Abstract
Amphibious fishes often emerse (leave water) when faced with unfavourable water conditions. How amphibious fishes cope with the risks of rising water temperatures may depend, in part, on the plasticity of behavioural mechanisms such as emersion thresholds. We hypothesized that the emersion threshold is reversibly plastic and thus dependent on recent acclimation history rather than on conditions during early development. Kryptolebias marmoratus were reared for 1 year at 25 or 30°C and acclimated as adults (one week) to either 25 or 30°C before exposure to an acute increase in water temperature. The emersion threshold temperature and acute thermal tolerance were significantly increased in adult fish acclimated to 30°C, but rearing temperature had no significant effect. Using a thermal imaging camera, we also showed that emersed fish in a low humidity aerial environment (30°C) lost significantly more heat (3.3°C min−1) than those in a high humidity environment (1.6°C min−1). In the field, mean relative humidity was 84%. These results provide evidence of behavioural avoidance of high temperatures and the first quantification of evaporative cooling in an amphibious fish. Furthermore, the avoidance response was reversibly plastic, flexibility that may be important for tropical amphibious fishes under increasing pressures from climatic change.
Keywords: developmental plasticity, thermal tolerance, behavioural thermoregulation, evaporative cooling
1. Introduction
Behavioural flexibility may be critical for animals facing rising temperatures [1,2]. In aquatic systems, behavioural avoidance is often the first line of defence for fishes. Amphibious fishes inhabiting warm tropical waters may leave water (emerse) to avoid aquatic temperature extremes, but few studies have quantified the thermal characteristics of emersion [3], and there is no knowledge of whether emersion thresholds are plastic.
Phenotypic plasticity is the capacity of an organism to express a range of phenotypes in response to environmental stimuli and can be reversible or irreversible [4]. Irreversible plasticity is often the result of environmental factors experienced during early development. If conditions during development are accurate predictors of the adult environment, irreversible plasticity should be favoured by selection [5,6]. In unpredictable environments however, reversible plasticity (flexibility) should be favoured. Both types of plasticity are important for determining the physiological tolerances of adult arthropods [7–9], frogs [10], birds [11] and zebrafish [12]. Although extensive research has investigated the thermal acclimation of physiological traits [13], the effects of thermal history on avoidance behaviour, the first line of defence for many animals, has been overlooked. We tested the hypothesis that in amphibious fishes inhabiting unstable thermal environments the emersion threshold is reversibly plastic and thus influenced primarily by their recent thermal environment. We investigated emersion plasticity and evaporative cooling in a eurythermal (7–38°C) amphibious fish, the mangrove rivulus (Kryptolebias marmoratus) that typically inhabits small pools or crab burrows in mangrove forests [14].
Plasticity in thermal tolerance would help fish cope with high water temperatures, but there is still the risk of overheating at temperature extremes. After observing fish emerse in the laboratory and in the field, we hypothesized that cutaneous evaporation could quickly lower body temperature and minimize thermal damage. This hypothesis predicts that fish emersing into low relative humidity (RH) would lose more body heat than fish in high RH environments even if water and air temperatures were similar.
2. Methods
(a). Emersion plasticity
Newly hatched K. marmoratus hermaphrodites from a single lineage were separated into four temperature groups in a fully crossed thermal history experimental design: (i) reared at 30°C and later tested at 30°C, (ii) reared and tested at 25°C, (iii) reared at 30°C and one week prior to experimentation, transferred to 25°C, and (iv) reared at 25°C and one week prior to experimentation, transferred to 30°C (n = 9–16 per group). Fish were fed Artemia nauplii three times per week.
To determine the emersion threshold temperature, individual fish in experimental containers (100 ml) were placed in a circulating water bath behind a visual barrier, and behaviour was recorded with a webcam (Logitech C905®, Newark, CA, USA). Temperature was increased by 1°C min−1 starting at the acclimation temperature of each group. Containers were aerated to ensure uniform oxygenation and temperature.
We also tested whether emersion temperature was correlated with the acute thermal tolerance of each group. The experiment was repeated, except a mesh barrier was placed at the air–water interface to prevent emersion (n = 11–18 per group). Acute thermal tolerance was defined as the temperature at which fish lost equilibrium, which provides a proxy for the physiological upper temperature limit [15]. All fish recovered fully.
