Abstract
ES-62 is a phosphorylcholine (PC)-containing glycoprotein secreted by the filarial nematode Acanthocheilonema viteae that acts to modulate the host immune response to promote the establishment of chronic helminth infection. Reflecting its anti-inflammatory actions, we have previously reported that ES-62 protects mice from developing Collagen-Induced Arthritis (CIA): thus, as this helminth-derived product may exhibit therapeutic potential in Rheumatoid Arthritis (RA), it is important to understand the protective immunoregulatory mechanisms triggered by ES-62 in this model in vivo. We have established to date that ES-62 acts by downregulating pathogenic Th17/IL-17-mediated responses and upregulating the regulatory cytokine IL-10. In addition, our studies have identified that IL-22, another member of the IL-10 family of cytokines, exerts dual pathogenic and protective roles in this model of RA with ES-62 harnessing the cytokine’s inflammation-resolving and tissue repair properties in the joint during the established phase of disease. Here, we discuss the counter-regulatory roles of IL-22 in the murine model of CIA and present additional novel data showing that ES-62 selectively induces γδ T cells with the capacity to induce IL-22 production and that γδ T cells with the capacity to produce IL-22, but not IL-17, induced during CIA can be identified by their expression of TLR4. Moreover, we also show that treatment of mice undergoing CIA with the active PC moiety of ES-62, in the form of PC conjugated to BSA, is not only sufficient to mimic the ES-62-dependent suppression of pathogenic IL-17 responses shown previously but also that of the IL-22 and IL-10 up-regulation observed with the parasitic worm product during CIA. These findings not only reinforce the potential of IL-22, firstly described as a Th17-related pro-inflammatory cytokine, as a protective factor in arthritis but also suggest that drugs based on the PC moiety found in ES-62 may be able to harness the joint-protecting activities of IL-22 therapeutically.
Introduction
ES-62 is a phosphorylcholine (PC)-containing glycoprotein secreted by the rodent filarial nematode Acanthocheilonema viteae and a homologue of excretory-secretory products that can be detected in the bloodstream of humans infected with Brugia malayi and Onchocerca volvulus [1]. Our early in vitro studies revealed that this parasite-derived molecule exhibits a broad range of immunomodulatory effects, for example the dampening of the pro-inflammatory responses of macrophages and dendritic cells [2, 3], that likely promote persistence of the parasite in the host without severely immunocompromising it. Although its mechanism of action is not yet fully understood, TLR4 is a major target of ES-62 [4] and most of its anti-inflammatory properties depend on its unusual post-translational addition of PC: thus, when PC is chemically linked to an irrelevant protein such as BSA or ovalbumin, as with ES-62, it inhibits full activation of macrophages and DCs following TLR stimulation in a TLR4-dependent manner [5].
In accordance with the Hygiene Hypothesis [6] that has led to the proposal that the recent dramatic increase in allergy and autoimmune diseases in industrialized countries is related to the eradication of parasitic infections, a wide range of studies have shown infection with helminths to be protective against such disorders in experimental models, suggesting that such parasites or their immunomodulatory products could be exploited therapeutically. Reflecting this, we have shown that treatment with ES-62, either prophylactically or therapeutically, reduces disease severity and incidence of Collagen-Induced Arthritis (CIA), a murine model of human Rheumatoid Arthritis (RA) [7] and PC-BSA is able to protect mice in a similar fashion to that of ES-62 [8, 9]. An increasing number of studies have shown that helminths and their secreted products exert their anti-inflammatory effects by actively modulating host immune effector mechanisms, for example, by reducing aberrant Th1 or Th2 responses and up-regulating IL-10-dependent regulatory pathways [10-12]. Indeed, some of these actions have been associated with the mechanism of action underlying ES-62-mediated protection in CIA. Thus, protection was first associated with suppressed Th1 responses, reduced levels of collagen-specific IgG2a antibodies and increased IL-10 production [7, 13]. However, ES-62 failed to protect against other Th1-driven models of disease [14, 15], suggesting that alternative pathways might be targeted in CIA. Indeed, ES-62-mediated protection was subsequently shown to be associated with downregulation of IL-17 production, a finding consistent with increasing evidence that this cytokine is a major pathogenic driver in RA as IL-17 induces hyperplasia and proliferation of synoviocytes, production of pro-inflammatory mediators and promotion of TLR-dependent responses and recruitment of immune system cells including T cells, B cells and neutrophils [16-18]. Interestingly, ES-62 down-regulates IL-17 production by a multi-pronged mechanism, inhibiting Th17 polarization both directly and indirectly, the latter via the suppression of IL-6 and IL-23 release by DCs, key cytokines in DC-priming of Th17 differentiation. ES-62 also targets DCs to modulate the cross-talk between γδ T cells and DCs, resulting in reduced levels of IL-17 production by the innate lymphocytes. Collectively, these actions result in downregulated expression of IL-17 in the joints of mice with CIA and reduced synovial inflammation [19]. Importantly, as biologics targeting IL-17 have resulted in some opportunistic fungal infections under clinical trial, IL-17-producing NK cells which have been shown to be protective in such infections remain unaffected in ES-62-treated mice undergoing CIA [20]. Thus ES-62 attenuates IL-17-mediated responses without fully inhibiting them, as a result suppressing pathogenic, but not infection-combating sources of IL-17 and these findings may go some way to explaining why helminth infections or ES-62-based treatments do not substantially immunocompromise the host.
More recently, we have investigated the effect of ES-62 on another component of the IL-23/IL-17 inflammatory axis in autoimmune arthritis, namely IL-22 which had also been reported to be a pro-inflammatory cytokine in RA [21, 22]. Consistent with this, we showed that neutralisation of IL-22 prior to onset of disease blocks joint pathology. However, we have also shown that IL-22 is required for ES-62’s anti-arthritic effects, via a mechanism in which IL-22-signalling desensitizes synovial fibroblast (SF) responses to pro-inflammatory factors such as IL-17 in the joint [23]. As a result, SFs produce less IL-6 and the number of neutrophils infiltrating the joints is significantly reduced. These findings are consistent with increasing evidence suggesting that IL-22 can play dual pathogenic and, reflecting its wound healing properties [24], protective roles in RA. It is therefore essential to fully understand the role(s) of IL-22 in the pathogenesis of arthritis and the resolution of inflammation and protection against joint damage induced by the helminth product in order to translate these potentially therapeutic actions to the clinic. The aim of this report is therefore to provide further insight into the sources of IL-22 modulated by ES-62 in mice with CIA and in particular we show that a subset of γδ T cells that express TLR4 in vivo is a major potential producer of such protective IL-22. In addition, we also report that PC-BSA mimics the ability of ES-62 to up-regulate IL-22 production during protection from CIA: thus as we have recently provided proof of principle that small drug-like PC-based compounds can be protective in mouse models of arthritis and asthma [9, 25], this latter finding highlights the novel potential of parasite-derived products to harness the therapeutic properties of IL-22 in RA.
