Abstract
Insect resistance to plant toxins is widely assumed to have evolved in response to using defended plants as a dietary resource. We tested this hypothesis in the milkweed butterflies (Danaini) which have progressively evolved higher levels of resistance to cardenolide toxins based on amino acid substitutions of their cellular sodium–potassium pump (Na+/K+-ATPase). Using chemical, physiological and caterpillar growth assays on diverse milkweeds (Asclepias spp.) and isolated cardenolides, we show that resistant Na+/K+-ATPases are not necessary to cope with dietary cardenolides. By contrast, sequestration of cardenolides in the body (as a defence against predators) is associated with the three levels of Na+/K+-ATPase resistance. To estimate the potential physiological burden of cardenolide sequestration without Na+/K+-ATPase adaptations, we applied haemolymph of sequestering species on isolated Na+/K+-ATPase of sequestering and non-sequestering species. Haemolymph cardenolides dramatically impair non-adapted Na+/K+-ATPase, but had systematically reduced effects on Na+/K+-ATPase of sequestering species. Our data indicate that major adaptations to plant toxins may be evolutionarily linked to sequestration, and may not necessarily be a means to eat toxic plants. Na+/K+-ATPase adaptations thus were a potential mechanism through which predators spurred the coevolutionary arms race between plants and insects.
Keywords: sequestration, coevolution, milkweed butterflies, cardenolide, adaptation, tritrophic interaction
1. Introduction
The long-standing model of coevolution posits that reciprocal adaptation in species interactions can result in an arms race of escalating phenotypes [1,2]. Accordingly, adaptations of insect herbivores to host plant toxins are typically interpreted as an evolutionary response to plant defence, crucial for the use of particular plant species as a dietary resource [3–8]. By contrast, the evolution of specific adaptations to facilitate sequestration of plant toxins (i.e. the absorption of toxins via the gut and their retention in the body [9]) implicates predators and parasitoids (the third trophic level), or top-down control, as a driving force in plant–herbivore coevolution. Despite the widespread appreciation of sequestration as an extreme component of host plant specialization and adaptation against predation [9,10], the mechanisms of how sequestration integrates into coevolutionary theory [3,11] are barely understood. To test the relative importance of coping with a toxic diet versus sequestration of toxins in shaping insect adaptations, here we assess the functional consequences of an evolutionarily escalating resistance trait across the milkweed butterflies [12,13].
Cardenolides (cardiac glycosides) are highly potent plant toxins that specifically inhibit Na+/K+-ATPase, an essential animal cation transporter [14,15]. Remarkably, molecular adaptation to cardenolides via altered Na+/K+-ATPases (target site insensitivity) has evolved convergently in at least five orders of herbivorous insects [16–18]. Insect Na+/K+-ATPase resistance is based on specific amino acid substitutions altering cardenolide binding properties of Na+/K+-ATPase, and was first described in the monarch butterfly (Danaus plexippus) [19,20].
The monarch butterfly also represents a classic case of acquired defence, where caterpillars not only tolerate, but also sequester cardenolides from their milkweed host plants (Asclepias spp., Apocynaceae) and transfer these toxins to the butterfly stage for their own defence against predators and parasites [21–23]. Virtually all of the ≈160 milkweed butterflies in the tribe Danaini (Nymphalidae) are specialized on host plants in the Apocynaceae, which often produce cardenolides [15], suggesting a long and shared evolutionary history. Within the Danaini, however, Na+/K+-ATPase resistance evolved in a stepwise manner, resulting in three forms of Na+/K+-ATPase with increasing cardenolide resistance. Among the 16 species of Danaini whose Na+/K+-ATPase genes were sequenced and Na+/K+-ATPases physiologically assayed, resistance to cardenolides was determined by three discrete molecular forms [12].
Here we report on studies of three representative species of milkweed butterflies, each having one of these different forms of Na+/K+-ATPase [12], ranging from sensitive to highly resistant to cardenolides. Caterpillars of the common crow (Euploea core; sensitive Na+/K+-ATPase), the queen butterfly (Danaus gilippus; intermediate resistance Na+/K+-ATPase) and the monarch butterfly (highly resistant Na+/K+-ATPase) were initially reared on eight species of Asclepias (incarnata, syriaca, hallii, cordifolia, viridis, curassavica, linaria and asperula), which vary approximately 14-fold in their foliar cardenolide concentration, to test for an impact of dietary cardenolides on caterpillar growth. Although the eight plant species vary in more traits than cardenolides, this critical experiment employed plants with diverse cardenolide mixtures, including several species with extreme toxicity [24]. Because we employed three caterpillar species with increasing levels of molecular resistance (altered Na+/K+-ATPase) to cardenolides, growth differences were predicted to be especially pronounced on plant species having high cardenolide concentrations. As we found plant cardenolide concentrations not to affect caterpillar growth, this correlative approach was confirmed using isolated cardenolides that were fed to caterpillars. We next analysed sequestration of cardenolides into the caterpillars' haemolymph, gut and body tissues to assess potential differences in uptake between the three caterpillar species representing the three forms of Na+/K+-ATPase and found that the level of Na+/K+-ATPase resistance indeed is associated with the amount of cardenolides sequestered. Finally, we mechanistically tested the hypothesis that Na+/K+-ATPase alterations are physiologically required to facilitate sequestration by examining the relative toxicological burden of sequestered cardenolides. Using a novel in vitro assay, we show that cardenolides present in the haemolymph of sequestering species dramatically impair Na+/K+-ATPase of a sensitive species, but had attenuated effects on the donor Na+/K+-ATPase which had altered molecular resistance. By injecting cardenolides into caterpillars we show that this pattern revealed on the level of proteins translates to the whole organism.
Despite the general acceptance of tritrophic interactions as an important contributor to plant–insect interactions [25,26], it is unknown how the third trophic level can influence reciprocal adaptation between plants and insects [3]. It is well established that cardenolides protect monarch butterflies from bird attacks [21] and both monarch and queen butterflies, are mimicry models for the viceroy (Limenitis archippus) [27,28] underlining the effectiveness of cardenolide sequestration as a defence. Moreover, it was shown that across milkweed butterfly species (D. plexippus, the plain tiger, D. chrysippus and E. core) emetic potency acting on predators is linked to the amount of cardenolides stored [29]. Our study thus suggests a mechanism of how the third trophic level can engage in plant–insect coevolution by favouring altered tolerance and use of toxins by herbivores. Indeed, the milkweed butterflies are a model for advancing our knowledge of how complex community interactions have shaped the evolution of species at the molecular and physiological levels.
