Abstract
Semen samples were collected from 1213 fertile men whose partners had a time-to-pregnancy (TTP) ≤12 months in Guangdong Province in Southern China, and semen parameters including semen volume, sperm concentration, total counts, motility, and morphology were evaluated according to the World Health Organization (WHO) 2010 guideline. All semen parameters analyzed were normal in ~62.2% of the total samples, whereas ~37.8% showed at least one of the semen parameters below normal threshold values. The fifth centiles (with 95% confidence intervals) were 1.3 (1.2–1.5) ml for semen volume, 20 × 106 (18×106–20×106) ml−1 for sperm concentration, 40 × 106 (38×106–44×106) per ejaculate for total sperm counts, 48% (47%–53%) for vitality, 39% (36%–43%) for total motility, 25% (23%–27%) for sperm progressive motility, 5.0% (4%–5%) for normal morphology. The pH values ranged from 7.2 to 8.0 with the mean ± standard deviation at 7.32 ± 0.17. No effects of age and body mass index were found on semen parameters. Occupation, smoking and alcohol abuse, varicocele appeared to decrease semen quality. Sperm concentration, but not sperm morphology, is positively correlated with TTP, whereas vitality is negatively correlated with TTP. Our study provides the latest reference values for the semen parameters of Chinese fertile men in Guangdong Province, which are close to those described in the new WHO guidelines (5th Edition).
Keywords: Chinese fertile men, semen parameters, semen quality, time-to-pregnancy
INTRODUCTION
Semen quality has been commonly regarded as a measure of male fecundity in clinical andrology, male fertility, reproductive toxicology, epidemiology, and pregnancy risk assessments.1 Since the publication of a meta-analysis showing a major decline in sperm counts over a period of 50 years after the second world war,2 many retrospective, comparative studies on semen quality around the world have been published over the past decade. Several reports suggested a decline,3,4,5,6,7,8 whereas others showed no significant changes in semen quality in men.9,10,11,12,13,14 Similar debatable data on the semen quality in China have also been published; by reviewing 115 reports published between 1985 and 2009, Huang et al.14 analyzed the data from 23 126 healthy Chinese subjects and demonstrated a possible decline in sperm concentration. In contrast, Wen et al.15 reported no evidence of decline in sperm concentration during a period of 18 years in Guangdong Province in Southern China.
Semen quality has been considered as one of the most sensitive indicators of the adverse effects of environmental pollution.16 In addition to physical environments, semen quality is also affected by other factors, such as age,17,18 occupation,19 cigarette smoking,20,21 and lifestyle.22,23 Most of the previous studies used less defined semen samples from laboratories in fertility clinics, whereas two recent studies from China investigated well-defined groups representative of the population of healthy young men.24,25 Other deficiencies of previous studies, especially those published in the earlier years,26,27,28 include the lack of comparable laboratory techniques and inappropriate statistical analyses. The present study was aimed at a comprehensive assessment of the current status of semen parameters in Chinese fertile men whose partners had time-to-pregnancy (TTP) ≤12 months in Guangdong area according to the strict guideline of World Health Organization (WHO) laboratory manual for the examination and processing of human semen (2010, 5th Edition).
MATERIALS AND METHODS
Subjects
Husbands of pregnant women who had TTP ≤ 12 months from Heyuan, Jiangmen, Yangjiang, Zhanjiang, and Qingyuan areas in Guangdong province were invited to participate in our study from October 2010 to September 2012 by the local Family Planning Network. The total of 1258 fertile men were invited to participate and 1213 (96.4%) agreed. The eligibility criteria for the male participants were as follows: age 20–45 years at the time of invitation, residence of the local area near the hospital where he was recruited. In addition, the woman's current pregnancy had to be achieved by normal sexual intercourse rather than fertility treatment. Subjects with the following conditions were excluded: epididymitis, cryptorchidism, orchitis, genital tract surgery, chemotherapy, radiotherapy or chronic illness, previous treatment for infertility or reduced fertility, and unwanted pregnancy or prolonged TTP.
Questionnaires
Both the men and their pregnant wives were asked to complete a questionnaire. The questionnaire included detailed information on demography, education, lifestyle, occupational exposure, reproductive history, consumption of tobacco and alcohol, and previous or current diseases. The participants were informed of the purpose of the study, and possible benefits and risks of participating in the study. All participants were asked to sign an informed consent form if they agreed to take part in this study. The protocol for the use of human subjects was approved by the Human Subject Ethics Committee of the Research Institute of Family Planning of Guangdong Province.
Physical examination
Physical examinations of all subjects were performed by the same two experienced andrologists. The results of examinations were recorded in a standard form. Secondary sexual characteristics and the possible presence of a varicocele, a hydrocele, the location of the testis in the scrotum, and the consistency of the testis and epididymis were examined to exclude the subjects with reproductive or urological diseases. Weight (kg) and height (cm) were measured using the same calibrated instrument in each center.