Material and Methods
CIA
Animals were maintained in the Biological Services Units in accordance with the Home Office UK Licences PPL60/3580, PPL60/3119 and PIL60/12183 and the Ethics Review Boards of the Universities of Glasgow and Strathclyde. CIA was induced in male DBA/1 mice (8-10 weeks old; Harlan Olac; Bicester, UK) by intradermal immunization with bovine type II collagen (CII, MD Biosciences) in complete Freund’s adjuvant (FCA) and mice were treated with purified endotoxin-free ES-62 from A. viteae or PC-BSA (2 μg/dose) or PBS subcutaneously on days −2, 0 and 21 [7, 8]. Mice were monitored for clinical symptoms of arthritis: severity scores represent 0=normal; 1=erythema; 2=erythema plus swelling; 3=extension of swelling; 4=loss of function, and the disease score is the sum for the four limbs.
Ex vivo analysis
DLN cells (106/ml) were stimulated in the presence and absence of PMA (50 ng/ml) plus ionomycin (500 ng/ml) for 1 h before being incubated with 10 μg/ml Brefeldin A (Sigma-Aldrich, UK) for 5 h at 37°C with 5% CO2. Live cells were discriminated by the LIVE/DEAD fixable aqua dye (BioLegend, UK) and phenotypic markers labelled using FITC-conjugated anti-CD49b (BioLegend, UK), APC-conjugated anti-γδ TCR (eBioscience, UK) and biotin-anti TLR4 antibodies before the cells were fixed and permeabilized according to BioLegend protocols. Pacific Blue-conjugated streptavidin was used for detection of biotinylated antibodies. Cytokines were stained using PerCP-Cy5.5–conjugated anti–IL-17A (BioLegend, UK) or PE-conjugated anti–IL-22 antibodies (R&D Systems, UK) for 30 minutes prior to analysis by flow cytometry, with gating according to appropriate isotype controls [19, 23].
Cytokine analysis
Serum levels of IL-22 and IL-10 were determined by ELISA, according to the manufacturers' recommendations ((R&D Systems; Biolegend).
Statistical analysis
Data were analyzed by Student’s unpaired 1-tailed t-test. Articular scores were analysed by the Mann-Whitney test. P values *<0.05 were considered significant.
Results and Discussion
ES-62 differentially regulates IL-22 production by γδ and NK T cells in DLN from mice undergoing CIA
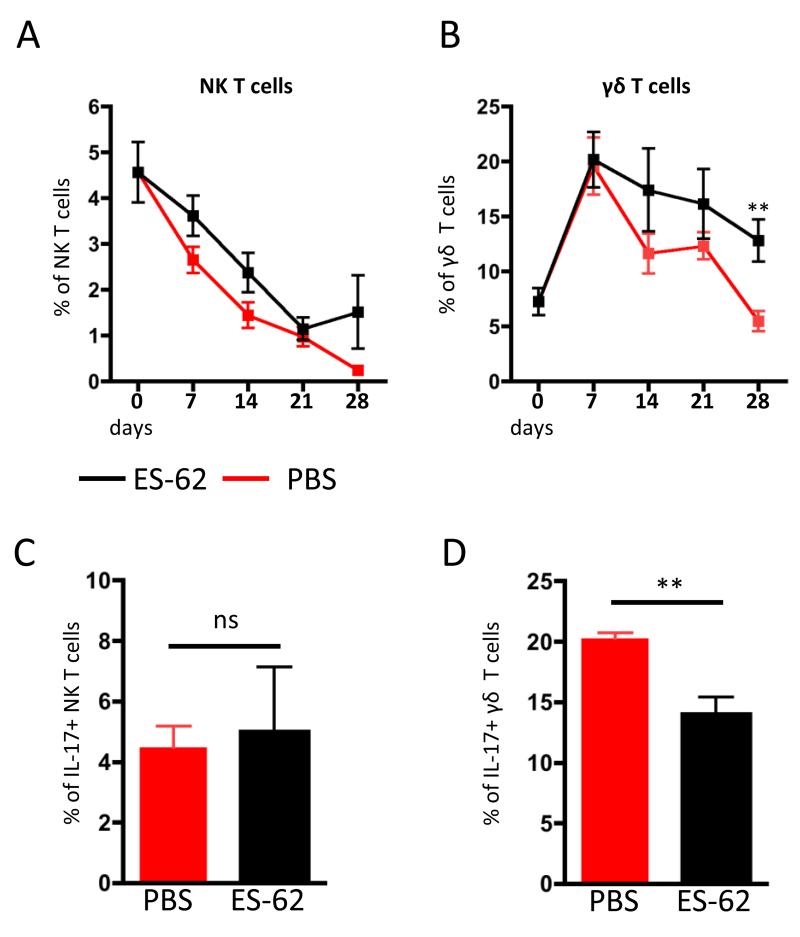
Protection by ES-62 in the CIA model has been associated with counter-regulation of IL-17 and IL-22 levels in serum and joints during the established phase of pathology, with exposure to the parasite product elevating IL-22 but reducing IL-17 levels [19, 23]. Similarly, exposure to ES-62 reduces IL-17, but not IL-22 production by DLN and joint cells ex vivo and analysis revealed these cytokines to be predominantly produced by distinct populations of cells at both sites [19, 23]. Moreover, whilst ES-62 inhibited the levels of both spontaneous and PMA/ionomycin-stimulated IL-17 production by CD4+ DLN cells, it only reduced the proportion of unstimulated CD4+ DLN cells expressing IL-22 [23]. However, although we have shown the parasite product to additionally suppress the levels of IL-17+ γδ T cells whilst leaving the levels of IL-17-producing NK cells unaffected [26], the effects of ES-62 on IL-22 production by these subsets of innate lymphocytes was not investigated. We therefore decided to evaluate this in order to potentially identify a source of protective IL-22 production in the DLN during CIA progression (Fig.1). Consistent with our previous observations that exposure to ES-62 appeared to have little effect on the levels of IL-22-producing DLN cells [23], whilst kinetic analysis suggested that the proportion of both NK and γδ T cells expressing IL-22 was elevated in those DLN from ES-62 exposed mice undergoing CIA following challenge (day 21; Fig. 1A & B), this only reached significance for γδ T cells (at d28; Fig. 1B). By contrast, the proportion of IL-17+ γδ but not NK IL-17+ T cells was reduced at this time point (Fig. 1C & D). These data suggest that γδ T cells, which by bridging innate and adaptive immunity play important immunoregulatory roles in RA [27], are selectively targeted by ES-62 to modulate the IL-17/IL-22 balance in the CIA model to mediate its protective effects. Interestingly, this kinetic analysis also revealed, consistent with IL-22 playing a pathogenic role during the initiation phase of disease, that the levels of IL-22-producing γδ T cells in the DLN peaked within about 7 days and then generally declined with disease progression in PBS-treated mice undergoing CIA. By contrast, and supporting their proposed