2. Material and methods
(a). Insects
Euploea core was maintained in a mesh cage in a greenhouse to obtain eggs. Danaus gilippus and D. plexippus eggs were purchased from commercial breeders. Work with E. core and D. gilippus was permitted under USDA permit number P526P-12-05106. Butterfly eggs were surface sanitized with a 100 ppm solution of oxine (Bio-Cide, International Inc., Norman, OK, USA) in water (0.01% ClO2) for 10 min to prevent potential transfer of parasites [30].
(b). Plants
Seeds of Asclepias (asperula, curassavica, cordifolia, hallii, linaria, incarnata, syriaca and viridis) were obtained commercially or collected in the field. Seeds were treated with household bleach (5%) for 5 min, nicked and cold stratified for 7 days. After germination at 28°C, seedlings were planted in 500 ml pots filled with potting soil (Metro-Mix; Sun Gro Horticulture, Canada CM Ltd, Vancouver, Canada). Plants were fertilized (NPK 21 : 5 : 20, 150 ppm N) after 10 and after 20 days and watered as needed. Plants (non-flowering) were used for experiments after four to seven weeks of growth in a controlled environment walk-in chamber (14 : 10 h day/night cycle, 400 microeinsteins m−2 s−1 of light, 27°C/24°C). Plants' spatial locations were randomized in the chamber.
(c). Caterpillar growth experiment
To test for the effects of cardenolides on caterpillar growth, neonate caterpillars of the three species were transferred individually to Asclepias plants (i.e. one plant received only one caterpillar). After 3 and 5 days in the environmental chamber under the same conditions as used for plant growth, caterpillar body weights were recorded using a micro balance (AT21 Comparator, Mettler Toledo, Columbus, OH, USA). After weighing, caterpillars were reapplied to the original plants. Also on day five, leaf samples for cardenolide analysis were taken and caterpillars on plants were caged within a mesh sleeve. Caterpillars remained on the plants until they had reached the fourth or fifth instar. For assessing caterpillar growth (5 days), caterpillars were kept on a single plant, and in no case was more than 50% of the foliage eaten. Later, plants were replaced with fresh plants of the same species, if necessary. In total, five blocks of the caterpillar growth experiment were performed. In every block, all eight Asclepias species were used. See the electronic supplementary material, table S1, for number of caterpillar weights obtained per block. Sample sizes for each combination of insect and plant species are listed in the electronic supplementary material, table S2.
(d). Haemolymph sampling
To assess sequestration of cardenolides into the body cavity, we examined larval haemolymph from all three caterpillar species, obtained from feeding 4th or 5th (last) instar larvae by cutting one or two tentacles (first pair). A defined volume of haemolymph (5–80 µl, with 77% of samples between 20 and 70 µl) was pipetted into 200 µl of methanol in 1.5 ml Eppendorf tubes, samples were mixed (vortex) and subsequently centrifuged at 20 000g for 3 min to precipitate haemolymph protein. Supernatants were transferred into 2 ml screw cap vials and stored at −20°C until further processing. After haemolymph sampling, caterpillars were frozen, stored at −80°C and eventually freeze-dried.
(e). Preparation of haemolymph samples for application on Na+/K+-ATPase
Supernatants (see above) were dried down in a vacuum concentrator and residues were dissolved in 200 µl methanol. After treating the samples for 10 min in an ultrasound bath and centrifugation (3 min at 5000g) supernatants were transferred into fresh tubes. An equivalent of 10 µl (D. gilippus and D. plexippus) or 5 µl (E. core) haemolymph was removed and dried down in a fresh tube (the rest of the original sample was also dried down for high-performance liquid chromatography analysis). The dried residue was dissolved in 20 µl (D. gilippus and D. plexippus) or 10 µl (E. core) DMSO (dimethyl sulfoxide) by vortexing and treating for 10 min in an ultrasound bath. Subsequently samples were mixed with 180 µl or 90 µl H2O to achieve a final concentration of 10% DMSO. Samples were stored at −20°C until application on Na+/K+-ATPase.
(f). Na+/K+-ATPase assays
Na+/K+-ATPase assays were performed as described in [12]. Instead of ouabain solutions [12], caterpillar haemolymph samples were applied on the Na+/K+-ATPase of E. core and on the Na+/K+-ATPase of the donor species (e.g. D. gilippus haemolymph on D. gilippus Na+/K+-ATPase) in parallel (on the same 96-well plate). Haemolymph samples obtained from E. core caterpillars were applied on E. core Na+/K+-ATPase, only. For details on Na+/K+-ATPase assays, see the electronic supplementary material. The numbers of haemolymph samples that were applied on Na+/K+-ATPase are listed in the electronic supplementary material, table S2.
(g). Preparation of haemolymph samples for high-performance liquid chromatography
Haemolymph extracts in methanol (see above) were dried down in a vacuum concentrator; 60 µl or 80 µl methanol containing 0.1 mg ml−1 digitoxin (Sigma, St Louis, MO, USA) as an internal standard was added and samples were incubated for 10 min in an ultrasound bath. Samples were centrifuged to remove undissolved matter and transferred into glass inserts of 1.8 ml high performance liquid chromatography (HPLC) vials and subjected to HPLC analysis. See the electronic supplementary material, table S2, for the number of haemolymph samples analysed.
(h). Preparation of food bolus and caterpillar body samples for high-performance liquid chromatography
Body tissues plus integument, the midgut epithelium (including the underlying musculature) and the food bolus (gut contents) were collected from freeze-dried caterpillars (a subset of the caterpillars which had been used for haemolymph sampling before, see above) and dissected under a stereomicroscope (see the electronic supplementary material, figure S1). Samples were homogenized, extracted in methanol containing digitoxin as internal standard and subjected to HPLC analysis. Details on sample sizes and preparation are given in the electronic supplementary material.
(i). Preparation of plant samples for cardenolide analysis
Leaf samples (typically two fully expanded leaves per plant, several of the needle-like leaves of A. linaria) taken at the fifth day of an experiment were oven dried (50°C) in coin-envelopes for several days and subsequently stored under ambient conditions. After grinding, samples were extracted with methanol (containing digitoxin as internal standard) and analysed by HPLC. Details on sample processing are described in the electronic supplementary material. Total numbers of replicates were A. incarnata = 13, A. syriaca = 13, A. hallii = 14, A. cordifolia = 15, A. viridis = 13, A. curassavica = 14, A. linaria = 9 and A. asperula = 12.