Semen samples
All subjects were asked to abstain from ejaculation for a period of 2–7 days. The period of ejaculation abstinence was calculated as the time between the current and previous ejaculations as reported by the subjects. The exact duration (in days) of abstinence was documented for each participant. The participants collected the ejaculates by masturbation at the local Family Planning Institutions into a sterile plastic container (MaleFree™SCD-II, BRED Life Science, Shenzhen, China) and immediately delivered the sample to a laboratory in the same building. The semen samples were marked with an anonymous serial number, and were then incubated in a water-bath at 37°C until analysis. The semen volume was measured after samples were liquefied. All samples were analyzed within 60 min after collection.
Sperm motility was analyzed using a computer-assisted semen analysis (CASA) system (SCA2000, Microptic, Barcelona, Spain). According to the WHO reference, percent of motile sperm was scored using category A (rapid progressive motility [PR]), category B (slow PR), category C (nonprogressive motility [NP]), and category D (immotility). Two types of motility were evaluated in the present study: PR and NP. Other CASA parameters analyzed included curvilinear velocity (VCL), straight-line velocity (VSL), average path velocity, beat cross frequency, and amplitude of lateral head displacement. The calculated parameters were linearity (LIN = VSL/VCL × 100) and straightness (STR = VSL/VCL). To avoid using CASA parameters that reflect different aspects of the same movement as described in previous studies,29,30 we selected VSL (for progression), VCL (for vigor), and LIN (for swimming pattern) for statistical analyses.
For morphological evaluation, semen smears were fixed with a mixture of absolute alcohol and acetone (at a ratio of 2:1), followed by staining using the modified Shorr solution. Assessment of sperm morphology was conducted according to the criteria published by the WHO, 2010, the 5th edition.
Quality control of semen analysis
To minimize variations in the assessment of sperm characteristics, samples from all five centers were analyzed by the same two well-trained technicians using the same instruments and methods. One technician evaluated appearance, viscosity, liquefaction time, semen volume, while the other measured sperm concentration, motility, and morphology. The two technicians participated in the continuous quality control system, an external quality control system established based on the WHO guidelines, supervised by the Quality Control Center of Guangdong Province. Semen quality was evaluated based on the recommendations by the WHO, 2010.
Statistical analyses
Because semen parameters follow markedly nonnormal distributions, unadjusted percentiles, and medians were calculated for the semen parameters. Percentages coincident with the criteria of WHO (2010) were also calculated. The data were also summarized using median, 5th percentiles, and were stratified by age, body mass index (BMI), occupation and lifestyle. Kruskal–Wallis analysis of variance, a nonparametric test, was used to compare medians between groups. A step-wise multiple regression analysis was used to determine which variables are the strongest independent correlates of TTP. A generalized linear model was used to examine the independent effect of risk factors on semen parameters. A full model that included all risk factors examined in the final regression was used. Independent variables entered into the regression model as dummy variables were as follows: (i) age: ≥35, 30–35, 25–30, and <25 as reference; (ii) BMI: >25.0, <18.5, and 18.5–25.0 as reference; (iii) occupation: others, farmers, and workers as reference; (iv) smoking: yes and no as reference; (v) alcohol consumption: yes and no as reference; (vi) urogenital disease history: yes and no as reference; (vii) varicocele: yes and no as reference. The level of significance was established at 0.05. All semen parameters were log-transformed to improve the normality as dependent variables in the generalized linear models.
RESULTS
Subject characteristics
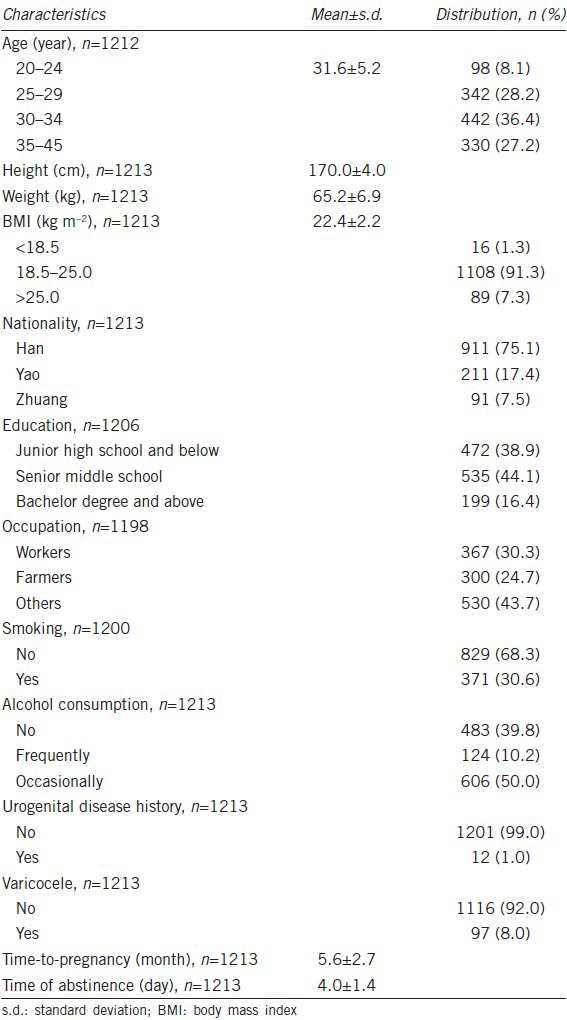
The mean age of the participants was 31.6 ± 5.2 years (range: 20–45 years). The demographic characteristics of the participants are presented in Table 1.
