Abstract
In many species, males rely on sexual ornaments to attract females. Females, by contrast, rarely produce ornaments. The glow-worm (Lampyris noctiluca) is an exception where wingless females glow to attract males that fly in search of females. However, little is known about the factors that promote the evolution of female ornaments in a sexual selection context. Here, we investigated if the female ornament of the glow-worm is a signal of fecundity used in male mate choice. In support of this, we found brightness to correlate with female fecundity, and males to prefer brighter dummy females. Thus, the glow emitted by females is a reliable sexual signal of female fecundity. It is likely that male preference for the fecundity-indicating ornament has evolved because of large variation among females in fecundity, and because nocturnal males cannot directly assess female size and fecundity. These results indicate that female ornamentation may evolve in capital breeders (i.e. those in which stored resources are invested in reproduction) when females vary significantly in fecundity and this variation cannot be assessed directly by males.
Keywords: female mate attraction, male mate choice, Lampyris noctiluca, sexual selection
1. Introduction
Female ornaments are comparatively rare in nature [1,2]. When they occur, they are often linked to resource competition or high male investment into offspring [3,4] rather than to sexual selection [4]. This is because investment into ornaments decreases fitness by taking resources away from fecundity, creating a trade-off between ornamentation and fecundity [3,5]. Moreover, fecundity is often correlated with body or abdomen size and the use of ornaments to advertise fecundity may then be superfluous. Female ornaments in a sexual selection context are more common when the species is sex-role reversed, or when the ornament is a consequence of a genetic correlation with male ornamentation [4,6].
Few studies have, however, tested the underlying reasons for the rarity of female ornaments in a sexual selection context. This is surprising, given that males can be choosy and could act on female ornamentation [7]. Even though there is a trade-off between fecundity and mate attraction, the evolution of fecundity-indicating ornaments may be owing to an inability of males to assess female size and fecundity directly. If females vary significantly in fecundity and mating is costly or mate encounter rate high, males would benefit from expressing a preference for a trait that indicates fecundity. The preference could in turn promote the evolution of female ornaments that reflect fecundity.
A potential example of a sexually ornamented female is the common glow-worm (Lampyris noctiluca, Lampyridae), a nocturnal capital breeder. Females attract males by glowing, usually only one or a few nights, and flying males search for females. However, sexual selection and female differences in mate attraction in glow-worms remain unknown [8–10]. We tested: (i) whether the brightness of the glow correlates with fecundity, and (ii) if males exert mate choice and prefer dummy females with brighter glow, in which case the ornament would be sexually selected.
2. Material and methods
(a). Study species
Common glow-worms (L. noctiluca) are nocturnal beetles. Adults are active from June to July [11] and generally live for less than two weeks without being able to eat. Females attract males by producing green (546–570 nm) light from tens of minutes to several hours each night [9,12,13]. Most females glow during one or two nights until one or a few males arrive. After mating, females lay their eggs and then die. However, some females are known to glow for weeks without attracting males [9]. It is common for several females to glow simultaneously within metres of one another. The adult sex ratio of glow-worms is unity [14]. The glow is produced in the lantern, which covers the underside of the sixth and seventh segments of the female's abdomen as well as two spots on the eighth segment. Males have previously been shown to prefer brighter females when held in small aquaria with no possibility of flying [15], but detailed examination of how males select females when flying is lacking.
(b). Female fecundity and lantern size
Female differences in fecundity and lantern size and the correlations between them were tested using females that were found glowing around Tvärminne research station (N 59°51′, E 23°14′) and in Nurmijärvi (N 60°34′, E 24°44′) in Finland. The selected areas were searched during consecutive nights to detect females that were glowing for the first time. These were brought to the laboratory, kept on a light rhythm that approximated natural conditions and allowed to mate. The number of eggs laid was counted, and the ventral side of the females (with the lantern) was photographed after death. The presence of fully grown eggs left in the ovaries was checked through dissection. Female mating status and the number of eggs laid before capture were unknown. However, females stop glowing after mating [9] and it is consequently unlikely that they had laid any eggs before capture. All females stopped glowing after mating in the laboratory.