(j). High-performance liquid chromatography analysis of samples
Fifteen microliters of extract were injected into an Agilent 1100 series HPLC and compounds were separated on a Gemini C18 reversed phase column (3 µm, 150 × 4.6 mm, Phenomenex, Torrance, CA, USA). Cardenolides were eluted on a constant flow of 0.7 ml min−1 with an acetonitrile–H2O gradient as follows: 0–2 min 16% acetonitrile, 25 min 70% acetonitrile, 30 min 95% acetonitrile, 35 min 95% acetonitrile, 10 min reconditioning at 16% acetonitrile. UV absorbance spectra were recorded from 200 to 400 nm by a diode array detector. Peaks with symmetrical absorption maxima between 216 and 222 nm were recorded as cardenolides and quantified at 218 nm [31]. Concentrations were calculated based on the peak area of the known digitoxin concentration. Details on evaluation of plant, haemolymph and food bolus samples are given in the electronic supplementary material.
(k). Leaf disc experiments
Leaf disc feeding assays were performed to allow for unambiguous testing for an effect of dietary cardenolides on caterpillar growth. Eggs of E. core and D. plexippus (obtained from colonies in a greenhouse) were treated with oxine as described above and caterpillars were allowed to feed on A. syriaca leaves until they had reached the 2nd instar. Leaves of A. syriaca were collected in the field and stored in a refrigerator. Leaf discs (19 mm diameter) were punched out with a cork borer and were painted either with solvent only (methanol, control), a solution of 1.275 mg ml−1 ouabain and 1.275 mg ml−1 digitoxin (high cardenolide concentration) 2.55 mg ml−1 ouabain and 2.55 mg ml−1 digitoxin (very high cardenolide concentration) using a 20 µl pipettor (10 µl on each side of the disc). One to one mixtures of the commercial cardenolides ouabain and digitoxin (based on weight) are about equimolar. Using this procedure, the cardenolide concentration of the leaf discs was adjusted to none (methanol only), 3 µg and 6 µg cardenolide per mg plant dry mass (endogenous cardenolides should be less than 1 µg mg−1 and were ignored, the dry weight of an A. syriaca 19 mm leaf disc was assumed to be 17 mg, fresh weights ranged from 41 to 72 mg, mean = 58 mg, s.e. = 0.63). These concentrations were chosen to encompass the range of cardenolide concentrations observed across the Asclepias species used. Ouabain (polar) and digitoxin (non-polar) were chosen to mimic the typical co-occurrence of polar and non-polar cardenolides in Asclepias. Leaf discs were placed on a piece of parafilm in a 9 cm Petri dish lined with dampened filter paper. After evaporation of methanol a pre-weighed 2nd instar caterpillar (AT21 Comparator, Mettler Toledo) of E. core or D. plexippus was included in the Petri dish which was subsequently sealed with parafilm and kept in a controlled environment (14 D : 10 N cycle, 27°C/24°C). Samples sizes and experimental details are reported in the electronic supplementary material.
(l). Injection experiment
To test for physiological effects of introducing cardenolides to the body cavity, 3rd or 4th instar caterpillars of E. core and D. plexippus were injected with the cardenolide ouabain dissolved in water (10−2 M) or with pure water as a control. Number of replicates was: E. core injected with H2O (n = 8) or ouabain (n = 13); D. plexippus injected with H2O (n = 7) or ouabain (n = 11). Prior to injection, caterpillars were anesthetized on ice for approximately 5 min. Subsequently, either 2 µl H2O or ouabain solution was injected into the abdominal dorsal vessel under a stereomicroscope using a 34-gauge needle attached to a 25 µl syringe (Hamilton, Reno, NV, USA). After injection caterpillars were kept in plastic cups with a piece of tissue and supplied with a piece of an Asclepias leaf. The next day caterpillars were inspected for recovery as measured by feeding activity.
(m). Statistical analysis
All data were analysed using a generalized least-squares approach in JMP Pro, v. 10.0 (SAS Institute, Cary, NC, USA). Data were checked for normality of residuals for analyses of variance and correlations. Data from the leaf disc experiment were analysed using an ANCOVA (JMP) approach suggested by Raubenheimer & Simpson [32]; initial caterpillar mass and plant biomass consumed were used as covariates with cardenolide treatment as the independent variable. We found no interactions between treatment and covariates, and therefore, removed interaction terms from analysis. Nonlinear curve fitting for the Na+/K+-ATPase activity assay was performed with Origin-Pro 9.1.0 (Originlab Corporation, Northampton, MA, USA) using a four-parameter logistic function (top asymptote set to 100, bottom asymptote set to 0).
3. Results and discussion
(a). Growth of the milkweed butterfly caterpillars is unaffected by cardenolides
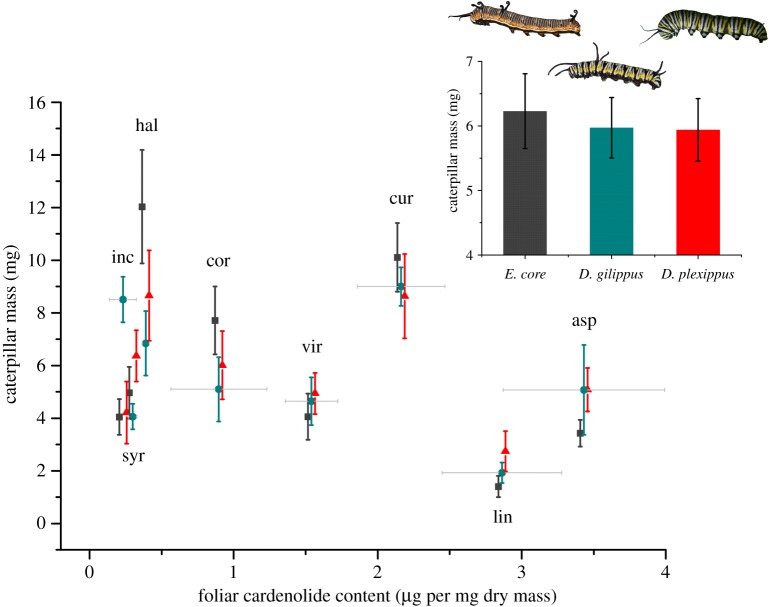
If escalating Na+/K+-ATPase resistance were an adaptation to resist dietary cardenolides, we would expect that caterpillar growth would be differentially impaired across the three caterpillar species, especially on Asclepias spp. with highly concentrated cardenolides. However, three lines of evidence indicate no negative impact of foliar cardenolides on caterpillar performance among the three milkweed butterfly species. First, their masses after 3 and 5 days of larval growth showed no declining trend over approximately 14-fold variation in foliar cardenolide concentrations (figure 1 and electronic supplementary material, figure S2, E. core: F1,6 = 1.147, p = 0.325, D. gilippus: F1,6 = 0.736, p = 0.424, D. plexippus: F1,6 = 0.676, p = 0.442, treating plant species as the unit of replication; the lack of an effect of cardenolides is upheld when treating each caterpillar individual as the unit of replication: E. core: F1,60 = 2.766, p = 0.102, D. gilippus: F1,48 = 1.234, p = 0.272, D. plexippus: F1,62 = 1.899, p = 0.173, see legend of the electronic supplementary material, figure S2, for statistics on caterpillar mass after 5 days). Indeed, one species with intermediate concentrations of cardenolides, A. viridis, was reported to kill sheep [33], but did not impair growth of the milkweed butterfly caterpillars. A similar result was found in other studies, including one where monarch caterpillars were reared on 50 different Asclepias species ([34]; see also [35,36]).