protective role, exposure to ES-62 helps maintain the levels of IL-22+ γδ T cells at time points where pathology is normally established in the joint. Although we do not know whether these cells can migrate to the joint and hence are representative of the IL-22-expressing lymphocyte-like cells previously shown to be elevated in ES-62-treated mice at this stage [23], these results could suggest that the observed increase of IL-22 in serum and joints of ES-62-treated mice might be a result of γδ T cell modulation rather than effects on conventional Th22 cells and may correlate with a report that CD4+ T cells isolated from synovial samples from RA patients were not able to produce IL-22 [22].
Figure 1. Kinetic analysis of IL-22-producing NK and γδ T cells in DLN of mice with CIA.
ES-62- and PBS-treated mice undergoing CIA were culled every 7 days (n=4 for each treatment group at each time-point) and IL-22 expression by DLN cells analysed by intracellular flow cytometry. Proportions of spontaneously IL-22-producing, CD49b+ NK T cells (A) and γδ+ TCR+ T cells (B) from individual mice were evaluated as described in the Material and Methods. The proportions of IL-17-producing NK (C) and γδ T cells (D) were also analysed at d28. Red symbols represent CIA mice treated with PBS whilst black symbols represent mice treated with ES-62. Values are the mean ± SEM of 4 individual mice at each time. *p<0.05, **p<0.01.
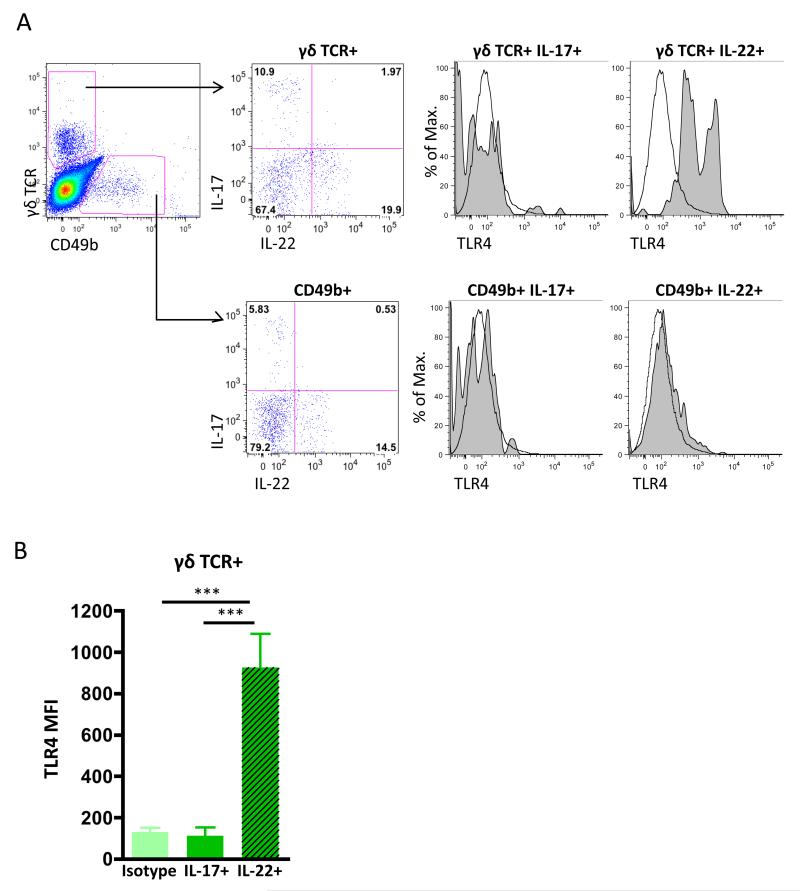
IL-22-producing γδ T cells, but not IL-17-producing γδ T cells, express Toll-like receptor 4 (TLR4)
It is not clear how ES-62 selectively targets γδ but not NK T cells, to render them respectively sensitive or resistant to modulation of IL-17/IL-22 production as both these cell types express pattern recognition receptors (PRR) important in CIA pathogenesis [28], such as TLR2 [29] and ES-62 has been shown to subvert TLR signalling by downregulation of MyD88 in a variety of cell types [4, 19, 30-33]. However, TLR4 is required for ES-62 action and although this PRR is not generally considered to be expressed by either γδ or NK T cells, TLR4+ γδ T cells have been described following burn injury [34] and we have recently reported that a population of these cells, but not NK T cells can be found in the DLN of mice undergoing CIA [20]. Thus, as TLR4 signalling has been reported to promote IL-17 production in γδ T cells isolated from lungs and lymphoid organs [35], we hypothesised that differential expression of this PRR may be involved in the selective regulation of IL-17/IL-22 production by γδ T cells and NK T cells in the CIA model. Although analysis of DLN cells from mice with CIA confirmed that TLR4+ γδ T cells did not express IL-17 [20, 28], perhaps surprisingly, but consistent with our previous findings that IL-17 and IL-22 were predominantly expressed by distinct populations of DLN cells, we observed a positive correlation between IL-22 expression and TLR4 on γδ, but not NK, T cells (Fig. 2A & B). This is the first observation of IL-22+TLR4+ γδ T cells in the CIA model, and this subset may be analogous to the population of IL-22+IL-17− γδ T cells that has been shown to be protective in models of colon inflammation [36] and lung fibrosis [37]. A similar association between other cell surface markers and cytokine profile expression in γδ T cells has previously been reported in that IL-17+ γδ T cells and IFNγ+ γδ T cells differentially express the markers CD25 and CD122 respectively [38, 39], suggesting that expression of particular cell sensors can dictate functional γδ T cell responses and thus, phenotyping may allow the possibility of distinguishing potentially protective IL-22+ γδ T cells from pathogenic IL-17+ γδ T cells. Whether ES-62 can induce “protective” IL-22+ γδ T cells via TLR4 signalling and/or differentially and temporally modulate IL-17 and IL-22 production by distinct subsets of γδ T cells is currently under investigation in our laboratory. Thus, although whether ES-62 acts via TLR4 to modulate IL-22 expression by γδ T cells during CIA is still unclear, it is an attractive hypothesis in terms of allowing selective modulation of the immune response given that we have previously shown that ES-62-mediated downregulation of IL-17 production by γδ T cells reflects inhibition of their activation by DCs [23] and that NK T cells, which do not express TLR4, are not affected by ES-62 in terms of either IL-17 or IL-22 production [20] (Fig. 1).
