Abstract
Trichomonas vaginalis infection is one of the most prevalent sexually transmitted infections in humans and is now recognized as an important cause of infertility in men. There is little information about the effect of extracellular polymeric substances (EPS) from T. vaginalis on sperm, but previous reports do not provide a conclusive description of the functional integrity of the sperm. To investigate the impact of EPS on the fertilizing capacity of sperm, we assessed sperm motility, acrosomal status, hypo-osmotic swelling, and in vitro fertilization rate after incubating the sperm with EPS in vitro using mice. The incubation of sperm with EPS significantly decreased sperm motility, viability, and functional integrity in a concentration and time-dependent manner. These effects on sperm quality also resulted in a decreased fertilization rate in vitro. This is the first report that demonstrates the direct negative impact of the EPS of T. vaginalis on the fertilization rate of sperm in vitro. However, further study should be performed using human sperm to determine if EPS has similar negative impact on human sperm fertilizing capacity in vitro.
Keywords: extracellular polymeric substances, fertilization, sperm, Trichomonas vaginalis
INTRODUCTION
Sexual and reproductive tract infections other than HIV are important global health priorities in their own right, not merely because they can facilitate HIV transmission. Depending on the disease, some untreated genital tract infections can lead to infertility, chronic pain or even death.1 Of the protozoans Trichomonas vaginalis (T. vaginalis) infection has been associated with a 4.7-fold increase in the risk of pelvic inflammatory disease and over half the 248 million new TV infections each year occur in men.2
T. vaginalis, which is responsible for one of the most prevalent sexually transmitted infections in humans, is restricted to the genito-urinary tract. The global incidence of trichomoniasis has been continuously increasing and has exceeded the combined rates of gonorrhea and chlamydia infections.2 Underestimates of trichomoniasis may be related to the asymptomatic nature of the infection and the lack of sensitive and specific diagnostic methods, especially for men.
T. vaginalis infection in women and men, regardless of the presence of symptoms, is now recognized as an important cause of infertility.3,4 In fact, T. vaginalis was found 5 times more often in infertile women than in control subjects3,4 and is probably associated with cervical and tubal factor in female infertility. On the other hand, among asymptomatic infertile men, approximately 4%–8% was found to be infected with T. vaginalis.5 Furthermore, several investigators have noted abnormalities in the sperm parameters of men infected with T. vaginalis, such as a decrease in sperm motility and viability,5,6,7,8,9 and a decline in the percentage of sperm cells with normal morphology.5 Subsequent studies have attempted to establish a mechanism by which these abnormalities arise.7,10 Almost all of the previous studies have concentrated on contact-dependent adhesion of T. vaginalis on sperm cells, whereas the possible consequences of substances secreted by T. vaginalis remain largely unexamined. Little information is available on the effect of the extracellular polymeric substances (EPS) of T. vaginalis on the functional integrity and fertilizing capacity of sperm.
Therefore, we evaluated whether the EPS of T. vaginalis impairs various aspects of fertilization and sperm physiology. To investigate the impact of EPS on the fertilizing capacity of the sperm in vitro, we incubated the sperm with EPS and assessed sperm viability, motility, functional integrity (by means of acrosomal status and the hypo-osmotic swelling [HOS] test) and the in vitro fertilization (IVF) rate.
MATERIALS AND METHODS
Animals
Male and female ICR mice, 8–10 weeks old (male = 30, female =25), were obtained from Samtako Biokorea (Kyunggi, South Korea). The weight range of the mice is 35–40 g for males and 30–35 g for females. All animals were kept under controlled humidity, temperature, and light conditions and were fed standard mouse chow ad libitum. Animal care followed institutional guidelines, and the Hanyang University IACUC approved all procedures involving the animals (HY-IACUC-09-043).
Semen collection and preparation
After exposure of the peritoneal cavity, the cauda epididymis of male mice (n = 30) of proven fertility was removed from each testis and the fat was removed. Then, the cauda epididymes were washed immediately in prewarmed 1 ml of collection medium (Whitten's HEPES-buffered medium), and transferred to a 200 ml drop of human tubal fluid medium (Quinn's Advantage® Fertilization, In vitro Fertilization Inc., Trumbull, Connecticut, USA) containing 10% fetal bovine serum in a 35 mm culture dish equilibrated overnight under embryo-tested mineral oil in a humidified atmosphere with 5% CO2 at 37°C. Sperm were gently squeezed out of the epididymis using a 26-gauge needle, and the residual caudal tissue was discarded. The sperm were then allowed to disperse for 15 min and large aggregations of immotile sperm in the culture drops were removed using a Pasteur pipette under a dissecting microscope.11 Sperm concentration was assessed with a Neubauer-improved counting chamber (Marienfeld Superior, Lauda-Konigshofen, Germany) and aliquots of the sperm suspension were diluted under oil to achieve a specific concentration necessary for the experiment.