(b). Evaporative cooling
To determine the role of evaporative cooling after emersion, emersion was induced by increasing water temperature (approx. 1°C min−1) as described above, but in these experiments, fish emersed onto a moist substrate. The entire experimental apparatus was contained within an incubator (Pelt-5, Sable Systems, NV, USA) to control air temperature (30°C) and RH. Emersed fish experienced one of two treatments, high (95 ± 5%) or low (65 ± 5%) RH (each n = 7). RH was regulated by aerating a separate water chamber within the incubator and continuously measured using a hygrometer (SperScientific, Scottsdale, AZ, USA). Thermal images were captured through a transparent Germanium window (10 cm diameter × 4 mm thickness; > 97% infrared (IR) transmittance) every second post-emersion using a thermal imaging camera (Mikron Instruments, Model 7515, Vista, CA, USA). Images were analysed using MikroSpecRT (Mikron) by taking the average temperature of the emersed fish along a line down the centre of the body. The total number of movements (jumps) per fish was also measured for 60 s after emersion to determine whether fish enhance cooling by increasing convection.
Finally, we measured RH in the field using the same hygrometer (see above). Daily RH measurements were taken between 15 and 20 December 2012 immediately adjacent to crab burrows from which K. marmoratus had been observed to emerse (Long Caye, Belize; N 17°13.08′; W 087°35.66′). Data were analysed using two-way ANOVA or two-way repeated measures ANOVA as appropriate (critical α = 0.05).
3. Results
Acutely increased water temperature caused emersion in all mangrove rivulus tested (65 out of 65 fish). Control fish (reared and acclimated to 25°C) emersed at temperatures between 28°C and 39.5°C (figure 1a). Fish acclimated to 30°C for 7 days emersed at significantly higher temperatures (approx. 