Lantern surface area was measured using ImageJ v. 1.48. It was correlated to fecundity using Pearson correlation, using both the number of laid eggs and the total number of eggs by including unlaid eggs. The results did not differ and we present only results for number of eggs laid.
To test whether lantern size correlates with brightness, we estimated the brightness of captive females on a five-point scale (1, barely visible; 5, very bright). The risk of observer bias was low as the identity of individuals could not be determined in the dark laboratory and as the individuals changed location each day. We used Spearman's rank correlation to test for a correlation between female brightness, lantern size and number of eggs. We chose the brightest glow of each female, because brightness declines with age (G. Baudry 2015, unpublished data).
(c). Male preference for brightness
We tested male preference for brightness in the field by using traps that simulated females of different brightness. We used pairs of traps with differing light intensity 1 m apart. Each trap was made of a 1.5 l plastic bottle cut in half with the top half inserted upside down into the lower half to make a funnel trap. At the top of each trap was a green 3 mm through-hole LED similar in wavelength (562 nm) to a female glow-worm (546–570 nm) [9,16]. We placed two to five trap pairs in different locations each night.
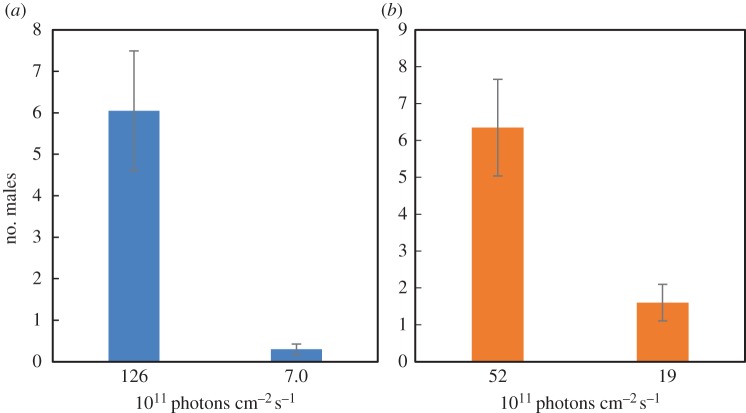
We varied the brightness of the traps by wiring varying amounts of 220 ohm resistors to the LEDs. Two different combinations of resistors were used: 220 and 440 ohms (called high variation treatment, approx. 1.26 × 1013 and 6.99 × 1011 photons cm−2 s−1, respectively), and 440 and 660 ohms (low-variation treatment, 5.22 × 1012 and 1.92 × 1012 photons cm−2 s−1, respectively). The brightness of the lights cannot be directly compared using resistance, as the total resistance in the two trap pairs was different (1100 ohm versus 660 ohm). These differences in relative brightness were reflective of the range found among real females (J. Hopkins 2014, personal observations).
The traps were placed in the same areas where females had been collected. Trapping was conducted in June and July 2014. The position of each trap in the pairs was chosen arbitrarily each night. Traps were not set during nights with high wind or rain when males are less likely to be active.
We used a nested ANOVA to test whether males preferred brighter light, with the number of males per trap each night as the dependent variable, and the two types of trap pairs and the two brightnesses of traps in them as fixed factors (brightness nested inside type of pair). All statistical analyses were done using R v. 3.1.1 for Windows.
3. Results
Females differed greatly in lantern area (7–19 mm2) and in fecundity (25–195 eggs, n = 26), and lantern area correlated positively with fecundity (r2 = 0.41, t25 = 4.14, p < 0.001; figure 1). Lantern area reflected perceived brightness (r2 = 0.22, S = 1382.0, n = 26, p < 0.05), and brightness consequently also correlated positively with fecundity (r2 = 0.36, S = 928.9, n = 25, p < 0.01). Female size, measured as pronotum width correlated positively with fecundity (r2 = 0.48, t21 = 4.436, p < 0.001) and with lantern size (r2 = 0.54, t21 = 4.933, p < 0.001). Nineteen females carried unlaid eggs on death. Four of them died before laying the eggs and another seven had 10% or less of the total number of eggs produced left inside the body. The remaining eight had larger numbers of unlaid eggs.
Figure 1.