Figure 2. TLR4 expression by γδ T cells is associated with their production of IL-22 in mice with Collagen-Induced Arthritis.
Representative plots of the gating strategy for analysis of intracellular IL-17 and IL-22 expression by γδ TCR+ and CD49b+ NK T cells in the DLN from a single CIA mouse following ex vivo stimulation with PMA/ionomycin are shown (A). Expression of TLR4 was determined according to appropriate isotype controls (tinted grey histograms) in γδ T cells and CD49b+ NK T cells respectively. Data represent Mean Fluorescence Intensity (MFI) ± SD of DLN cells (B) from 5 individual mice (***p<0.001) undergoing CIA from a single experiment representative of two independent experiments
Phosphorylcholine (PC) mimics the ability of ES-62 to increase IL-22 levels in the bloodstream of CIA animals
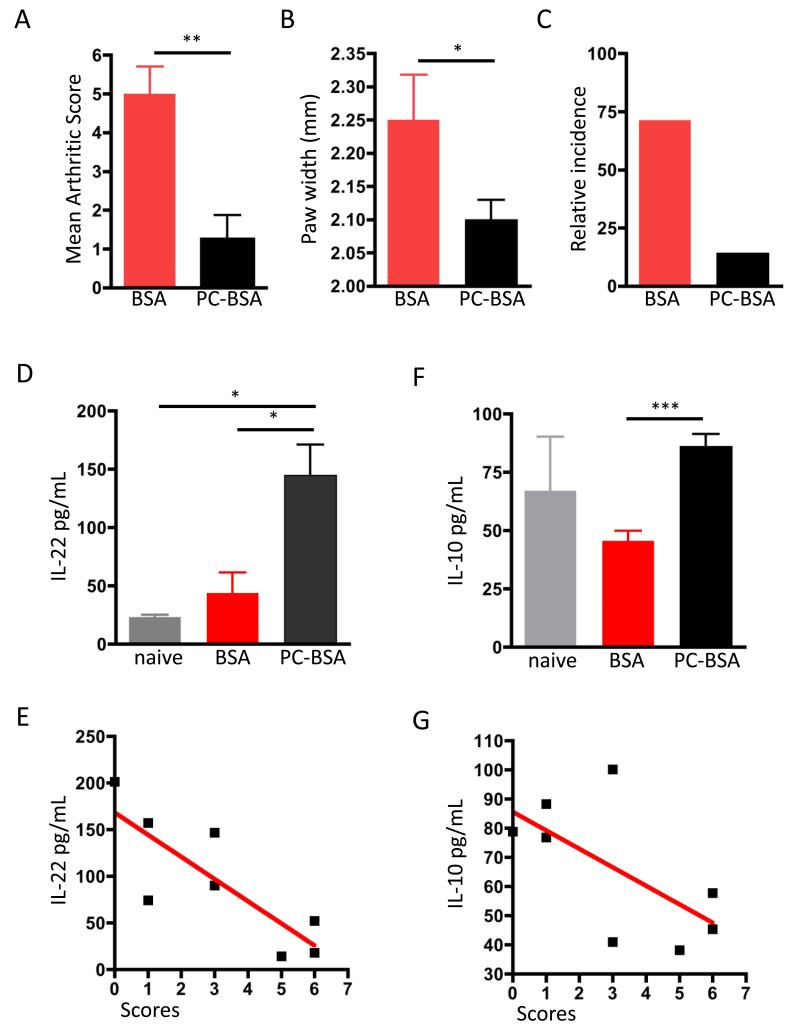
Collectively, our data indicate that ES-62 can selectively modify critical regulatory IL-17/IL-22 pathways in the CIA model. Of note, therapies based on its mode of action promoting resetting of the protective: pathogenic T cell effector balance of this inflammatory axis that focus on the differential suppression of IL-17/IL-22-producing innate cell populations are perhaps less likely to leave the host open to opportunistic fungal infections, than current strategies involving novel biological agents like monoclonal IL-17 neutralizing antibodies. This new generation of immunomodulators is in phase II clinical trials (secukinomab [40], ixekizumab [41] and brodalumab [42]), but already it seems clear that more studies are needed to establish their long-term safety as some reports showed increased rates of such infection concomitant with treatment [43-45]. ES-62 thus presents certain advantages as a potential anti-arthritic treatment: reduced risk of immunosuppression since IL-17-driven responses are limited but not abrogated, regulation of a complex network of cells rather than a key regulatory molecule and the additional ability to trigger damage repair mechanisms, such as IL-22-mediated pathways of inflammation resolution and wound healing. However, as a large potentially immunogenic protein, ES-62 still presents important limitations preventing its translation into the clinic. Nevertheless, new ES-62-based interventional approaches can still be developed as the bioactive PC moiety, when conjugated to an “inert” carrier protein such as BSA, can also protect mice against CIA (Fig. 3A-B). Indeed, studies involving PC-BSA were the starting point for our development of small molecular analogues (SMAs) that mimic the anti-inflammatory ability of ES-62 in targeting pathogenic IL-17 responses in mouse models of arthritis and asthma [9, 25] and provide prof of concept for helminth-based drugs in inflammatory disease.