2.3°C; p < 0.05) than fish acclimated to 25°C (figure 1b), but at a lower differential between acclimation and emergence temperature (25: 10°C versus 30: 8°C). Similarly, the acute thermal tolerance of fish acclimated to 30°C was significantly higher than that of fish acclimated to 25°C (p < 0.001; figure 1c), but again at a lower differential between acclimation and loss of equilibrium temperature (25: 15.5°C versus 30: 11.5°C). In addition, we performed an assessment of thermal tolerance using standard critical thermal maximum methods (heating rate of 0.3°C min−1) and the results were similar (electronic supplementary material, table S1). The duration of acute heating was different between 25°C and 30°C groups in both the emersion and thermal tolerance experiments. There was no effect of rearing temperature on the emersion threshold or thermal tolerance (p > 0.05; figure 1b,c). Overall, fish lost equilibrium at water temperatures that were 4–5°C warmer than the emersion threshold.
Figure 1.
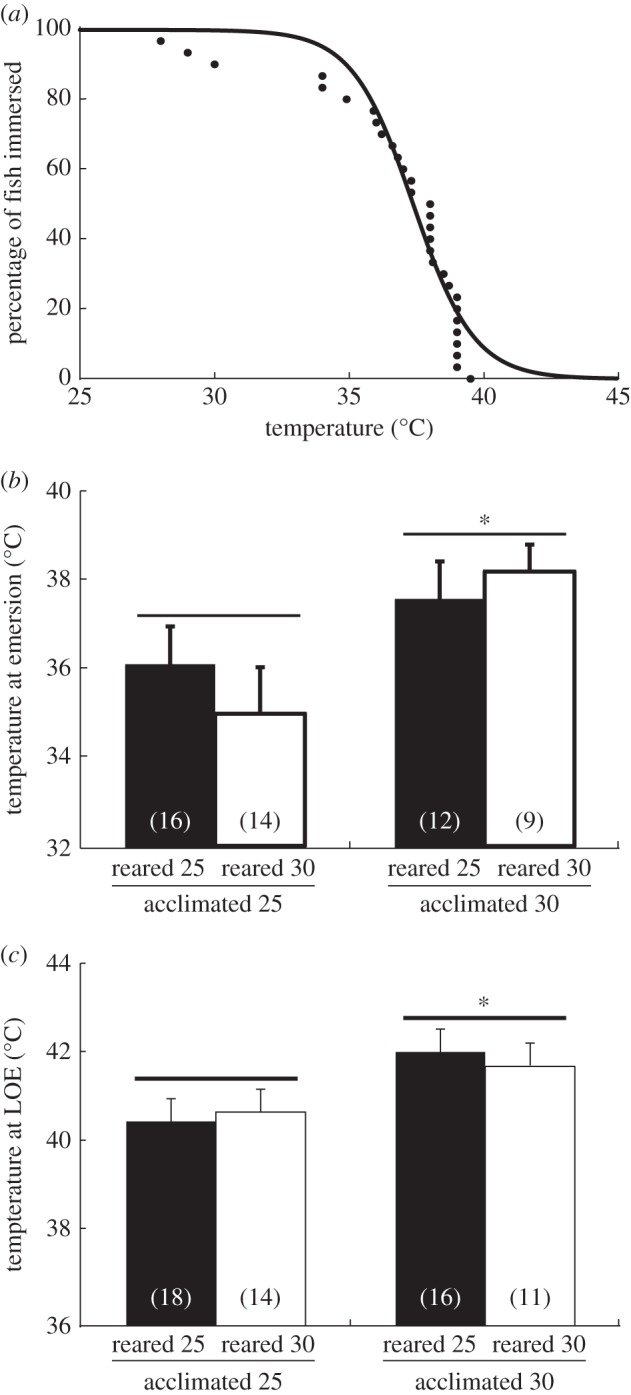
(a) Percentage of immersed (submerged) Kryptolebias marmoratus (control, reared and acclimated to 25°C) when exposed to rising water temperatures (approx. 1°C min−1). The line of best fit follows the sigmoidal curve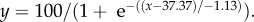 (b) Emersion temperatures and (c) loss of equilibrium (LOE) temperatures of K. marmoratus reared or acclimated to 25 or 30°C. Significant differences are indicated by an asterisk (means ± s.e.). Sample sizes are given in parentheses.
(b) Emersion temperatures and (c) loss of equilibrium (LOE) temperatures of K. marmoratus reared or acclimated to 25 or 30°C. Significant differences are indicated by an asterisk (means ± s.e.). Sample sizes are given in parentheses.
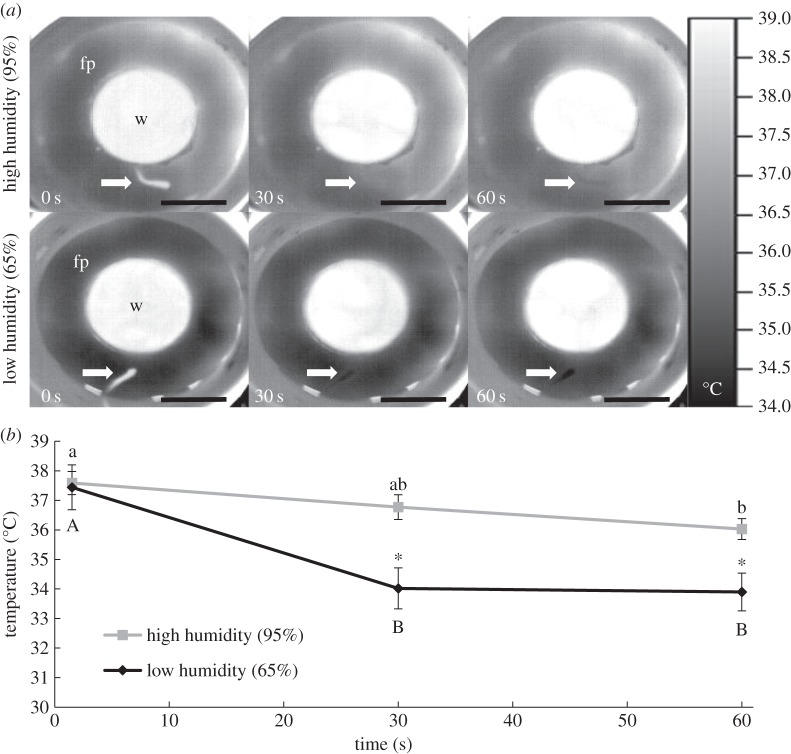
Emersed fish quickly cooled; by 30 s, it was difficult to distinguish fish from background in thermal images (figure 2a). By 60 s, the fish appeared to be slightly cooler than the surrounding filter paper, a difference that was more pronounced at low RH. The surface temperature of fish decreased significantly in both treatments, but cooled faster under low RH (p < 0.001; figure 2b). Terrestrial activity (jumps) post-emersion was not affected by RH (6.0 ± 2.8 at low RH versus 3.8 ± 1.4 at high RH, each n = 7, p > 0.05). In the field, RH immediately above crab burrows occupied by mangrove rivulus was 83.8 ± 1.5% (range 79–92%).