Trichomonas vaginalis culture and preparation of the extracellular polymeric substances
Trichomonas vaginalis (T016 isolate; provided by Prof. John F. Alderete [Department of Microbiology, University of Texas Health Science Center at San Antonio, TX, USA]) was grown in trypticase-yeast extract-maltose medium supplemented with 10% heat-inactivated horse serum at 37°C.12 EPS was prepared as follows: T. vaginalis was harvested in log-phase, washed 3 times with cold phosphate buffered saline (PBS, pH 7.4), suspended (107 trichomonads) in 1 ml of Hank's balanced salt solution (HBSS, pH 7.2) (Gibco, NY, USA) and incubated for 1 h at 37°C. Previous reports found that T. vaginalis secreted high concentrations of inflammatory factors after 1 h of incubation,13 and hence we applied the same method to obtain EPS. Following incubation, parasites were pelleted in a microcentrifuge at 10,000 g for 30 min, and the supernatants were passed through a 0.22 μm filter. The supernatants were collected as EPS of T. vaginalis and were either frozen at − 20°C or used directly to assess the impact on sperm.
Sperm viability
Sperm viability was measured to determine the appropriate incubation time and concentrations of EPS to be used in further experiments. To investigate the effects of EPS on sperm, sperm (2 × 106 ml−1) were incubated for 1–3 h at 37°C with various concentrations of EPS (10%, 25%, 50%, 75%, and 100%) diluted in HBSS. Viability tests were based on visual morphology under ×400 magnification, using vital staining with trypan blue; total or partly stained cells were considered dead, and the percentages of live sperm were evaluated. At least 200 sperm cells/smear were counted per group, and at least two smears per aliquot were assessed. The controls consisted of sperm incubated in HBSS without EPS and maintained at 37°C for 1–3 h.
Motility analysis
Based on the results of the analysis of viability, sperm motility was evaluated after the incubation of sperm (2 × 106 ml−1) in 25%, 50%, or 75% of EPS for 2 h. The motility of at least 200 sperm per group was evaluated subjectively using a phase contrast microscope, across five fields at ×400 magnification. A drop of sperm was kept on a prewarmed slide and motility was graded in four categories (Grade A: rapid progressive motility; Grade B: slow or sluggish progressive motility; Grade C: nonprogressive motility; and Grade D: immotility) (Figure 1).14 The assessment was then repeated in a separate 10 μl drop and the proportions of motility grades from those two independent counts were calculated.
Figure 1.
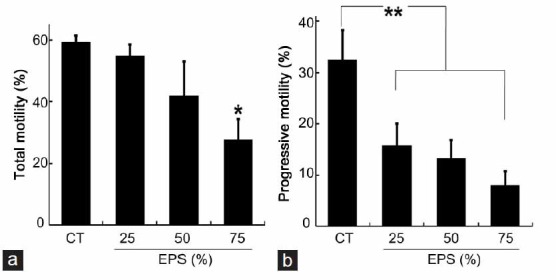
The effect of the EPS of Trichomonas vaginalis on the motility parameters of sperm. (a) The proportion of motile sperm with total motility after incubation for 2 h in 25%, 50%, or 75% of EPS of T. vaginalis diluted in Hank's balanced salt solution (HBSS). (b) The proportion of sperm with progressive motility after incubation for 2 h in 25%, 50%, or 75% EPS diluted in HBSS. Values are the mean ± standard deviation of three separate experiments; 200 cells were counted per treatment per experiment. Total motility: Grade A + B + C; Progressive motility: Grade A + B; Grade A: rapid progressive motility; Grade B: slow or sluggish progressive motility; Grade C: nonprogressive motility; Grade D: immotility; CT: control sperm cells were incubated at 37°C for 2 h in HBSS that did not contain EPS. EPS: extracellular polymeric substances prepared from T. vaginalis. *P < 0.02 compared with CT; **P < 0.04 compared with CT.