Figure 3. PC-BSA protection against CIA is associated with upregulation of serum IL-22 and IL-10.
Mice undergoing CIA were treated with BSA (n=4) or PC-BSA (n=4). Arthritis scores (A) at day 32 after initial collagen injections are shown, expressed as mean scores ± SEM for BSA- or PC-BSA-treatment groups. Disease incidence is also shown (B), indicated by the % of mice developing a severity score ≥2. ** = p< 0.01. IL-22 (C & E) and IL-10 (D & F) levels were evaluated by ELISA in serum of naïve (n=3), BSA- (n=4) and PC-BSA-treated (n=4) individual mice and plotted as mean values of triplicate analyses (C & D). *p<0.05 and ***p<0.001. The correlations between IL-22 (E; n = 8, r = 0.6726, p = 0.0127) and IL-10 (F; n = 8, r = 0.406, p = 0.088) serum concentrations for all mice undergoing CIA (BSA- and PC-BSA-treated groups) and clinical scores are shown (E & F).
Although protection by PC-BSA against CIA also reflects down-regulation of pathogenic IL-17-dependent responses [9], our earlier studies suggested that PC does not necessarily reproduce all of the potentially protective effects of ES-62. For example, whilst PC conjugated to ovalbumin (PC-OVA) also ameliorated joint pathology in CIA models, it did not reduce the levels of potentially pathogenic anti-collagen IgG2a antibodies that are additionally decreased in ES-62-treated mice [8]. Therefore, we decided to investigate whether PC-BSA-mediated protection against CIA also reflected the up-regulation of IL-22 required for protection by ES-62 [23]. Interestingly therefore, whilst as we have shown previously, CIA mice presented elevated levels of IL-22 in serum compared to naïve mice [23], it was found that such PC-BSA-treated mice exhibit significantly higher levels of IL-22 (3.5-fold increase, Fig. 3C) and these serum levels of IL-22 inversely correlate with the clinical scores of the mice with CIA (Fig. 3E). Taken together, these findings corroborate our previous results observed with ES-62 and further support our hypothesis that the high levels of IL-22 observed in the serum of such mice during established disease, reflect the resolution of inflammation and joint damage properties of this cytokine in the CIA model [23].
Increased levels of IL-22 are also associated with elevated levels of IL-10 in serum obtained from PC-BSA-treated CIA mice
We have previously shown that protection against CIA in mice afforded by ES-62 is associated not only with inflammation-resolving IL-22 responses, but also with elevated splenic IL-10 production [7] which may reflect restoration of the levels of B cells with potential (IL-10-dependent) regulatory capacity [13]. Interestingly, therefore, a recent study has suggested that IL-22-mediated amelioration of CIA severity is associated with increased levels of IL-10 [46]. Moreover, IL-22 has also recently been reported to suppress experimental autoimmune uveitis via generation of IL-10-producing regulatory CD11b+ antigen presenting cells [47]. Therefore, we decided to evaluate the levels of IL-10 in serum of PC-BSA-treated mice, to determine whether there was concomitant up-regulation of IL-22 and IL-10 levels, as observed with ES-62. Indeed, treatment with PC-BSA was found to restore the levels of serum IL-10 which had tended to be down-regulated in CIA mice to those observed in naive mice (Fig. 3D): moreover, and consistent with a protective role for this cytokine, such IL-10 levels also inversely correlated with CIA articular disease scores (Fig. 3F). These results suggest that PC-BSA is able to reproduce the ability of ES-62 to promote IL-10 production in the CIA model [8]: although the cell type responsible for such IL-10 production is still unknown, it is possible that PC-BSA promotes IL-10-producing regulatory B cells, rather than regulatory T cells, as with ES-62 in CIA mice [13]. Mechanistically, since neutralizing anti-IL-10 antibodies were shown to abrogate the protective effect of IL-22 in CIA [46], such restoration of IL-10 levels by PC-BSA might impact on DC-dependent priming of Th17 and IL-17-producing γδ T cells, consistent with the reduced levels of IL-17 observed in serum of PC-BSA-treated CIA mice [9]. Thus understanding the mechanisms responsible for controlling cytokine networks such as those resulting in the ES-62-mediated counter-regulation of IL-10/IL-22/IL-17 responses (Summarized in Fig. 4), rather than the analysis of the individual cytokines, may clarify the contradictory data relating to, for example, whether IL-22 is a pro- or anti-inflammatory mediator in RA.
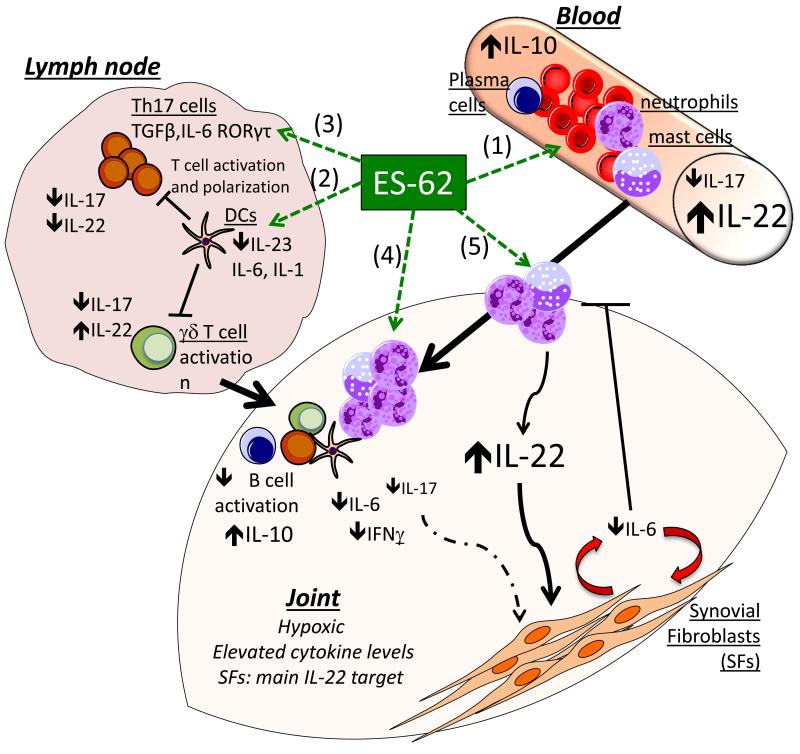
Fig. 4. Protective effects of ES-62 observed in the CIA model.