Figure 1.
Caterpillar weights after 3 days on eight species of milkweed. Caterpillars of Euploea core (grey, squares), Danaus gilippus (blue, circles) and D. plexippus (red, triangles) were reared from hatching on eight species of Asclepias. Mean cardenolide concentrations (±s.e.) in leaves are shown on the x-axis. Species codes: inc, Asclepias incarnata; syr, A. syriaca; hal, A. hallii; cor, A. cordifolia; vir, A. viridis; cur, A. curassavica; lin, A. linaria; asp, A. asperula. Inset: mean caterpillar mass (±s.e.) was not different among the three caterpillar species. (Online version in colour.)
Second, caterpillar mass did not differ across the three caterpillar species (F2,18 = 0.028, p = 0.973; figure 1, inset), indicating that the differences in Na+/K+-ATPase resistance were not reflected in caterpillar growth, even on plants with the highest cardenolide concentrations (interaction term between plant cardenolide content and caterpillar species: F2,18 = 0.169, p = 0.846). When considering each plant species separately, the caterpillars only had different masses on A. incarnata, which was the lowest cardenolide plant species (F2,19 = 7.525, p = 0.004); larval masses on all other plant species were not significantly different 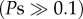 . Finally, we found no toxicity of isolated cardenolides when fed to E. core (treatment: F2,34 = 0.952, p = 0.396; initial caterpillar mass: F1,35 = 15.743, p < 0.001; leaf mass consumed: F1,35 = 159.535, p < 0.001) and D. plexippus (treatment: F2,34 = 0.597, p = 0.556; initial caterpillar mass: F1,34 = 18.157, p < 0.001; leaf mass consumed: F1,34 = 185.088, p < 0.001) caterpillars in painted leaf disc assays (figure 2), demonstrating that Na+/K+-ATPase substitutions are not necessary to protect caterpillars from the toxic effects of dietary cardenolides.
. Finally, we found no toxicity of isolated cardenolides when fed to E. core (treatment: F2,34 = 0.952, p = 0.396; initial caterpillar mass: F1,35 = 15.743, p < 0.001; leaf mass consumed: F1,35 = 159.535, p < 0.001) and D. plexippus (treatment: F2,34 = 0.597, p = 0.556; initial caterpillar mass: F1,34 = 18.157, p < 0.001; leaf mass consumed: F1,34 = 185.088, p < 0.001) caterpillars in painted leaf disc assays (figure 2), demonstrating that Na+/K+-ATPase substitutions are not necessary to protect caterpillars from the toxic effects of dietary cardenolides.
Figure 2.
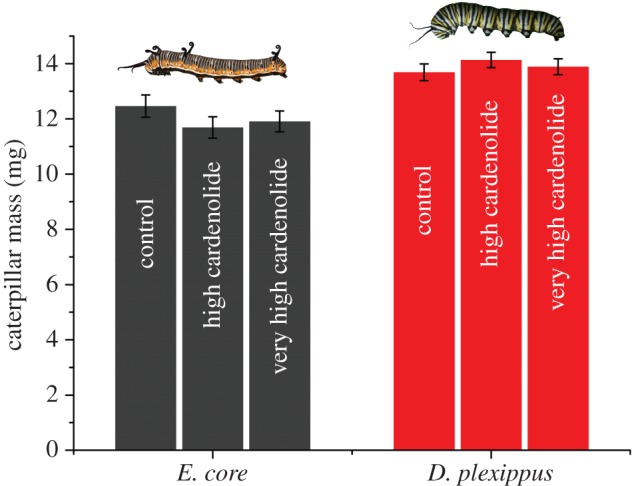
Caterpillar growth on leaf discs painted with isolated toxins. Caterpillars of E. core (grey) and D. plexippus (red) were fed A. syriaca leaf discs painted with an equimolar mixture of ouabain and digitoxin. Control, painted with methanol only; high cardenolide, 3 µg cardenolide per mg dry weight; very high cardenolide, 6 µg cardenolide per mg dry weight. Bars represent least square means ± s.e. (corrected for initial mass and plant mass consumed). (Online version in colour.)
(b). Escalating sequestration of cardenolides into caterpillar haemolymph
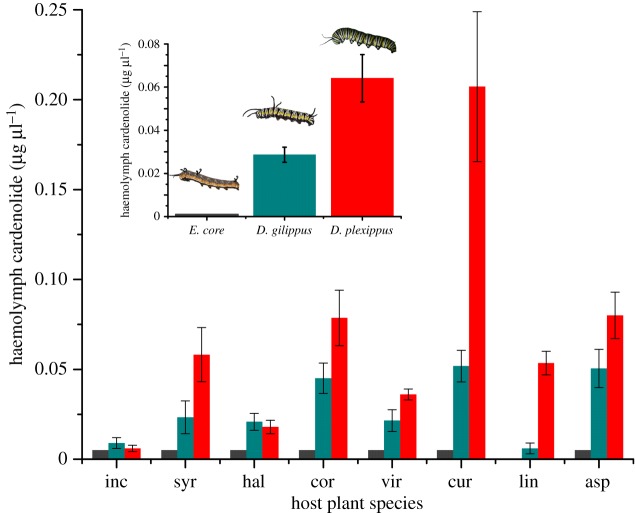
While growth of the three caterpillar species did not differ in response to cardenolides in their diet, there was substantial differentiation in the uptake of cardenolides into their body tissues. From the perspective of plant defence and toxicology, cardenolides that enter the larval haemolymph may be the most relevant as they are mobile and may reach neural Na+/K+-ATPase by diffusion [37]. Nonetheless, our HPLC analyses showed that the haemolymph of E. core did not contain cardenolides when reared on any of the plant species, indicating that the midgut epithelium of E. core is impermeable to cardenolides. By contrast, haemolymph from D. gilippus and D. plexippus caterpillars contained substantial amounts of cardenolides (figure 3), with D. plexippus caterpillars sequestering twice as much as D. gilippus (D. gilippus versus D. plexippus controlling for plant species: F1,67 = 18.488, p < 0.001). Using the sensitive E. core Na+/K+-ATPase as a pharmacological detector for haemolymph cardenolides verified the findings obtained by HPLC; D. gilippus and D. plexippus show a stepwise pattern of increasing cardenolide sequestration (electronic supplementary material, figure S3, inset: controlling for plant species, F2,104 = 134.974, p < 0.001; D. gilippus versus D. plexippus: F1,67 = 11.954, p = 0.001, see also the electronic supplementary material, figure S5). As in previous studies of whole D. plexippus animals [34,38], both of these sequestering species show an increase in sequestration when raised on diets with increasing levels of cardenolides; sequestration saturates, however, on mid-cardenolide plants, and some specificity and selectivity clearly exists with altered sequestration on particular species (e.g. A. curassavica and A. linaria, figure 3) [39].