Assessment of acrosomal status
Acrosomal status was assessed after the incubating sperm (2 × 106 ml−1) in 25%, 50% or 75% EPS for 2 h. Sperm were air-dried onto glass slides, fixed with 5% paraformaldehyde in PBS (pH 7.4) for 15 min, and washed once with PBS. The slides were stained for 5 min with aqueous 0.25% Coomassie brilliant blue R-250 (Amresco Inc, Solon, Ohio) in 10% glacial acetic acid and 25% methanol. Sperm were then rinsed with water and covered with cover slips under mounting medium (90% glycogen). This method stains the acrosomal cap blue in acrosome-intact sperm, but does not stain the acrosome region in acrosome-reacted sperm.15 Each acrosome status was assessed by counting 200 sperm across five fields twice.
Hypo-osmotic swelling test
Sperm (2 × 106 ml−1) were incubated as above and the HOS test was performed as described by Jeyendran et al.16; the pretreated sperm were incubated in 2 ml of hypo-osmotic solution (7.35 g sodium citrate·2H2O and 13.51 g fructose mixed with 1 L distilled H2O) for 1 h at 37°C. The percentage of sperm undergoing tail swelling was determined under high power magnification (×400) on a phase contrast microscope. A minimum of 200 sperm were counted twice across three to five slides.
In vitro fertilization
Because HOS was impaired by 75% EPS, the same concentration of EPS was applied to evaluate fertilization. Female mice (n = 25) were superovulated at 8–10 weeks of age by intraperitoneal injection of 5 IU of pregnant mare serum gonadotrophin (PMSG; Sigma, St. Louise, MO, USA) followed 48 h later by an intraperitoneal injection of 5 IU of human chorionic gonadotropin (hCG; Sigma). The oviducts were removed at 12 h after hCG injection and placed in a prewarmed collection medium. Cumulus–oocyte complexes, found in the ampullar oviduct at the site of the cumulus bulge, were collected by tearing the oviduct using a 26-gauge needle. The IVF procedure was carried out in 100 μl drops of the insemination medium under mineral oil. Sperm, preincubated as described above, were gently added to the drops containing cumulus–oocyte complexes to yield a final motile sperm concentration of 1 × 105 per oocyte. After incubation with the sperm suspension at 37°C under 5% CO2 for 8 h, the oocytes were transferred to fresh medium and cultured for 12–14 h. They were evaluated for fertility using an inverted phase contrast microscope counting two-cell embryos.
Statistical analysis
Data are presented as the mean ± standard deviation of duplicate measurements, and each experiment was repeated 3–5 times. Significant differences between data sets were assessed by t-tests and one-way analyses of variance using the Statistical Package for Social Sciences 13.5 (SPSS Inc., Chicago, IL, USA). Significance was accepted at the level of P < 0.05.
RESULTS
The effect of the extracellular polymeric substances of Trichomonas vaginalis on sperm viability
Fresh sperm were approximately 52% viable before interaction (Figure 2). The proportion of viable sperm in vitro decreased over time, and the percentage of viable sperm was reduced by half in the control samples after 3 h of incubation, indicating that this incubation time was too long. In addition, the significant decrease in the viable sperm percentage after 2 h of incubation with EPS-10 (36.6% ±4.96% vs 51.5% ±7.56% before incubation, P < 0.002), or EPS-25 (30.8% ±9.46% vs 55.3% ±9.61% before incubation, P < 0.005) were observed. As shown in Figure 2, a profound effect of EPS on sperm viability was observed after 1 h of incubation in EPS-50 (37.4% ±5.90% vs 47.9% ±8.66% for the control, P < 0.05), whereas a significant difference between EPS-25 and the control was noted after 2 h of incubation (30.8% ±9.46% vs 42.0% ±7.91%, P < 0.05). Incubation in EPS-100 markedly decreased the percentage of viable sperm after 1 h of incubation (14.6% ±3.06% vs 47.9% ±8.66% for the control) and seemed to be too cytotoxic. We decided to adopt 2 h of incubation and 25%, 50% or 75% of EPS for the following experiments investigating the effect of EPS on sperm fertilizing capacity.
Figure 2.