ES-62 targets a complex cell network to regulate cytokine production and effector responses in the joint of CIA mice. In serum, ES-62/PC-BSA up-regulates IL-10 and IL-22, but strongly down-regulates IL-17 (1). In draining lymph nodes, IL-17 production by γδ T cells and conventional CD4 T cells, is downregulated by ES-62 via modulation of DC responses (2). This is reflected in vitro by ES-62 targeting DCs to down-regulate TLR-mediated production of IL-23, IL-6 and IL-1, resulting in reduced Th17 priming and inhibited induction of IL-17-producing γδ T cells. In addition, ES-62 directly inhibits IL-1-dependent differentiation of Th17 in vitro by downregulation of MyD88 (3). Analysis of joint cells by flow cytometry and immunohistochemistry (4) revealed that at the site of inflammation, ES-62 down-regulates the levels of antibody producing B cells and restores the levels of IL-10 producing B cells. In addition, the IL-17/IL-22 balance is reset with protective IL-22 responses desensitising synovial fibroblasts to IL-17, further suppressing IL-17-dependent inflammatory pathways, including the IL-6 production by synoviocytes and the subsequent recruitment of pro-inflammatory cells like neutrophils (5). Furthermore, the residual neutrophils infiltrating the joint exhibit a modulated phenotype and produce higher amounts of IL-22 (and lower levels of IL-17/IFNγ and IL-6), to perpetuate the protective mechanisms of ES-62 in the joint.
Concluding Remarks
IL-22 was originally described as a pro-inflammatory mediator in RA. This reflects findings that mice deficient in this cytokine were resistant to developing CIA [48] as well as reports that IL-22 levels and Th22 cells are elevated in the serum of RA patients [21] and also that IL-22 has been shown to induce proliferation of human SF and promote RANKL production and osteoclastogenesis in vitro [49]. However, whilst IL-22 levels are up-regulated in RA patients and IL-22 has been shown to be involved in bone damage and RANKL production, certain studies report that such IL-22 upregulation does not contribute to the induction of synovial inflammation [21, 49-51]. Moreover, supporting our proposal of dual pathogenic and protective roles for IL-22 in RA based on the protective action of ES-62 in CIA [23], recent studies have also shown that IL-22 can play protective roles in CIA, via enhancing IL-10-mediated immunoregulation [46] and, as with ES-62, desensitizing SFs to IL-17 [52]. Collectively, these data suggest that the precise role of IL-22 in RA - protective versus pathogenic - probably depends on the inflammatory context. This is likely to especially be the case with respect to its differential effects on SFs, which in RA are subject to a complex pro-inflammatory cocktail involving cytokines, chemokines and alarmins generated by the hypoxic conditions pertaining in the arthritic joint. These results therefore question the hypothesis that serum IL-22 levels provide a pathogenic biomarker in established RA and may even suggest that IL-22 is upregulated in a homeostatic mechanism to limit and resolve joint inflammation and promote tissue repair. In any case, they dictate that therapies targeting IL-22 should be reconsidered while the role of the cytokine in the initiation and progression of synovial inflammation in RA awaits full understanding.
Acknowledgements
This work was supported by the Wellcome Trust, grant number 086852/Z/08/Z.
List of abbreviations used
- RA
Rheumatoid Arthritis
- CIA
Collagen-Induced Arthritis
- DC
Dendritic Cell
- IL-22
Interleukin 22
- IL-17
Interleukin 17
- IL-10
Interleukin 10
- NK cell
Natural Killer cell
- TLR4
Toll-Like Receptor 4
- PKCα
Protein kinase C α
- IFNγ
Interferon γ
- LPS
Lipopolysaccharide
- RANKL
Receptor activator of nuclear factor kappa-B ligand
- BSA
Bovine Serum Albumin
- DLN
Draining Lymph Node
Footnotes
Disclosures
The authors have no conflict of interest.