Figure 3.
Concentration of toxins in caterpillar haemolymph. Haemolymph from caterpillars raised on eight species of Asclepias was analysed by HPLC; plant species codes are given in figure 1; plant species are arranged from lowest to highest foliar cardenolide concentration. Bars represent means ± s.e. Grey bars, Euploea core; blue bars, Danaus gilippus; red bars, D. plexippus. No cardenolides were detectable in the haemolymph of E. core (grey bars are shown for clarity). The grey bar for A. linaria is lacking as no E. core haemolymph sample was obtained. Inset: means ± s.e. of haemolymph cardenolides over all plant species (Online version in colour.)
(c). The toxicological burden of sequestration
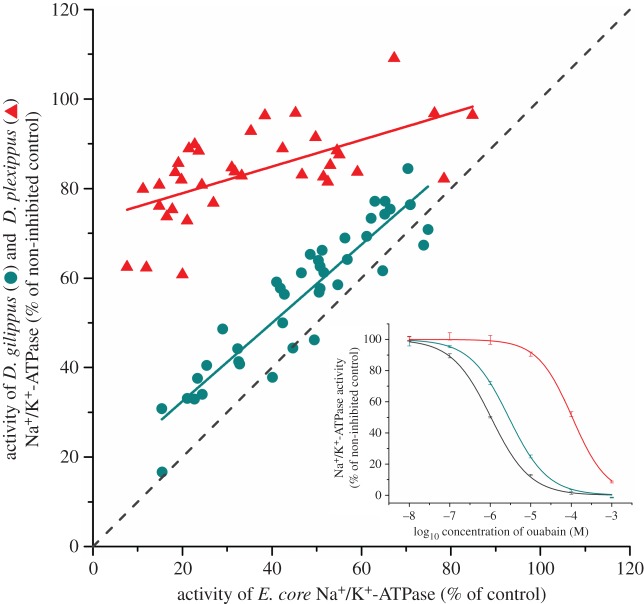
If Na+/K+-ATPase alterations evolved to facilitate sequestration, we predict a toxicological burden of cardenolides for an insect with a susceptible Na+/K+-ATPase. Accordingly, we employed ‘forbidden combinations' to assess this potential burden by applying cardenolides from haemolymph sequestered by D. plexippus and D. gilippus caterpillars on the sensitive Na+/K+-ATPase of E. core (as well as on the donor D. plexippus and D. gilippus Na+/K+-ATPase). Haemolymph cardenolides sequestered by D. gilippus and D. plexippus revealed systematically stronger inhibition of E. core Na+/K+-ATPase compared with that on the donor's enzyme (figure 4, E. core versus D. gilippus: d.f. = 39, paired t = −8.782, p < 0.001; D. gilippus versus D. plexippus: F1,72 = 393.883, p < 0.001) and inhibition of E. core Na+/K+-ATPase and Na+/K+-ATPase of the donor species (D. plexippus or D. gilippus) caused by the identical haemolymph sample was significantly correlated (E. core versus D. plexippus: F1,34 = 21.058, p < 0.001, E. core versus D. gilippus: F1,38 = 212.279, p < 0.001). To test how this result translates from the level of isolated Na+/K+-ATPase to the whole organism, we injected caterpillars of D. plexippus and E. core with the standard cardenolide ouabain: E. core did not tolerate cardenolides within the body cavity (only 23% of the injected caterpillars recovered, Williams corrected G = 13.940, p < 0.001), while D. plexippus caterpillars were unaffected (figure 5). This result confirms that butterfly resistance to cardenolide toxins was associated with sequestration into the body, not dietary tolerance of the compounds. Several studies had suggested a physiological burden of cardenolide sequestration in D. plexippus based on negative correlations between body weight and amount of cardenolides stored [40,41]; here we show with stronger inference that costs of sequestration could lead to the evolution of cardenolide resistance of Na+/K+-ATPase.
Figure 4.
Toxic impact of sequestered cardenolides on adapted and non-adapted Na+/K+-ATPase. The impact of sequestered cardenolides on D. gilippus and D. plexippus Na+/K+-ATPase activity was assessed and compared with the effect on E. core Na+/K+-ATPase (lower activity indicates greater enzyme inhibition). Haemolymph cardenolide samples from D. gilippus (blue) and D. plexippus (red) caterpillars raised on eight Asclepias species were applied on the isolated Na+/K+-ATPase of the donor species and on the Na+/K+-ATPase of E. core in in vitro assays. Each dot represents the activity of two different butterfly Na+/K+-ATPases exposed to the same haemolymph sample. The dashed grey line represents the 1 : 1 relationship. Inset: sensitivity of E. core (IC50: 1.04 × 10−6 M) (grey), D. gilippus (IC50: 2.88 × 10−6 M) (blue) and D. plexippus (red) Na+/K+-ATPase (IC50 1.06 × 10−4 M) to the standard cardenolide ouabain. (Online version in colour.)
Figure 5.

Recovery of caterpillars upon toxin injection. Caterpillars of E. core (grey) and D. plexippus (red) were either injected with water (control) or the standard cardenolide ouabain (10−2 M in water). Caterpillars which had resumed feeding by the next day were scored as recovered. Inset: typical appearance of an E. core caterpillar which is paralysed after injection with ouabain (response observed in 11 of 13 injected caterpillars). Sample size ranged from seven to 13 caterpillars. (Online version in colour.)