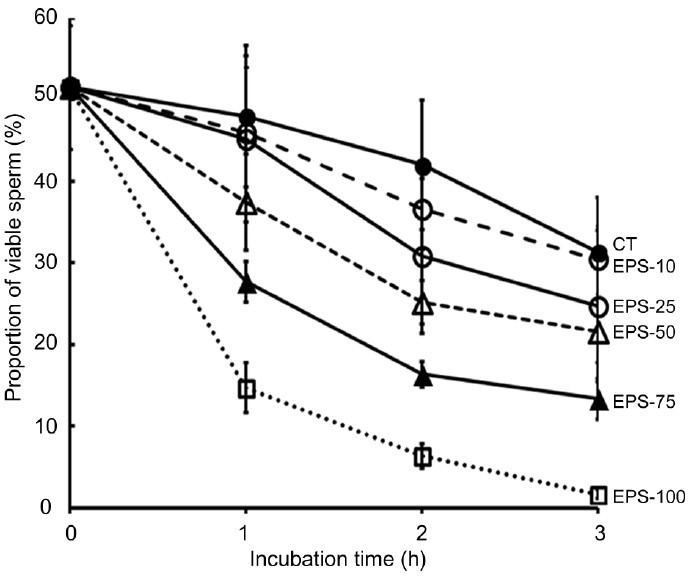
The effect of extracellular polymeric substances (EPS) of Trichomonas vaginalis on the viability of sperm. Viability was analyzed based on sperm morphology and vital staining with trypan blue. Data are presented at the mean ± standard deviation percent of viable sperm (relative to the total sperm numbers set at 100%) in three separate experiments, in which 200 cells were counted per treatment per experiment. EPS-10, -25, -50, -75, -100: sperm cells were incubated for 1, 2, or 3 h in 10%, 25%, 50%, 75%, or 100% of EPS prepared from T. vaginalis were diluted in Hank's balanced salt solution (HBSS). CT: control sperm cells were incubated at 37°C for 1, 2, or 3 h in HBSS that did not contain EPS.
The effect of the extracellular polymeric substances of Trichomonas vaginalis on sperm motility
Sperm motility was evaluated after 2 h of incubation with 25%, 50% or 75% of EPS. No significant differences were observed between the control and EPS-25 or EPS-50 with respect to total motility, which includes fast progressive motility, slow progressive motility and nonprogressive motility (Figure 1a). However, incubation with 75% EPS led to a decrease in the proportion of motile sperm (27.7% ±6.66% vs 59.2% ±2.25% in the control, P = 0.0148) (Figure 1a). The percentage of progressive motile sperm (Grade A + B) declined in EPS-25 (15.8% ±4.25% vs 32.5% ±5.77% in the control, P = 0.0326) and was reduced further at EPS-50 and EPS-75 (13.2% ±3.65% and 8.0% ±2.68%, respectively) (Figure 1b). These results indicate that progressive motility is impaired even by a low concentration of EPS.
The effect of the extracellular polymeric substances of Trichomonas vaginalis on acrosome status
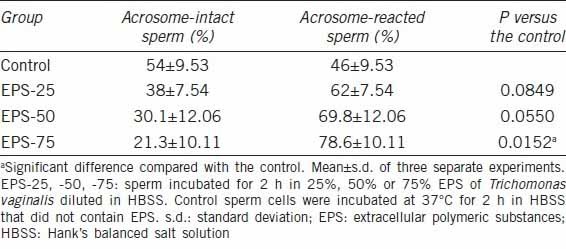
Fertility is closely related to acrosomal status, and the loss of the acrosomal content by either premature sperm activation or the breakdown of membranes would prevent normal sperm-egg interactions during fertilization.17 Acrosomal status may, therefore, be a significant factor in infertility, and we assessed the acrosomal status of sperm as a contributor to fertilization. We noted that the sperm incubated with EPS was less likely to have an intact acrosome than the controls (Table 1). After incubation with 25% EPS, an average of 38% of the sperm had an intact acrosome, and this percentage fell to 30.1% and 21.3% after incubation with 50% and 75% EPS, respectively, compared with an average of 54.0% in the control samples. The percentage of sperm with an intact acrosome at EPS-75 was significantly lower than in the control samples (P = 0.01).
Table 1.
The effect of the EPS of Trichomonas vaginalis on acrosome status
The proportion of sperm positive for the HOS test decreased as the concentration of EPS increased, as shown in Figure 3. When incubated with 25% and 50% EPS, the percentage of swollen sperm decreased to 33.5% ±9.26% and 31.5% ±9.76%, respectively. Although these values were not significantly different from the control samples (44.2% ±11.77%, P > 0.1) (Figure 3), the decline with 75% EPS was significantly different (23.7% ±8.08%, P = 0.0338). In addition, the proportion of sperm positive for the HOS test was similar to the proportion of sperm cells with intact acrosomes (Table 1).
Figure 3.