Figure 2.
(a) Representative thermal images showing the decrease in temperature of Kryptolebias marmoratus (arrows) after 30 and 60 s of emersion. W, water; fp, filter paper. Scale bar, 5 cm. (b) Mean (±s.e.) body temperature of emersed fish in high and low humidity treatments (each n = 7). Starting water temperature was 25°C and air temperature was constant at 30°C. Different letters indicate significant differences within a treatment over time (p < 0.001). Asterisks denote significant differences between treatments (p < 0.01).
4. Discussion
Kryptolebias marmoratus reap the benefits of an amphibious lifestyle because they can escape stressful thermal conditions through emersion and evaporatively cool to prevent overheating. In the field, RH was below 100% (and between our test conditions of 65–95% RH) and therefore body heat would be lost to the environment in the wild through evaporation even if water and air temperatures were similar. Fish appeared cooler than the substrate, suggesting that evaporative cooling was more rapid than conductive loss of heat through direct contact. Although emersion appears to be a thermoregulatory avoidance response, we found no evidence to suggest that fish continued to actively thermoregulate by jumping to increase convection. Evidence for behavioural thermoregulation has been reported in amphibians that move to cooler environments to reduce water loss under arid conditions [16]. Behavioural thermoregulation in amphibious fishes was suspected in earlier studies [17,18], but ours, to our knowledge, is the first direct evidence.
Our results demonstrate that emersion behaviour is reversibly plastic in adult K. marmoratus. Increased emersion temperatures after acclimation to 30°C were associated with an increased acute thermal tolerance, suggesting a parallel response in behaviour and physiological traits. The ability for K. marmoratus to safely remain in relatively warm water after acclimation would allow fish to continue to forage for aquatic prey and avoid terrestrial predators.
Interestingly, we found no evidence of irreversible plasticity in acute thermal tolerance as has been reported in other fishes [12,19]. Plasticity acts at many levels of biological organization, often interactively, and thus it is possible that different developmental temperatures irreversibly altered some physiological traits in K. marmoratus but these are hidden by other, reversible phenotypes [11,20]. Overall, our results suggest that amphibious fishes may be at an advantage during peak aquatic temperatures, as behavioural avoidance by emersion and subsequent rapid evaporative cooling provide a mitigation strategy unavailable to fully aquatic species.
Supplementary Material
Supplementary Material
Acknowledgements
We thank Dr Fred Laberge and two anonymous reviewers for helpful comments, Irene Yin-Liao for experimental work, Laurie J. MacNevin for editorial assistance and Lori Ferguson for typographical help.
Ethics
Experiments were approved by the University of Guelph Animal Care Committee (no. 2239).
Data accessibility
Data have been uploaded as the electronic supplementary material.
Authors' contributions
D.G., E.S., A.T., G.T. and P.W. designed the experiments. D.G. and E.S. carried out the work. D.G. and P.W. wrote the draft manuscript. D.G., E.S., A.T., G.T. and P.W. edited the manuscript. All authors approve the final version and agree to be held accountable for all aspects of the work.
Competing interests
We have no competing interests.
Funding
This research was supported by Natural Sciences and Engineering Council of Canada Discovery grants to P.A.W. and G.J.T. A.J.T. was supported by an NSERC PhD scholarship.