Table 1.
Characteristics of participants
Semen analysis
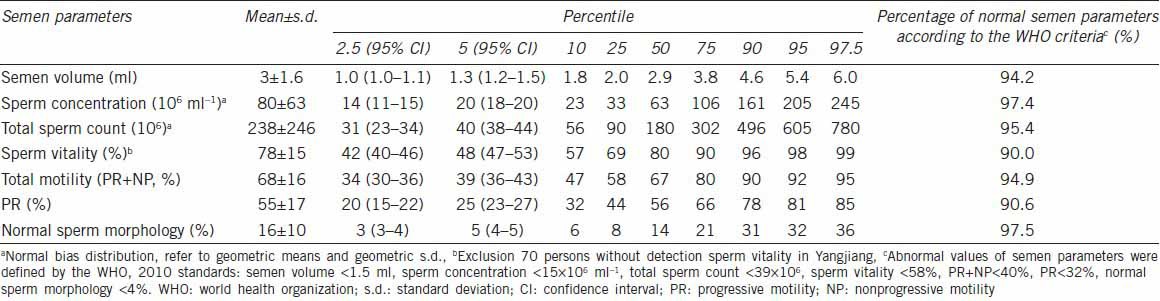
All semen samples were gray or grayish yellow in color. The liquefaction time was within 60 min. The thread length was no more than 2 cm. the pH values ranged from 7.2 to 8.0, and the mean and standard deviation was 7.32 ± 0.17. All samples were analyzed within 60 min after collection. Table 2 shows semen characteristics of the 1213 subjects. The normal sperm morphology and sperm concentration were within high-normal values (97.5% and 97.4%, respectively) according to the WHO criteria. Of the 1213 semen samples evaluated, 62.2% (755/1213) displayed normal semen parameters according to the WHO criteria (WHO, 2010), but 37.8% showed below normal threshold values in at least one of the semen parameters, including semen volume, sperm concentration, total count, vitality, PR, and normal morphology.
Table 2.
Summary of semen parameters
Risk factors for the semen quality of fertile men
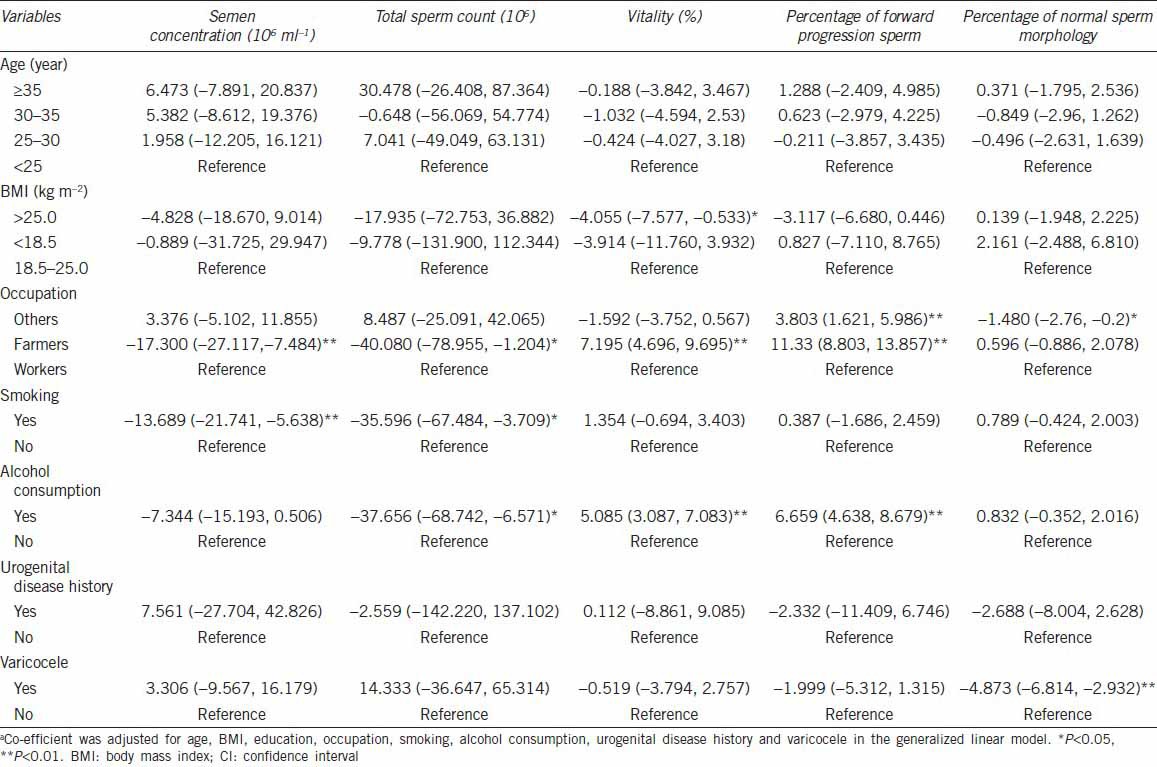
The semen samples were grouped based on age, BMI, occupation, smoking, alcohol consumption, urogenital disease history, and varicocele. The semen parameters were correlated with these variables using step-wise multiple regression analyses. Table 3 shows adjusted regression coefficients and 95% confidence interval for all possible risk factors in relation to semen parameters. Ages and BMI appeared to have no effects on the semen quality, whereas occupations seemed to affect sperm concentration, total sperm counts, vitality and percentage of forward progression sperm. In addition, smoking had effects on sperm concentration and total sperm counts, and alcohol intake could impact sperm vitality and percentage of forward progression sperm. Interestingly, history of urogenital diseases did not seem to affect the semen quality except that varicocele had a negative impact on sperm morphology.
Table 3.
Effects of potential risk factors on semen parameters of fertile mena