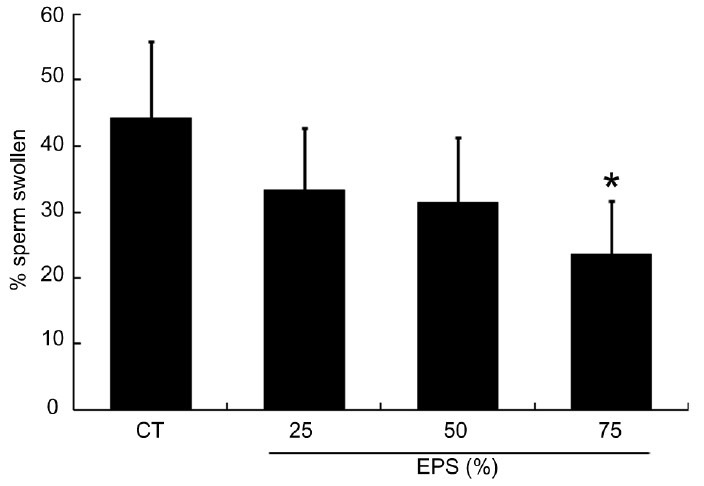
The effect of extracellular polymeric substances (EPS) of Trichomonas vaginalis on the proportion of sperm reacting positively to the hypo-osmotic swelling test. The percentage of swollen sperm was greatly reduced in sperm incubated with 75% EPS prepared from T. vaginalis. Sperm were incubated for 2 h in 25%, 50%, or 75% of EPS diluted in Hank's balanced salt solution (HBSS). CT: control sperm were incubated at 37°C for 2 h in HBSS that did not contain EPS. Values are the mean ± standard deviation of three separate experiments; 200 cells were counted per treatment per experiment. *P = 0.033 compared with the control.
The effect of the extracellular polymeric substances of Trichomonas vaginalis on the in vitro fertilization rate of sperm
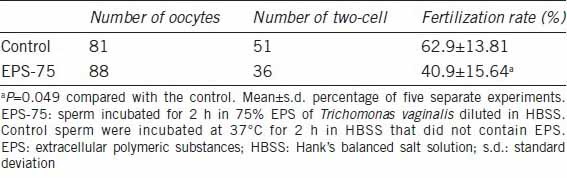
We also assessed fertilization 18 h after incubation of the ova with sperm suspensions. As both the acrosomal status and HOS test, which are highly correlated with fertilization capacity, were significantly compromised by incubation with 75% EPS, we used this concentration to evaluate the fertilization rate. Oocytes with two-cells were considered normally fertilized. The results of the IVF procedures in Table 2 show that the percentage of fertilized eggs was significantly lower in the EPS group than in the control group (40.9% ±15.64% vs 62.9% ±13.81%, P < 0.05).
Table 2.
In vitro fertilization of mouse oocytes with sperm preincubated with or without Trichomonas vaginalis EPS
DISCUSSION
Here, we have shown that, in vitro, the EPS produced from T. vaginalis has a negative impact on sperm fertilizing capacity. If this effect also occurs in vivo, it would lead to a decline in fertility. This result may help to clarify the mechanism that causes infertility or subfertility in women and men infected by T. vaginalis. Infection of the male accessory glands may compromise sperm quality, depending on the underlying inflammation and affected site. In fact, infections of the genito-urinary tract account for up to 15% of male infertility.18 T. vaginalis has also been found to infect the urethra and accessory glands,8,9 as well as the testis, in which case it can induce azoospermia or hypogonadism.19 In addition, chronic urethritis has also been shown to have a negative impact on semen quality. Although the semen have a short exposure time to trichomonads in the male urethra after ejaculation, sperm can be exposed to T. vaginalis or EPS contained in the ejaculates for several days through the female tract.20
There is conflicting information on the effects of the interaction between T. vaginalis and sperm cells, but the majority of in vitro studies have demonstrated a detrimental effect of T. vaginalis on sperm motility.6,21 This could be due to the direct binding of T. vaginalis to the sperm,22 to the circular whirling movement of T. vaginalis interrupting the normal horizontal movement of sperm within the vagina,23 or to the contact-independent cytopathic mechanisms induced by substances secreted by T. vaginalis.24 Our results show that incubation of sperm with EPS markedly decreases sperm motility (Figure 1), confirming that, in vitro, T. vaginalis adherence is not the sole factor affecting sperm motility. In fact, T. vaginalis has been shown to release molecules that are capable of acidifying the growth medium,25 or lysing host cells26 under triggering conditions. A number of biological agents have also been isolated from T. vaginalis; for example proteinases,27 phospholipases,28 acid phosphatase,29 and peroxide10 have been identified and are involved in cytotoxicity,30 hemolysis,28 and cytoadherence.30
An inhibitory role of EPS on sperm motility, similar to the effect of live T. vaginalis, has been reported in previous studies,6,7,10 although incubation medium and time, and the protocol for preparing EPS, differed between studies. Before analyzing sperm motility, we evaluated sperm viability after 1, 2, and 3 h of incubation with different concentrations of EPS. Our results indicate that longer incubation times and higher concentrations of EPS have an increasing detrimental impact on sperm viability. We also observed a profound decrease in sperm motility after 2 h of incubation with EPS, which is consistent with a previous in vitro study showing that sperm motility decreased after 2 h of incubation with trichomonads.6