References
- 1.Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LEB, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320, 800–803. ( 10.1126/science.1157174) [DOI] [PubMed] [Google Scholar]
- 2.Reed TE, Schindler DE, Waples RS. 2010. Interacting effects of phenotypic plasticity and evolution on population persistence in a changing climate. Conserv. Biol. 25, 56–63. ( 10.1111/j.1523-1739.2010.01552.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sayer MDJ. 2005. Adaptations of amphibious fish for surviving life out of water. Fish Fish. 6, 186–211. ( 10.1111/j.1467-2979.2005.00193.x) [DOI] [Google Scholar]
- 4.Piersma T, van Gils JA. 2011. The flexible phenotype: a body-centred integration of ecology, physiology, and behaviour, 256 p. New York, NY: Oxford University Press. [Google Scholar]
- 5.Auld JR, Agrawal AA, Relyea RA. 2009. Re-evaluating the costs and limits of adaptive phenotypic plasticity. Proc. R. Soc. B 277, 503–511. ( 10.1098/rspb.2009.1355) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateson P, Gluckman P, Hanson M. 2014. The biology of developmental plasticity and the predictive adaptive response hypothesis. J. Physiol. 592, 2357–2368. ( 10.1113/jphysiol.2014.271460) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer K, Eenhoorn E, Bot ANM, Brakefield PM, Zwaan BJ. 2003. Cooler butterflies lay larger eggs: developmental plasticity versus acclimation. Proc. R. Soc. Lond. B 270, 2051–2056. ( 10.1098/rspb.2003.2470) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krstevska B, Hoffmann A. 1994. The effects of acclimation and rearing conditions on the response of tropical and temperate populations of Drosophila melanogaster and D. simulans to a temperature gradient (Diptera: Drosophilidae). J. Insect Behav. 7, 279–288. ( 10.1007/BF01989735) [DOI] [Google Scholar]
- 9.Terblanche JS, Chown SL. 2006. The relative contributions of developmental plasticity and adult acclimation to physiological variation in the tsetse fly, Glossina pallidipes (Diptera, Glossinidae). J. Exp. Biol. 209, 1064–1073. ( 10.1242/jeb.02129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seebacker F, Grigalchik VS. 2015. Developmental thermal plasticity of prey modifies the impact of predation. J. Exp. Biol. 218, 1402–1409. ( 10.1242/jeb.116558) [DOI] [PubMed] [Google Scholar]
- 11.Burness G, Huard JR, Malcolm E, Tattersall GJ. 2013. Post-hatch heat warms adult beaks: irreversible physiological plasticity in Japanese quail. Proc. R. Soc. B 280, 20131436 ( 10.1098/rspb.2013.1436) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaefer J, Ryan A. 2006. Developmental plasticity in the thermal tolerance of zebrafish Danio rerio. J. Fish Biol. 69, 722–734. ( 10.1111/j.1095-8649.2006.01145.x) [DOI] [Google Scholar]
- 13.Fry FEJ. 1947. Effect of the environment on animal activity. Univ. Toronto Stud. Biol. Ser. 55, 1–62. [Google Scholar]
- 14.Taylor DS. 2012. Twenty-four years in the mud: what have we learned about the natural history and ecology of the mangrove rivulus, Kryptolebias marmoratus? Integr. Comp. Biol. 52, 724–736. ( 10.1093/icb/ics062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beitinger TL, Lutterschmidt WI. 2011. Measures of thermal tolerance. In Encyclopedia of fish physiology: from genome to environment (ed. Farrell AP.), 2272 p. San Diego, CA: Academic Press. [Google Scholar]
- 16.Malvin GM, Wood SC. 1991. Behavioral thermoregulation of the toad, Bufo marinus: effects of air humidity. J. Exp. Zool. 326, 322–326. ( 10.1002/jez.1402580307) [DOI] [PubMed] [Google Scholar]
- 17.Graham JB. 1973. Terrestrial life of the amphibious fish Mnierpes macrocephalus. Mar. Biol. 23, 83–91. ( 10.1007/BF00394114) [DOI] [Google Scholar]
- 18.Hendy IW, Eme J, Dabruzzi TF, Nembhard RV, Cragg SM, Bennett WA. 2013. Dartfish use teredinid tunnels in fallen mangrove wood as a low-tide refuge. Mar. Ecol. Prog. Ser. 486, 237–245. ( 10.3354/meps10370) [DOI] [Google Scholar]
- 19.Scott GR, Johnston IA. 2012. Temperature during embryonic development has persistent effects on thermal acclimation capacity in zebrafish. Proc. Natl. Acad. Sci. USA 109, 14 247–14 252. ( 10.1073/pnas.1205012109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulte PM, Healy TM, Fangue NA. 2011. Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr. Comp. Biol. 51, 691–702. ( 10.1093/icb/icr097) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data have been uploaded as the electronic supplementary material.