471, 51–57. ( 10.1038/nature09678) [DOI] [PubMed] [Google Scholar]
- 4.Thomas CD, et al. 2004. Extinction risk from climate change. Nature 427, 145–148. ( 10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 5.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. 2012. Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377. ( 10.1111/j.1461-0248.2011.01736.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warren R, et al. 2013. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Change 3, 678–682. ( 10.1038/nclimate1887) [DOI] [Google Scholar]
- 7.Parmesan C, Yohe G. 2003. A globally coherent fingerprint of climate change impacts across natural systems. Nature 421, 37–42. ( 10.1038/nature01286) [DOI] [PubMed] [Google Scholar]
- 8.Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333, 1024–1026. ( 10.1126/science.1206432) [DOI] [PubMed] [Google Scholar]
- 9.Davey CM, Chamberlain DE, Newson SE, Noble DG, Johnston A. 2012. Rise of the generalists: evidence for climate driven homogenization in avian communities. Glob. Ecol. Biogeogr. 21, 568–578. ( 10.1111/j.1466-8238.2011.00693.x) [DOI] [Google Scholar]
- 10.Devictor V, et al. 2012. Differences in the climate debts of birds and butterflies at a continental scale. Nat. Clim. Change 2, 121–124. ( 10.1038/nclimate1347) [DOI] [Google Scholar]
- 11.Gregory RD et al. 2009. An indicator of the impact of climatic change on European bird populations. PLoS ONE 4, e4678 ( 10.1371/journal.pone.0004678) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cahill AE, et al. 2013. How does climate change cause extinction? Proc. R. Soc. B 280, 21231890 (doi:10/1098.rspb.2012.1890) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foden W, et al. 2012. Identifying the worlds’ most climate change vulnerable species: a systematic trait-based assessment of all birds, amphibians and corals. PLoS ONE 8, e65427 ( 10.1371/journal.pone.0065427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce-Higgins JW. 2011. Modelling conservation management options for a southern range-margin population of golden plover Pluvialis apricaria vulnerable to climate change. Ibis 153, 345–356. ( 10.1111/j.1474-919X.2011.01108.x) [DOI] [Google Scholar]
- 15.Carroll MJ, Dennis P, Pearce-Higgins JW, Thomas CD. 2011. Maintaining northern peatland ecosystems in a changing climate: effects of soil moisture, drainage and drain blocking on craneflies. Glob. Change Biol. 17, 2991–3001. ( 10.1111/j.1365-2486.2011.02416.x) [DOI] [Google Scholar]
- 16.Ockendon N, et al. 2014. Mechanisms underpinning climatic impacts on natural populations: altered species interactions are more important than direct effects. Glob. Change Biol. 20, 2221–2229. ( 10.1111/gcb.12559) [DOI] [PubMed] [Google Scholar]
- 17.Sunday JM, Bates AE, Dulvy NK. 2012. Thermal tolerance and the global redistribution of animals. Nat. Clim. Change 2, 686–690. ( 10.1038/nclimate1539) [DOI] [Google Scholar]
- 18.Menzel A, et al. 2006. European phenological response to climate change matches the warming pattern. Glob. Change Biol. 12, 1969–1976. ( 10.1111/j.1365-2486.2006.01193.x) [DOI] [Google Scholar]
- 19.Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. 2006. The distributions of a wide range of taxonomic groups are expanding polewards. Glob. Change Biol. 12, 450–455. ( 10.1111/j.1365-2486.2006.01116.x) [DOI] [Google Scholar]
- 20.O'Brien EM. 1998. Water-energy dynamics, climate, and prediction of woody plant species richness: an interim general model. J. Biogeogr. 25, 379–398. ( 10.1046/j.1365-2699.1998.252166.x) [DOI] [Google Scholar]
- 21.Hawkins BA, et al. 2003. Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117. ( 10.1890/03-8006) [DOI] [Google Scholar]
- 22.Tylianakis JM, Didham RK, Bascompte J, Wardle DA. 2008. Global change and species interactions in terrestrial ecosystems. Ecol. Lett. 11, 1351–1363. ( 10.1111/j.1461-0248.2008.01250.x) [DOI] [PubMed] [Google Scholar]
- 23.Jovani R, Tella JL. 2004. Age-related environmental sensitivity and weather mediated nestling mortality in white storks. Ecography 27, 611–618. ( 10.1111/j.0906-7590.2004.03925.x) [DOI] [Google Scholar]
- 24.Kunin WE. 2008. On comparative analyses involving non-heritable traits: why half a loaf is sometimes worse than none. Evol. Ecol. Res. 10, 787–796. [Google Scholar]
- 25.Simpson DC, Harveson LA, Brewer CE, Walser RE, Sides AR. 2007. Influence of precipitation on pronghorn demography in Texas. J. Wildl. Manag. 71, 906–910. ( 10.2193/2005-753) [DOI] [Google Scholar]