The impact on sperm quality may be dependent on the protocol used to prepare EPS. Sperm exposed to seminal plasma or vaginal secretions harboring T. vaginalis are exposed to the harsh environment within the vagina. For in vitro studies it, therefore, seemed reasonable to incubate the sperm in HBSS instead of T. vaginalis culture medium. Furthermore, EPS composition within the vagina may be different from that in infected semen, as the secretory activity from T. vaginalis could be pH-dependent.28 To maximize the secretory activity of T. vaginalis for preparing EPS we used HBSS and a short incubation time.
Most studies limit semen evaluation to the observation of sperm motility, viability, and morphology. Thus, the putative detrimental effect of EPS on the fertilizing capacity of the sperm is unknown. Although sperm motility reflects a large number of biochemical functions such as sperm metabolism and microtubular action in the tail fibers,16 it does not provide any indication of the fertilizing capacity of the sperm. In fact, 30% of all patients with normal semen analyses have abnormal sperm function.31 Among the functional tests of sperm, the evaluation of acrosomal status and the HOS test are indicative of normal membrane integrity and function.32 Membrane and acrosome integrity are strongly associated with fertilization rate;16 hence we decided to look at those three parameters in vitro. As shown in Table 1, the incubation of sperm with EPS reduced the proportion of sperm cells with an intact acrosome. Reactive oxygen species such as peroxide have been identified in EPS,10 and sperm membrane peroxidation could be responsible for the breakdown of the acrosomal membrane.
Furthermore, it has been reported that the proportion of sperm showing HOS was markedly reduced in infected semen.5 Consistent with this decrease, we also observed a reduction in HOS, indicating that the integrity of the sperm membrane is affected by EPS. A previous study showed that the percentage of sperm cells positive for the HOS test was positively correlated with the percentage of intact acrosomes.32 Consistent with this, our results in 75% EPS yielded a similar proportion of sperm cells positive in the HOS test and with intact acrosomes (23.6% ±8.08% and 21.3% ±10.11%, respectively).
Because the HOS test reflects the functional integrity of the sperm cell membrane and is closely related to the IVF ability of sperm,16 our results indicate that EPS contributes to the ultimate loss of fertilizing capacity. As predicted, incubation with EPS significantly reduced the sperms’ fertilization rates. Some clinical cases suggest that the sperm-mucus interaction in men infected with T. vaginalis4 may not impair the sperms’ ability to travel and fertilize egg. However, our study suggests that only 2 h of incubation of sperm in the semen or a vagina harboring T. vaginalis could impair the fertilizing capacity of sperm.
This is the first report demonstrating a direct negative impact of the EPS from T. vaginalis on the fertilization rate of the sperm in vitro. However, further study should be performed using human sperm to determine if EPS has similar negative impact on sperm fertilizing capacity in vitro. Sperm fertilizing capacity may however not be affected by a single component of EPS and the secretions of T. vaginalis could be affected by its microenvironment. Thus, further research is needed to determine the molecular mechanisms responsible for the reduction of sperm fertilizing capacity by T. vaginalis.
AUTHOR CONTRIBUTIONS
JR participated in design, data analysis, and development of the manuscript; YSL, MYS and YC participated in the experiments and data analysis; JSR participated in the design of the study, data analysis, and supervision. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGEMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology (No. 2009-0067578).