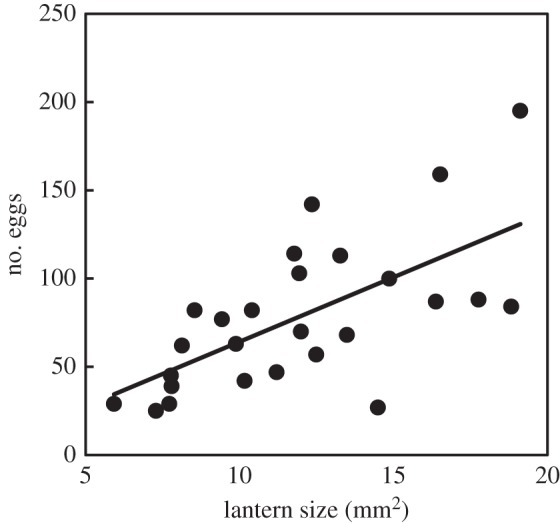
Relationship between number of eggs laid and lantern size.
Males were significantly more likely to select the brighter trap in each pair of traps (F1,76 = 27.23, p < 0.001; figure 2). In the high variation trap pairs, 121 males were trapped in the brighter trap and six in the duller. This pattern remained in the low-variation trap pairs (127 versus 32 males; figure 2). There were no significant differences between the trap pairs in the number of males trapped (F1 = 182.8, p = 0.43).
Figure 2.
Number of males (mean ± 1 s.e.) attracted by paired green LED-lights differing in brightness. (a) High variation treatment and (b) low-variation treatment. (Online version in colour.)
4. Discussion
Our results suggest that male glow-worms prefer females with brighter glow, and that the glow reliably indicates female fecundity. This suggests that the glow is a sexually selected female ornament. Male mate choice for the ornament has probably evolved because females differ greatly in fecundity—there was an eightfold difference in fecundity between females—as males may then gain considerable benefits by selecting the most fecund females. Moreover, as males cannot directly assess female size during nights, sexual selection has probably promoted the evolution of a preference for a trait that indicates fecundity also in the dark.
However, glow-worm eggs are fully developed only sometime after mating [9], and the possibility remains that females can use eggs as a source of energy for glowing, which would reduce the benefit of glowing [17]. In addition, glowing could increase predation risk and reduce survival and, hence, the probability of mating. Yet, the benefit of glowing probably offsets these costs for large females, as brighter dummy females attracted many more males than dull dummy females. Large females will therefore have more males to choose from than smaller females and may glow for shorter time, which could have favoured the evolution of the ornament. Unsuccessful females can glow for several weeks in the attempt to attract males [9]. Whether female mate choice occurs in this species has not been studied but it would be expected, as large dummy females attracted many males.
These results suggest that larger glow-worm females gain dual benefits from their size. They produce more eggs and mate sooner than small females. As they are capital breeders, smaller females cannot increase their fecundity. This in turn may have promoted the evolution of female flightlessness. Wingless females do not waste time or energy in producing wings, but rather invest the resources into fecundity and size.
The number of males we collected in the light traps may overestimate female attraction in nature, as our brightest lights were brighter than females detected in nature, whereas our dullest light was, to a human eye, similar in brightness to real females. In a previous study, males were found to prefer natural brightnesses [16]. The difference between these results could be explained by the different types and wavelengths of light used in the studies. Nevertheless, our results show that males prefer brighter females than found in the field, which indicates that the preference could initially have evolved through sensory drive [18].
To conclude, our results indicate that the female ornament of the glow-worm is sexually selected. It could have evolved through strong male preference for a fecundity-indicating ornament, given the high variation in female fecundity and the difficulty inherent in directly assessing female fecundity.
Acknowledgements
We thank Bob Wong and three anonymous referees for comments. Tvärminne Zoological Station provided working facilities.
Ethics
This study complies with all national regulations on using live animals in research.
Data accessibility
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.jp35r.
Authors' contributions
Concept and design: all authors. Acquisition and interpretation of data: J.H., A.K., G.B. Drafting and revising the manuscript: all authors. All authors approved the final version of the manuscript.
Competing interests
The authors declare that they have no conflicting interests.
Funding
This study was supported by a grant from Otto A. Malm foundation to J.H.