(d). Processing cardenolides for sequestration and following their fate
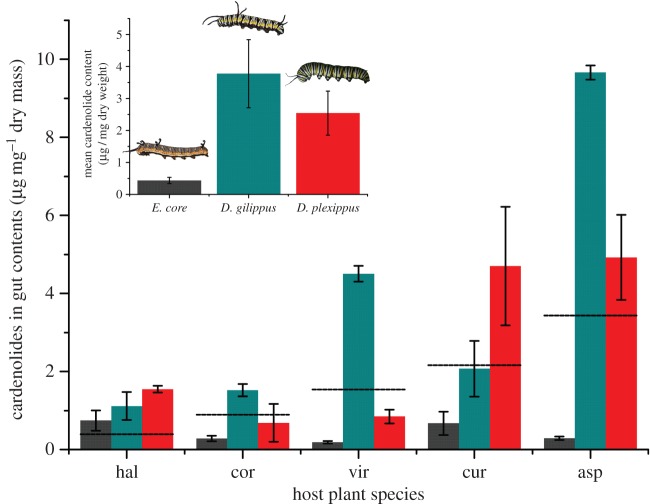
Although the larval haemolymph is a critical physiological compartment for movement of sequestered toxins, the process of sequestration begins in the gut and the toxins are eventually stored in the integument [42]. We found that cardenolides accumulate in the midgut lumen of D. gilippus and D. plexippus but not in the midgut of the non-sequestering E. core (figure 6, F2,23 = 8.13, p = 0.002, no difference between D. gilippus and D. plexippus: F1,14 = 2.373, p = 0.146). Indeed, gut contents of E. core seemed to have cardenolide levels below plant material potentially indicating degradation (figure 6). Thus, not only do sequestering butterflies not degrade plant toxins, but very early accumulation might fuel cardenolide diffusion via the gut epithelium and may be a necessary precondition for sequestration. Chemical analysis of caterpillar bodies (i.e. integument and adhering tissues, excluding the gut) revealed a concordant pattern of sequestration as in the haemolymph across the three butterflies (electronic supplementary material, figure S4). Our work demonstrates that the process of sequestration begins in the midgut, in a second step toxins enter the haemolymph, and are eventually retained in the body tissues. Previous observations had suggested that D. gilippus and D. chrysippus store lower amounts of cardenolides than monarchs [40,43,44] and that E. core does not sequester the compounds [29,45]. Here we conclusively demonstrate these patterns experimentally and show that sequestration is associated with the three discrete and progressive modifications of the Na+/K+-ATPase [12].
Figure 6.
Cardenolide concentration of caterpillar midgut contents. Grey bars, E. core; blue bars, D. gilippus; red bars, D. plexippus. Dashed horizontal lines indicate mean plant cardenolide concentration. Inset: means ± s.e. of cardenolides of gut contents over five plant species. For quantitative evaluation of cardenolides in the gut contents, we focused on the dominant peaks (see Material and methods, the trend that midgut contents of E. core have cardenolide concentrations below plant material is upheld when all cardenolide peaks detected in E. core are taken into account). (Online version in colour.)
(e). Synthesis
Although all herbivores have adaptations to cope with plant defences, we know relatively little about the evolutionary origin of those traits. Indeed, most such traits have been interpreted as a coevolutionary answer to plant defence [3–8]. And yet many herbivorous insects actively sequester plant defences in their own defence [46], and there is some evidence that sequestration explains the predominance of specialized over generalist feeding habits [11,26]. Our data suggest that the evolution of a major insect resistance trait in the milkweed butterflies, which is highly specific to cardenolide plant toxins, is more likely to be associated with sequestration that confers protection against predators, rather than as a dietary necessity. For other well-studied sequestered plant defences like iridoid glycosides [47], pyrrolizidine alkaloids [48] and aristolochic acids [49], the relative importance of sequestration in the evolution of toxin resistance is unclear. Adaptations to toxins driven by sequestration could be a mechanistic link for how sequestration leads to dietary specialization [50].
For the rest of the milkweed insect community, it is an open question whether cardenolide resistance of the Na+/K+-ATPase also evolved in association with sequestration or as an adaptation to tolerate dietary cardenolides. There are species that sequester cardenolides (e.g. arctiid moths) but still possess a sensitive Na+/K+-ATPase [37], while other species (e.g. Labidomera clivicollis) have an insensitive Na+/K+-ATPase, but do not appear to sequester cardenolides [15,16]. Nonetheless, by examining three progressive levels of Na+/K+-ATPase resistance in a single clade, our results bear on the long-standing debate of two versus three trophic level interactions dominating plant–herbivore coevolution [3,25]. Although a tritrophic view has been widely accepted for ecological interactions, we have now shown that such interactions, e.g. the evolution of sequestration, could be an important mechanism through which the third trophic level spurs coevolutionary escalation.
Supplementary Material
Acknowledgements
This manuscript was improved by discussions with members of the Phytophagy Laboratory at Cornell University (www.herbivory.com), especially Rayko Halitschke and Tobias Züst who also gave technical advice. We thank Lara Flacht for technical support and performing preliminary experiments leading to the discovery of accumulation of cardenolides in the caterpillar midgut. We thank Jaap de Roode and Michael Boppré for sending D. gilippus and E. core, respectively. We highly appreciate comments on this manuscript by Michael Boppré, Robert A. Raguso and Heiko Vogel. We greatly acknowledge technical support by Carolyn Creneti, Daniel Fines and Amy P. Hastings.
Data accessibility
The raw data reported in this paper were deposited in Dryad: http://dx.doi.org/10.5061/dryad.3jm4q.
Authors' contributions
G.P. and A.A.A. conceived and designed the study. G.P. performed the research. G.P. and A.A.A. discussed and interpreted the results, analysed data and wrote the paper.
Competing interests
We have no competing interests to declare.
Funding
This work was supported by the German Research Foundation (G.P., PE 2059/1-1) and the Templeton Foundation (A.A.A.).