REFERENCES
- 1.Low N, Broutet N, Adu-Sarkodie Y, Barton P, Hossain M, et al. Global control of sexually transmitted infections. Lancet. 2006;368:2001–16. doi: 10.1016/S0140-6736(06)69482-8. [DOI] [PubMed] [Google Scholar]
- 2.Poole DN, McClelland RS. Global epidemiology of Trichomonas vaginalis. Sex Transm Infect. 2013;89:418–22. doi: 10.1136/sextrans-2013-051075. [DOI] [PubMed] [Google Scholar]
- 3.El-Shazly AM, El-Naggar HM, Soliman M, El-Negeri M, El-Nemr HE, et al. A study on Trichomoniasis vaginalis and female infertility. J Egypt Soc Parasitol. 2001;31:545–53. [PubMed] [Google Scholar]
- 4.Soper D. Trichomoniasis: under control or undercontrolled? Am J Obstet Gynecol. 2004;190:281–90. doi: 10.1016/j.ajog.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 5.Gopalkrishnan K, Hinduja IN, Kumar TC. Semen characteristics of asymptomatic males affected by Trichomonas vaginalis. J In Vitro Fert Embryo Transf. 1990;7:165–7. doi: 10.1007/BF01135682. [DOI] [PubMed] [Google Scholar]
- 6.Tuttle JP, Jr, Holbrook TW, Derrick FC. Interference of human spermatozoal motility by Trichomonas vaginalis. J Urol. 1977;118:1024–5. doi: 10.1016/s0022-5347(17)58285-3. [DOI] [PubMed] [Google Scholar]
- 7.Jarecki-Black JC, Lushbaugh WB, Golosov L, Glassman AB. Trichomonas vaginalis: preliminary characterization of a sperm motility inhibiting factor. Ann Clin Lab Sci. 1988;18:484–9. [PubMed] [Google Scholar]
- 8.Hobbs MM, Kazembe P, Reed AW, Miller WC, Nkata E, et al. Trichomonas vaginalis as a cause of urethritis in Malawian men. Sex Transm Dis. 1999;26:381–7. doi: 10.1097/00007435-199908000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Skerk V, Schönwald S, Krhen I, Markovinovic L, Beus A, et al. Aetiology of chronic prostatitis. Int J Antimicrob Agents. 2002;19:471–4. doi: 10.1016/s0924-8579(02)00087-0. [DOI] [PubMed] [Google Scholar]
- 10.Kranjcic-Zec I, Dzamic A, Mitrovic S, Arsic-Arsenijevic V, Radonjic I. The role of parasites and fungi in secondary infertility. Med Pregl. 2004;57:30–2. doi: 10.2298/mpns0402030k. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Ahuja KK. An investigation using lectins of glycocomponents of mouse spermatozoa during capacitation and sperm-zona binding. J Reprod Fertil. 1987;80:65–74. doi: 10.1530/jrf.0.0800065. [DOI] [PubMed] [Google Scholar]
- 12.Han IH, Park SJ, Ahn MH, Ryu JS. Involvement of mast cells in inflammation induced by Trichomonas vaginalis via crosstalk with vaginal epithelial cells. Parasite Immunol. 2012;34:8–14. doi: 10.1111/j.1365-3024.2011.01338.x. [DOI] [PubMed] [Google Scholar]
- 13.Nam YH, Min A, Kim SH, Lee YA, Kim KA, et al. Leukotriene B(4) receptors BLT1 and BLT2 are involved in interleukin-8 production in human neutrophils induced by Trichomonas vaginalis-derived secretory products. Inflamm Res. 2012;61:97–102. doi: 10.1007/s00011-011-0425-3. [DOI] [PubMed] [Google Scholar]
- 14.Jedrzejczak P, Taszarek-Hauke G, Hauke J, Pawelczyk L, Duleba AJ. Prediction of spontaneous conception based on semen parameters. Int J Androl. 2008;31:499–507. doi: 10.1111/j.1365-2605.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- 15.Feng H, Sandlow JI, Sandra A. Expression and function of the c-kit proto-oncogene protein in mouse sperm. Biol Reprod. 1997;57:194–203. doi: 10.1095/biolreprod57.1.194. [DOI] [PubMed] [Google Scholar]
- 16.Jeyendran RS, Van der Ven HH, Perez-Pelaez M, Crabo BG, Zaneveld LJ. Development of an assay to assess the functional integrity of the human sperm membrane and its relationship to other semen characteristics. J Reprod Fertil. 1984;70:219–28. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 17.Saacke RG, Marshall CE. Observations on the acrosomal cap of fixed and unfixed bovine spermatozoa. J Reprod Fertil. 1968;16:511–4. doi: 10.1530/jrf.0.0160511. [DOI] [PubMed] [Google Scholar]