References
- 1.Clutton-Brock T. 2007. Sexual selection in males and females. Science 318, 1882–1885. ( 10.1126/science.1133311) [DOI] [PubMed] [Google Scholar]
- 2.Clutton-Brock T. 2009. Sexual selection in females. Anim. Behav. 77, 3–11. ( 10.1016/j.anbehav.2008.08.026) [DOI] [Google Scholar]
- 3.Rosenqvist G, Berglund A. 2011. Sexual signals and mating patterns in Syngnathidae. J. Fish Biol. 78, 1647–1661. ( 10.1111/j.1095-8649.2011.02972.x) [DOI] [PubMed] [Google Scholar]
- 4.Tobias JA, Montgomerie R, Lyon BE. 2012. The evolution of female ornaments and weaponry: social selection, sexual selection and ecological competition. Phil. Trans. R. Soc. B 367, 2274–2293. ( 10.1098/rstb.2011.0280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzpatrick S, Berglund A, Rosenqvist G. 1995. Ornaments or offspring: costs to reproductive success restrict sexual selection processes. Biol. J. Linn. Soc. 55, 251–260. ( 10.1111/j.1095-8312.1995.tb01063.x) [DOI] [Google Scholar]
- 6.LeBas NR. 2006. Female finery is not for males. Trends Ecol. Evol. 21, 170–172. ( 10.1016/j.tree.2006.01.005) [DOI] [PubMed] [Google Scholar]
- 7.Bonduriansky R. 2001. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. Camb. Philos. Soc. 76, 305–339. ( 10.1017/S1464793101005693) [DOI] [PubMed] [Google Scholar]
- 8.Day JC, Tisi LC, Bailey MJ. 2004. Evolution of beetle bioluminescence: the origin of beetle luciferin. Luminescence 19, 8–20. ( 10.1002/bio.749) [DOI] [PubMed] [Google Scholar]
- 9.Tyler J. 2002. The glow-worm. Kent, UK: Lakeside Printing Ltd. [Google Scholar]
- 10.Shimomura O. 2012. Bioluminescence: chemical principles and methods. Singapore: World Scientific. [Google Scholar]
- 11.Albrecht A, Karjalainen S, Salokannel J, Coleoptera TFEG. 2010. Suomen Kuoriasatlas/Atlas över Finlands skalbaggar/Atlas of the beetles of Finland 3: Lucanidae—Scraptiidae.
- 12.De Cock R. 2004. Larval and adult emission spectra of bioluminescence in three European firefly species. Photochem. Photobiol. 79, 339–342. ( 10.1562/2003-11-11-RA.1) [DOI] [PubMed] [Google Scholar]
- 13.Bird S, Parker J. 2014. Low levels of light pollution may block the ability of male glow-worms (Lampyris noctiluca L.) to locate females. J. Insect Conserv. 18, 737–743. ( 10.1007/s10841-014-9664-2) [DOI] [Google Scholar]
- 14.Tyler J. 2013. Determining the sex ratio in the glow-worm Lampyris noctiluca (L.) (Coleoptera: Lampyridae). Lampyrid 3, 43–48. [Google Scholar]
- 15.Booth D, Stewart AJ, Osorio D. 2004. Colour vision in the glow-worm Lampyris noctiluca (L.) (Coleoptera: Lampyridae): evidence for a green-blue chromatic mechanism. J. Exp. Biol. 207, 2373–2378. ( 10.1242/jeb.01044) [DOI] [PubMed] [Google Scholar]
- 16.Schwalb HH. 1961. Beiträge zur Biologie der einheimischen Lampyriden Lampyris noctiluca Geoffr. und Phausis splendidula Lec. und experimetelle Analyse ihres Beutefang- und Sexualverhaltens. Zool. Jahrbucher/Abteilung für Syst. Ökologie und Geogr. der Tiere 88, 399–550. [Google Scholar]
- 17.Jönsson KI. 1997. Capital and income breeding as alternative tactics of resource use in reproduction. Oikos 78, 57–66. ( 10.2307/3545800) [DOI] [Google Scholar]
- 18.Endler J, Basolo A. 1998. Sensory ecology, receiver biases and sexual selection. Trends Ecol. Evol. 13, 415–420. ( 10.1016/S0169-5347(98)01471-2) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available from the Dryad Digital Repository: http://dx.doi.org/10.5061/dryad.jp35r.