References
- 1.Ehrlich PR, Raven PH. 1964. Butterflies and plants: a study in coevolution. Evolution 18, 586–608. ( 10.2307/2406212) [DOI] [Google Scholar]
- 2.van Valen L. 1973. A new evolutionary law. Evol. Theory 1, 1–30. [Google Scholar]
- 3.Singer MS, Stireman JO. 2005. The tri-trophic niche concept and adaptive radiation of phytophagous insects. Ecol. Lett. 8, 1247–1255. ( 10.1111/j.1461-0248.2005.00835.x) [DOI] [Google Scholar]
- 4.Wittstock U, Agerbirk N, Stauber EJ, Olsen CE, Hippler M, Mitchell-Olds T, Gershenzon J, Vogel H. 2004. Successful herbivore attack due to metabolic diversion of a plant chemical defense. Proc. Natl Acad. Sci. USA 101, 4859–4864. ( 10.1073/pnas.0308007101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jongsma MA, Bolter C. 1997. The adaptation of insects to plant protease inhibitors. J. Insect Physiol. 43, 885–895. ( 10.1016/S0022-1910(97)00040-1) [DOI] [PubMed] [Google Scholar]
- 6.Krieger RI, Feeny PP, Wilkinson CF. 1971. Detoxication enzymes in the guts of caterpillars: an evolutionary answer to plant defenses? Science 172, 579–581. ( 10.1126/science.172.3983.579) [DOI] [PubMed] [Google Scholar]
- 7.Engler HS, Spencer KC, Gilbert LE. 2000. Preventing cyanide release from leaves. Nature 406, 144–145. ( 10.1016/S1360-1385(00)01775-1) [DOI] [PubMed] [Google Scholar]
- 8.Dussourd DE, Denno RF. 1994. Host range of generalist caterpillars: trenching permits feeding on plants with secretory canals. Ecology 75, 69–78. ( 10.2307/1939383) [DOI] [Google Scholar]
- 9.Duffey SS. 1980. Sequestration of plant natural products by insects. Annu. Rev. Entomol. 25, 447–477. ( 10.1146/annurev.en.25.010180.002311) [DOI] [Google Scholar]
- 10.Bowers MD, Collinge SK. 1992. Fate of iridoid glycosides in different life stages of the buckeye, Junonia coenia (Lepidoptera: Nymphalidae). J. Chem. Ecol. 18, 817–831. ( 10.1007/BF00988322) [DOI] [PubMed] [Google Scholar]
- 11.Dyer LA. 1995. Tasty generalists and nasty specialists? Antipredator mechanisms in tropical lepidopteran larvae. Ecology 76, 1483–1496. ( 10.2307/1938150) [DOI] [Google Scholar]
- 12.Petschenka G, Fandrich S, Sander N, Wagschal V, Boppré M, Dobler S. 2013. Stepwise evolution of resistance to toxic cardenolides via genetic substitutions in the Na+/K+-ATPase of milkweed butterflies (Lepidoptera: Danaini). Evolution 67, 2753–2761. ( 10.1111/evo.12152) [DOI] [PubMed] [Google Scholar]
- 13.Aardema ML, Zhen Y, Andolfatto P. 2012. The evolution of cardenolide-resistant forms of Na+,K+-ATPase in Danainae butterflies. Mol. Ecol. 21, 340–349. ( 10.1111/j.1365-294X.2011.05379.x) [DOI] [PubMed] [Google Scholar]
- 14.Schatzmann H-J. 1953. Herzglykoside als Hemmstoffe für den aktiven Kalium- und Natriumtransport durch die Erythrocytenmembran. Helv. Physiol. Pharmacol. Acta 11, 346–354. [PubMed] [Google Scholar]
- 15.Agrawal AA, Petschenka G, Bingham RA, Weber MG, Rasmann S. 2012. Toxic cardenolides: chemical ecology and coevolution of specialized plant-herbivore interactions. New Phytol. 194, 28–45. ( 10.1111/j.1469-8137.2011.04049.x) [DOI] [PubMed] [Google Scholar]
- 16.Dobler S, Dalla S, Wagschal V, Agrawal AA. 2012. Community-wide convergent evolution in insect adaptation to toxic cardenolides by substitutions in the Na,K-ATPase. Proc. Natl Acad. Sci USA 109, 13 040–13 045. ( 10.1073/pnas.1202111109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhen Y, Aardema ML, Medina EM, Schumer M, Andolfatto P. 2012. Parallel molecular evolution in an herbivore community. Science 337, 1634–1637. ( 10.1126/science.1226630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobler S, Petschenka G, Wagschal V, Flacht L. 2015. Convergent adaptive evolution: how insects master the challenge of cardiac glycoside-containing host plants. Entomol. Exp. Appl. 157, 30–39. ( 10.1111/eea.12340) [DOI] [Google Scholar]
- 19.Vaughan GL, Jungreis AM. 1977. Insensitivity of lepidopteran tissues to ouabain: physiological mechanisms for protection from cardiac glycosides. J. Insect Physiol. 23, 585–589. ( 10.1016/0022-1910(77)90052-X) [DOI] [Google Scholar]
- 20.Holzinger F, Frick C, Wink M. 1992. Molecular basis for the insensitivity of the monarch (Danaus plexippus) to cardiac glycosides. FEBS Lett. 314, 477–480. ( 10.1016/0014-5793(92)81530-Y) [DOI] [PubMed] [Google Scholar]
- 21.Brower LP, Ryerson WN, Coppinger LL, Glazier SC. 1968. Ecological chemistry and the palatability spectrum. Science 161, 1349–1350. ( 10.1126/science.161.3848.1349) [DOI] [PubMed] [Google Scholar]
- 22.Glendinning JI. 1990. Responses of three mouse species to deterrent chemicals in the monarch butterfly. II. Taste tests using intact monarchs. Chemoecology 1, 124–130. [Google Scholar]
- 23.Sternberg ED, Lefèvre T, Li J, de Castillejo CLF, Li H, Hunter MD, de Roode JC. 2012. Food plant derived disease tolerance and resistance in a natural butterfly-plant-parasite interactions. Evolution 66, 3367–3376. ( 10.1111/j.1558-5646.2012.01693.x) [DOI] [PubMed] [Google Scholar]
- 24.Agrawal AA, Fishbein M. 2008. Phylogenetic escalation and decline of plant defense strategies. Proc. Natl Acad. Sci. USA 105, 10 057–10 060. ( 10.1073/pnas.0802368105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price PW, Bouton CE, Gross P, McPheron BA, Thompson JN, Weis AE. 1980. Interactions among three trophic levels: influence of plants on interactions between insect herbivores and natural enemies. Annu. Rev. Ecol. Syst. 11, 41–65. ( 10.1146/annurev.es.11.110180.000353) [DOI] [Google Scholar]