References
- 1.Goodridge HS, Stepek G, Harnett W, Harnett MM. Signalling mechanisms underlying subversion of the immune response by the filarial nematode secreted product ES-62. Immunology. 2005;115:296–304. doi: 10.1111/j.1365-2567.2005.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodridge HS, Wilson EH, Harnett W, Campbell CC, Harnett MM, Liew FY. Modulation of macrophage cytokine production by ES-62, a secreted product of the filarial nematode Acanthocheilonema viteae. J Immunol. 2001;167:940–945. doi: 10.4049/jimmunol.167.2.940. [DOI] [PubMed] [Google Scholar]
- 3.Whelan M, Harnett MM, Houston KM, Patel V, Harnett W, Rigley KP. A filarial nematode-secreted product signals dendritic cells to acquire a phenotype that drives development of Th2 cells. J Immunol. 2000;164:6453–6460. doi: 10.4049/jimmunol.164.12.6453. [DOI] [PubMed] [Google Scholar]
- 4.Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, et al. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol. 2005;174:284–293. doi: 10.4049/jimmunol.174.1.284. [DOI] [PubMed] [Google Scholar]
- 5.Goodridge HS, Marshall FA, Wilson EH, Houston KM, Liew FY, Harnett MM, et al. In vivo exposure of murine dendritic cell and macrophage bone marrow progenitors to the phosphorylcholine-containing filarial nematode glycoprotein ES-62 polarizes their differentiation to an anti-inflammatory phenotype. Immunology. 2004;113:491–498. doi: 10.1111/j.1365-2567.2004.01993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McInnes IB, Leung BP, Harnett M, Gracie JA, Liew FY, Harnett W. A novel therapeutic approach targeting articular inflammation using the filarial nematode-derived phosphorylcholine-containing glycoprotein ES-62. J Immunol. 2003;171:2127–2133. doi: 10.4049/jimmunol.171.4.2127. [DOI] [PubMed] [Google Scholar]
- 8.Harnett MM, Kean DE, Boitelle A, McGuiness S, Thalhamer T, Steiger CN, et al. The phosphorycholine moiety of the filarial nematode immunomodulator ES-62 is responsible for its anti-inflammatory action in arthritis. Ann Rheum Dis. 2008;67:518–523. doi: 10.1136/ard.2007.073502. [DOI] [PubMed] [Google Scholar]
- 9.Al-Riyami L, Pineda MA, Rzepecka J, Huggan JK, Khalaf AI, Suckling CJ, et al. Designing anti-inflammatory drugs from parasitic worms: a synthetic small molecule analogue of the Acanthocheilonema viteae product ES-62 prevents development of collagen-induced arthritis. Journal of medicinal chemistry. 2013;56:9982–10002. doi: 10.1021/jm401251p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harnett W. Secretory products of helminth parasites as immunomodulators. Mol Biochem Parasitol. 2014;195:130–136. doi: 10.1016/j.molbiopara.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Maizels RM, Yazdanbakhsh M. Immune regulation by helminth parasites: cellular and molecular mechanisms. Nat Rev Immunol. 2003;3:733–744. doi: 10.1038/nri1183. [DOI] [PubMed] [Google Scholar]
- 12.McSorley HJ, Hewitson JP, Maizels RM. Immunomodulation by helminth parasites: defining mechanisms and mediators. Int J Parasitol. 2013;43:301–310. doi: 10.1016/j.ijpara.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers DT, Pineda MA, McGrath MA, Al-Riyami L, Harnett W, Harnett MM. Protection against collagen-induced arthritis in mice afforded by the parasitic worm product, ES-62, is associated with restoration of the levels of interleukin-10-producing B cells and reduced plasma cell infiltration of the joints. Immunology. 2014;141:457–466. doi: 10.1111/imm.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Riyami L, Egan CA, Bradley JE, Lustigman S, Harnett W. Failure of ES-62 to inhibit T-helper type 1 responses to other filarial nematode antigens. Parasite Immunol. 2008;30:304–308. doi: 10.1111/j.1365-3024.2008.01024.x. [DOI] [PubMed] [Google Scholar]
- 15.Al-Riyami L, Wilson EH, Watson CA, Harnett W. T-helper type 1 responses to the BCG vaccine component PPD in mice are unaffected by the filarial nematode immunomodulatory molecule ES-62. J Parasitol. 2009;95:1201–1204. doi: 10.1645/GE-2017.1. [DOI] [PubMed] [Google Scholar]
- 16.van Hamburg JP, Asmawidjaja PS, Davelaar N, Mus AM, Colin EM, Hazes JM, et al. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 17.Hu F, Li Y, Zheng L, Shi L, Liu H, Zhang X, et al. Toll-like receptors expressed by synovial fibroblasts perpetuate Th1 and th17 cell responses in rheumatoid arthritis. PLoS One. 2014;9:e100266. doi: 10.1371/journal.pone.0100266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parsonage G, Filer A, Bik M, Hardie D, Lax S, Howlett K, et al. Prolonged, granulocyte-macrophage colony-stimulating factor-dependent, neutrophil survival following rheumatoid synovial fibroblast activation by IL-17 and TNFalpha. Arthritis Res Ther. 2008;10:R47. doi: 10.1186/ar2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pineda MA, McGrath MA, Smith PC, Al-Riyami L, Rzepecka J, Gracie JA, et al. The parasitic helminth product ES-62 suppresses pathogenesis in collagen-induced arthritis by targeting the interleukin-17-producing cellular network at multiple sites. Arthritis Rheum. 2012;64:3168–3178. doi: 10.1002/art.34581. [DOI] [PubMed] [Google Scholar]
- 20.Pineda MA, Al-Riyami L, Harnett W, Harnett MM. Themed Issue 2014: Lessons from helminth infections: ES-62 highlights new interventional approaches in rheumatoid arthritis. Clin Exp Immunol. 2014;177:13–23. doi: 10.1111/cei.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leipe J, Schramm MA, Grunke M, Baeuerle M, Dechant C, Nigg AP, et al. Interleukin 22 serum levels are associated with radiographic progression in rheumatoid arthritis. Ann Rheum Dis. 2011;70:1453–1457. doi: 10.1136/ard.2011.152074. [DOI] [PubMed] [Google Scholar]
- 22.Ikeuchi H, Kuroiwa T, Hiramatsu N, Kaneko Y, Hiromura K, Ueki K, et al. Expression of interleukin-22 in rheumatoid arthritis: potential role as a proinflammatory cytokine. Arthritis Rheum. 2005;52:1037–1046. doi: 10.1002/art.20965. [DOI] [PubMed] [Google Scholar]