- 26.Pérez-Ramos IM, Ourcival JM, Limousin JM, Rambal S. 2010. Mast seeding under increasing drought: results from a long-term data set and from a rainfall exclusion experiment. Ecology 91, 3057–3068. ( 10.1890/09-2313.1) [DOI] [PubMed] [Google Scholar]
- 27.Kullmann L. 2007. Tree line population monitoring of Pinus sylestris in the Swedish Scandes, 1973–2005: implications for tree line theory and climate change ecology. J. Ecol. 95, 41–52. ( 10.1111/j.1365-2745.2006.01190.x) [DOI] [Google Scholar]
- 28.Bradbury RB, Pearce-Higgins JW, Wotton SR, Conway GJ, Grice PV. 2011. The influence of climate and topography in patterns of territory establishment in a range-expanding bird. Ibis 153, 336–344. ( 10.1111/j.1474-919X.2011.01106.x) [DOI] [Google Scholar]
- 29.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669. ( 10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 30.Tewksbury JJ, Huey RB, Deutsch CA. 2008. Putting the heat on tropical animals. Science 320, 1296–1297. ( 10.1126/science.1159328) [DOI] [PubMed] [Google Scholar]
- 31.Deutsch CA, et al. 2009. Impacts of climate warming on terrestrial ectotherms across latitude. Proc. Natl Acad. Sci. USA 105, 6668–6672. ( 10.1073/pnas.0709472105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.VanderWal J, et al. 2013. Focus on poleward shifts in species’ distribution underestimates the fingerprint of climate change. Nat. Clim. Change 3, 239–243. ( 10.1038/nclimate1688) [DOI] [Google Scholar]
- 33.Freeman BG, Freeman AMC. 2014. Rapid upslope shifts in New Guinean birds illustrate strong distributional responses of tropical montane species to global warming. Proc. Natl Acad. Sci. USA 111, 4490–4494. ( 10.1073/pnas.1318190111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holt RE, Jørgensen C. 2015. Climate change in fish: effects of respiratory constraints on optimal life history and behaviour. Biol. Lett. 11, 20141032 ( 10.1098/rsbl.2014.1032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pearce-Higgins JW, Eglington SE, Martay B, Chamberlain DE. 2015. Drivers of climate change impacts on bird communities. J. Anim. Ecol. 84, 943–954. ( 10.1111/1365-2656.12364) [DOI] [PubMed] [Google Scholar]
- 36.Whittaker RJ, Nogues-Bravo D, Araujo MB. 2007. Geographical gradients of species richness: a test of the water-energy conjecture of Hawkins et al. (2003) using European data for five taxa. Glob. Ecol. Biogeogr. 16, 76–89. ( 10.1111/j.1466-8238.2006.00268.x) [DOI] [Google Scholar]
- 37.Field R, et al. 2009. Spatial species-richness gradients across scales: a meta-analysis. J. Biogeogr. 36, 132–147. ( 10.1111/j.1365-2699.2008.01963.x) [DOI] [Google Scholar]
- 38.Gillings S, Balmer DE, Fuller RJ. 2015. Directionality of recent bird distribution shifts and climate change in Great Britain. Glob. Change Biol. 21, 2155–2168. ( 10.1111/gcb.12823) [DOI] [PubMed] [Google Scholar]
- 39.Mair L, Hill JK, Fox R, Botham M, Brereton T, Thomas CD. 2014. Abundance changes and habitat availability drive species’ responses to climate change. Nat. Clim. Change 4, 127–131. ( 10.1038/nclimate2086) [DOI] [Google Scholar]
- 40.Solomon S, Plattner G-K, Knutti R, Friedlingstein P. 2009. Irreversible climate change due to carbon dioxide emissions. Proc. Natl Acad. Sci. USA 106, 1704–1709. ( 10.1073/pnas.0812721106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai A. 2013. Increasing drought under global warming in observations and models. Nat. Clim. Change 3, 52–58. ( 10.1038/nclimate1633) [DOI] [Google Scholar]
- 42.Davies RG, et al. 2007. Topography, energy and the global distribution of bird species richness. Proc. R. Soc. B 274, 1189–1197. ( 10.1098/rspb.2006.0061) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hilton-Taylor C, Pollock C, Chanson J, Butchart SHM, Oldfield T, Katariya V. 2008. Status of the world's species. In Wildlife in a changing world. An analysis of the 2008 IUCN Red List of Threatened Species (eds Vié J-C, Hilton-Taylor C, Stuart SN), pp. 15–42. Gland, Switzerland: IUCN. [Google Scholar]
- 44.Amano T, Sutherland WJ. 2013. Four barriers to the global understanding of biodiversity conservation: wealth, language, geographical location and security. Proc. R. Soc. B 280, 20122649 ( 10.1098/rspb.2012.2649) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Full details of the references identified by the literature search, and from which information was extracted, are listed in the electronic supplementary material, table S1. The extracted dataset used in the analysis is available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.3p93t.