- 26.Camara MD. 1997. Physiological mechanisms underlying the costs of chemical defence in Junonia coenia Hübner (Nymphalidae): a gravimetric and quantitative genetic analysis. Evol. Ecol. 11, 451–469. ( 10.1023/A:1018436908073) [DOI] [Google Scholar]
- 27.Brower JVZ. 1958. Experimental studies of mimicry in some North American butterflies: Part I. The monarch, Danaus plexippus, and viceroy, Limenitis archippus. Evolution 12, 32–47. [Google Scholar]
- 28.Ritland DB, Brower LP. 1991. The viceroy butterfly is not a Batesian mimic. Nature 350, 497–498. ( 10.1038/350497a0) [DOI] [Google Scholar]
- 29.Dixon CA, Erickson JM, Kellett DN, Rothschild M. 1978. Some adaptations between Danaus plexippus and its food plant, with notes on Danaus chrysippus and Euploea core (Insecta: Lepidoptera). J. Zool. 185, 437–467. ( 10.1111/j.1469-7998.1978.tb03344.x) [DOI] [Google Scholar]
- 30.McLaughlin RE, Myers J. 1970. Ophryocystis elektroscirrha sp. n., a neogregarine pathogen of the monarch butterfly Danaus plexippus (L.) and the Florida queen butterfly D. gilippus berenice Cramer. J. Protozool. 17, 300–305. ( 10.1111/j.1550-7408.1970.tb02375.x) [DOI] [Google Scholar]
- 31.Rasmann S, Johnson MD, Agrawal AA. 2009. Induced responses to herbivory and jasmonate in three milkweed species. J. Chem. Ecol. 35, 1326–1334. ( 10.1007/s10886-009-9719-0) [DOI] [PubMed] [Google Scholar]
- 32.Raubenheimer D, Simpson SJ. 1992. Analysis of covariance: an alternative to nutritional indices. Entomol. Exp. Appl. 62, 221–231. ( 10.1007/BF00353441) [DOI] [Google Scholar]
- 33.Smith RA, Scharko P, Bolin D, Hong CB. 2000. Intoxication of sheep exposed to ozark milkweed (Asclepias viridis Walter). Vet. Hum. Toxicol. 42, 349–350. [PubMed] [Google Scholar]
- 34.Agrawal AA, Ali JG, Rasmann S, Fishbein M. 2015. Macroevolutionary trends in the defense of milkweeds against monarchs: latex, cardenolides, and tolerance of herbivory. In Monarchs in a changing world: biology and conservation of an iconic insect (eds Oberhauser KS, Nail KR, Altizer S). Ithaca, NY: Cornell University Press. [Google Scholar]
- 35.Erickson JM. 1973. The utilization of various Asclepias species by larvae of the monarch butterfly, Danaus plexippus. Psyche 80, 230–244. ( 10.1155/1973/28693) [DOI] [Google Scholar]
- 36.Seiber JN, Tuskes PM, Brower LP, Nelson CJ. 1980. Pharmacodynamics of some individual milkweed cardenolides fed to larvae of the monarch butterfly (Danaus plexippus L.). J. Chem. Ecol. 6, 321–339. ( 10.1007/BF01402911) [DOI] [Google Scholar]
- 37.Petschenka G, Offe JK, Dobler S. 2012. Physiological screening for target site insensitivity and localization of Na+/K+-ATPase in cardenolide-adapted Lepidoptera. J. Insect Physiol. 58, 607–612. ( 10.1016/j.jinsphys.2011.12.012) [DOI] [PubMed] [Google Scholar]
- 38.Malcolm S. 1994. Milkweeds, monarch butterflies and the ecological significance of cardenolides. Chemoecology 5–6, 101–117. ( 10.1007/BF01240595) [DOI] [Google Scholar]
- 39.Malcolm SB, Brower LP. 1989. Evolutionary and ecological implications of cardenolide sequestration in the monarch butterfly. Cell. Mol. Life Sci. 45, 284–295. ( 10.1007/bf01951814) [DOI] [Google Scholar]
- 40.Cohen JA. 1985. Differences and similarities in cardenolide contents of queen and monarch butterflies in Florida and their ecological and evolutionary implications. J. Chem. Ecol. 11, 85–103. ( 10.1007/BF00987608) [DOI] [PubMed] [Google Scholar]
- 41.Brower LP, Moffitt CM. 1974. Palatability dynamics of cardenolides in the monarch butterfly. Nature 249, 280–283. ( 10.1038/249280b0) [DOI] [PubMed] [Google Scholar]
- 42.Roeske CN, Seiber JN, Brower LP, Moffitt CM. 1976. Milkweed cardenolides and their comparative processing by monarch butterflies (Danaus plexippus L.). Recent Adv. Phytochem. 10, 93–167. ( 10.1007/978-1-4684-2646-5_3) [DOI] [Google Scholar]
- 43.Brower LP, Gibson DO, Moffitt CM, Panchen AL. 1978. Cardenolide content of Danaus chrysippus butterflies from three areas of East Africa. Biol. J. Linn. Soc. 10, 251–273. ( 10.1111/j.1095-8312.1978.tb00015.x) [DOI] [Google Scholar]
- 44.Mebs D, Wagner M, Toennes S, Wunder C, Boppré M. 2012. Selective sequestration of cardenolide isomers by two species of Danaus butterflies (Lepidoptera: Nymphalidae: Danainae). Chemoecology 22, 269–272. ( 10.1007/s00049-012-0109-7) [DOI] [Google Scholar]
- 45.Malcolm S, Rothschild M. 1983. A danaid Mullerian mimic, Euploea core amymone (Cramer) lacking cardenolides in the pupal and adult stages. Biol. J. Linn. Soc. 19, 27–33. ( 10.1111/j.1095-8312.1983.tb00774.x) [DOI] [Google Scholar]
- 46.Opitz S, Müller C. 2009. Plant chemistry and insect sequestration. Chemoecology 19, 117–154. ( 10.1007/s00049-009-0018-6) [DOI] [Google Scholar]
- 47.Dobler S, Petschenka G, Pankoke H. 2011. Coping with toxic plant compounds: the insect's perspective on iridoid glycosides and cardenolides. Phytochemistry 72, 1593–1604. ( 10.1016/j.phytochem.2011.04.015) [DOI] [PubMed] [Google Scholar]
- 48.Boppré M. 1990. Lepidoptera and pyrrolizidine alkaloids. Exemplification of complexity in chemical ecology. J. Chem. Ecol. 16, 165–185. ( 10.1007/BF01021277) [DOI] [PubMed] [Google Scholar]
- 49.Nishida R. 2002. Sequestration of defensive substances from plants by Lepidoptera. Annu. Rev. Entomol. 47, 57–92. ( 10.1146/annurev.ento.47.091201.145121) [DOI] [PubMed] [Google Scholar]
- 50.Camara MD. 1997. A recent host range expansion in Junonia coenia Hübner (Nymphalidae): oviposition preference, survival, growth, and chemical defense. Evolution 51, 873–884. ( 10.2307/2411162) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data reported in this paper were deposited in Dryad: http://dx.doi.org/10.5061/dryad.3jm4q.