- 23.Pineda MA, Rodgers DT, Al-Riyami L, Harnett W, Harnett MM. ES-62 protects against collagen-induced arthritis by resetting IL-22 towards resolution of inflammation in the joints. Arthritis Rheumatol. 2014;66(6):1492–503. doi: 10.1002/art.38392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, et al. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–1472. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rzepecka J, Coates ML, Saggar M, Al-Riyami L, Coltherd J, Tay HK, et al. Small molecule analogues of the immunomodulatory parasitic helminth product ES-62 have anti-allergy properties. Int J Parasitol. 2014 doi: 10.1016/j.ijpara.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pineda MA, Al-Riyami Lamyaa., Harnett W, Harnett M. Themed Issue 2014: Lessons from helminth infections: ES-62 highlights new interventional approaches in rheumatoid arthritis. Clin Exp Immunol. 2014;177:13–23. doi: 10.1111/cei.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Su D, Shen M, Li X, Sun L. Roles of gammadelta T cells in the pathogenesis of autoimmune diseases. Clinical & developmental immunology. 2013;2013:985753. doi: 10.1155/2013/985753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 29.Pietschmann K, Beetz S, Welte S, Martens I, Gruen J, Oberg HH, et al. Toll-like receptor expression and function in subsets of human gammadelta T lymphocytes. Scandinavian journal of immunology. 2009;70:245–255. doi: 10.1111/j.1365-3083.2009.02290.x. [DOI] [PubMed] [Google Scholar]
- 30.Pineda MA, Lumb F, Harnett MM, Harnett W. ES-62, a therapeutic anti-inflammatory agent evolved by the filarial nematode Acanthocheilonema viteae. Mol Biochem Parasitol. 2014;194(1-2):1–8. doi: 10.1016/j.molbiopara.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Harnett W, Goodridge HS, Allen JM, Harnett M. Receptor usage by the Acanthocheilonema viteae-derived immunomodulator, ES-62. Exp Parasitol. 2012;132:97–102. doi: 10.1016/j.exppara.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Harnett W, Goodridge HS, Harnett MM. Subversion of immune cell signal transduction pathways by the secreted filarial nematode product, ES-62. Parasitology. 2005;130(Suppl):S63–68. doi: 10.1017/S0031182005008164. [DOI] [PubMed] [Google Scholar]
- 33.Ball DH, Tay HK, Bell KS, Coates ML, Al-Riyami L, Rzepecka J, et al. Mast Cell Subsets and Their Functional Modulation by the Acanthocheilonema viteae Product ES-62. Journal of parasitology research. 2013;2013:961268. doi: 10.1155/2013/961268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwacha MG, Daniel T. Up-regulation of cell surface Toll-like receptors on circulating gammadelta T-cells following burn injury. Cytokine. 2008;44:328–334. doi: 10.1016/j.cyto.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reynolds JM, Martinez GJ, Chung Y, Dong C. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci U S A. 2012;109:13064–13069. doi: 10.1073/pnas.1120585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mielke LA, Jones SA, Raverdeau M, Higgs R, Stefanska A, Groom JR, et al. Retinoic acid expression associates with enhanced IL-22 production by gammadelta T cells and innate lymphoid cells and attenuation of intestinal inflammation. J Exp Med. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simonian PL, Wehrmann F, Roark CL, Born WK, O'Brien RL, Fontenot AP. gammadelta T cells protect against lung fibrosis via IL-22. J Exp Med. 2010;207:2239–2253. doi: 10.1084/jem.20100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas JD, Gonzalez FH, Schmitz S, Chennupati V, Fohse L, Kremmer E, et al. CCR6 and NK1.1 distinguish between IL-17A and IFN-gamma-producing gammadelta effector T cells. Eur J Immunol. 2009;39:3488–3497. doi: 10.1002/eji.200939922. [DOI] [PubMed] [Google Scholar]
- 39.Shibata K, Yamada H, Nakamura R, Sun X, Itsumi M, Yoshikai Y. Identification of CD25+ gamma delta T cells as fetal thymus-derived naturally occurring IL-17 producers. J Immunol. 2008;181:5940–5947. doi: 10.4049/jimmunol.181.9.5940. [DOI] [PubMed] [Google Scholar]
- 40.Genovese MC, Durez P, Richards HB, Supronik J, Dokoupilova E, Mazurov V, et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis. 2013;72:863–869. doi: 10.1136/annrheumdis-2012-201601. [DOI] [PubMed] [Google Scholar]
- 41.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, et al. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 42.Papp KA, Leonardi C, Menter A, Ortonne JP, Krueger JG, Kricorian G, et al. Brodalumab, an anti-interleukin-17-receptor antibody for psoriasis. N Engl J Med. 2012;366:1181–1189. doi: 10.1056/NEJMoa1109017. [DOI] [PubMed] [Google Scholar]
- 43.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61:1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spuls PI, Hooft L. Brodalumab and ixekizumab, anti-interleukin-17-receptor antibodies for psoriasis: a critical appraisal. The British journal of dermatology. 2012;167:710–713. doi: 10.1111/bjd.12025. discussion 714-715. [DOI] [PubMed] [Google Scholar]
- 45.Leonardi C, Matheson R, Zachariae C, Cameron G, Li L, Edson-Heredia E, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190–1199. doi: 10.1056/NEJMoa1109997. [DOI] [PubMed] [Google Scholar]
- 46.Sarkar S, Zhou X, Justa S, Bommireddy SR. IL-22 reduces the severity of collagen arthritis in association with increased levels of IL-10. Arthritis Rheum. 2013;65(4):960–71. doi: 10.1002/art.37849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ke Y, Sun D, Jiang G, Kaplan HJ, Shao H. IL-22-induced regulatory CD11b+ APCs suppress experimental autoimmune uveitis. J Immunol. 2011;187:2130–2139. doi: 10.4049/jimmunol.1100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Geboes L, Dumoutier L, Kelchtermans H, Schurgers E, Mitera T, Renauld JC, et al. Proinflammatory role of the Th17 cytokine interleukin-22 in collagen-induced arthritis in C57BL/6 mice. Arthritis Rheum. 2009;60:390–395. doi: 10.1002/art.24220. [DOI] [PubMed] [Google Scholar]
- 49.Kim KW, Kim HR, Park JY, Park JS, Oh HJ, Woo YJ, et al. Interleukin-22 promotes osteoclastogenesis in rheumatoid arthritis through induction of RANKL in human synovial fibroblasts. Arthritis Rheum. 2012;64:1015–1023. doi: 10.1002/art.33446. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Li JM, Liu XG, Ma DX, Hu NW, Li YG, et al. Elevated Th22 cells correlated with Th17 cells in patients with rheumatoid arthritis. Journal of clinical immunology. 2011;31:606–614. doi: 10.1007/s10875-011-9540-8. [DOI] [PubMed] [Google Scholar]
- 51.van Hamburg JP, Corneth OB, Paulissen SM, Davelaar N, Asmawidjaja PS, Mus AM, et al. IL-17/Th17 mediated synovial inflammation is IL-22 independent. Ann Rheum Dis. 2013;72:1700–1707. doi: 10.1136/annrheumdis-2012-202373. [DOI] [PubMed] [Google Scholar]
- 52.Carrion M, Juarranz Y, Martinez C, Gonzalez-Alvaro I, Pablos JL, Gutierrez-Canas I, et al. IL-22/IL-22R1 axis and S100A8/A9 alarmins in human osteoarthritic and rheumatoid arthritis synovial fibroblasts. Rheumatology (Oxford) 2013;52:2177–2186. doi: 10.1093/rheumatology/ket315. [DOI] [PubMed] [Google Scholar]