- 18.Pellati D, Mylonakis I, Bertoloni G, Fiore C, Andrisani A, et al. Genital tract infections and infertility. Eur J Obstet Gynecol Reprod Biol. 2008;140:3–11. doi: 10.1016/j.ejogrb.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Lloyd G, Case JR, De Frias D, Brannigan RE. Trichomonas vaginalis orchitis with associated severe oligoasthenoteratospermia and hypogonadism. J Urol. 2003;170:924. doi: 10.1097/01.ju.0000080375.18547.cc. [DOI] [PubMed] [Google Scholar]
- 20.Aitken RJ. Sperm function tests and fertility. Int J Androl. 2006;29:69–75. doi: 10.1111/j.1365-2605.2005.00630.x. [DOI] [PubMed] [Google Scholar]
- 21.Mali BN, Hazari KT, Meherji PK. Interaction between Trichomonas vaginalis and human spermatozoa in the female genital tract: papanicolaou-stained cervical smear findings. Acta Cytol. 2006;50:357–9. [PubMed] [Google Scholar]
- 22.Benchimol M, de Andrade Rosa I, da Silva Fontes R, Burla Dias AJ. Trichomonas adhere and phagocytose sperm cells: adhesion seems to be a prominent stage during interaction. Parasitol Res. 2008;102:597–604. doi: 10.1007/s00436-007-0793-3. [DOI] [PubMed] [Google Scholar]
- 23.Wiwanitkit V. Counteraction during movement of spermatozoa by Trichomonas vaginalis observed by visual image analysis: a possible cause of female infertility. Fertil Steril. 2008;90:528–30. doi: 10.1016/j.fertnstert.2007.07.1306. [DOI] [PubMed] [Google Scholar]
- 24.Chen WL, Chen JF, Zhong XR, Liang P, Lin W. Ultrastructural and immunohistochemical studies on Trichomonas vaginalis adhering to and phagocytizing genitourinary epithelial cells. Chin Med J (Engl) 2004;117:376–81. [PubMed] [Google Scholar]
- 25.Pindak FF, Mora de Pindak M, Gardner WA., Jr Contact-independent cytotoxicity of Trichomonas vaginalis. Genitourin Med. 1993;69:35–40. doi: 10.1136/sti.69.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fiori PL, Rappelli P, Addis MF, Sechi A, Cappuccinelli P. Trichomonas vaginalis haemolysis: pH regulates a contact-independent mechanism based on pore-forming proteins. Microb Pathog. 1996;20:109–18. doi: 10.1006/mpat.1996.0010. [DOI] [PubMed] [Google Scholar]
- 27.Neale KA, Alderete JF. Analysis of the proteinases of representative Trichomonas vaginalis isolates. Infect Immun. 1990;58:157–62. doi: 10.1128/iai.58.1.157-162.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vargas-Villarreal J, Mata-Cárdenas BD, Palacios-Corona R, González-Salazar F, Cortes-Gutierrez EI, et al. Trichomonas vaginalis: identification of soluble and membrane-associated phospholipase A1 and A2 activities with direct and indirect hemolytic effects. J Parasitol. 2005;91:5–11. doi: 10.1645/GE-3338. [DOI] [PubMed] [Google Scholar]
- 29.de Jesus JB, Podlyska TM, Hampshire A, Lopes CS, Vannier-Santos MA, et al. Characterization of an ecto-phosphatase activity in the human parasite Trichomonas vaginalis. Parasitol Res. 2002;88:991–7. doi: 10.1007/s00436-001-0583-2. [DOI] [PubMed] [Google Scholar]
- 30.Arroyo R, Alderete JF. Two Trichomonas vaginalis surface proteinases bind to host epithelial cells and are related to levels of cytoadherence and cytotoxicity. Arch Med Res. 1995;26:279–85. [PubMed] [Google Scholar]
- 31.Sigman M. Laboratory testing in the evaluation of male infertility. A rational approach. World J Urol. 1993;11:96–101. doi: 10.1007/BF00182036. [DOI] [PubMed] [Google Scholar]
- 32.Kumaresan A, Kadirvel G, Bujarbaruah KM, Bardoloi RK, Das A, et al. Preservation of boar semen at 18 degrees C induces lipid peroxidation and apoptosis like changes in spermatozoa. Anim Reprod Sci. 2009;110:162–71. doi: 10.1016/j.anireprosci.2008.01.006. [DOI] [PubMed] [Google Scholar]