Summary of time-to-pregnancy
The minimum and maximum TTP of 1213 fertile men whose partners achieved pregnancy within 12 months of unprotected sexual intercourse was 0 and 12 months, respectively. Distribution of the TTP frequency among 1213 women whose male partners participated in this study is shown in Figure 1. More than 50% couples achieved pregnancy within 6 months. The step-wise multiple regression analyses were used to determine which variables are the strongest independent correlates of TTP. Statistical analyses showed that sperm concentration was positively correlated to TTP (t = −4.801, P < 0.001), whereas vitality was negatively correlated to TTP (t = 3.954, P < 0.001).
Figure 1.
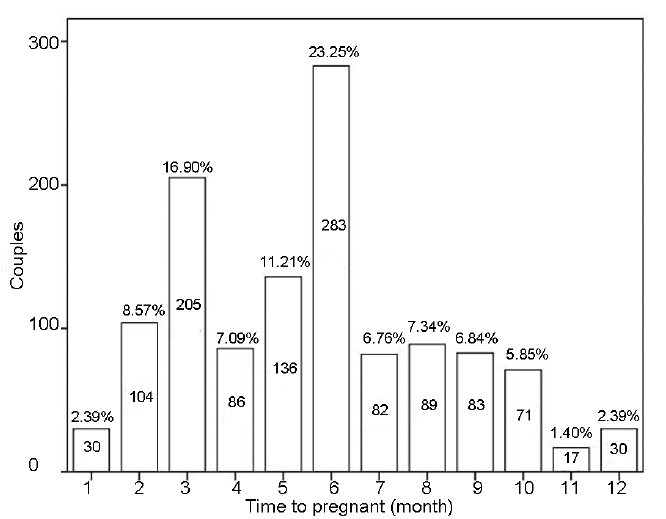
The time-to-pregnancy frequency distribution of 1213 women whose male partners participated in this study. The number in the bar represent the number of couples establish pregnancy at this month; the percentage above the bar represent the percentage of couples establish pregnancy at this month. More than 50% couples achieved pregnancy within 6 months.
DISCUSSION
Our data represent the current semen parameters of Chinese fertile men whose partners had TTP ≤ 12 months in Guangdong area. Subjects in the previous reports on semen quality in China were only defined as healthy people or sperm donors, with no or little information on TTP of their female partners.24,25,31 Subjects in our study were all whose partners achieving pregnancy within 12 months. Moreover, our results are not in complete agreement with those reported previously in several large studies in Chinese men.24,25,31,32,33,34 As shown in Table 4, the mean values of semen volume and concentration in our study (3.0 ml and 80 × 106 ml−1, respectively) are in agreement with those of others (2.5–3.0 ml and 55.9 × 106–99.2 × 106 ml−1, respectively), whereas the mean values of total sperm counts in our study (238 × 106) are markedly higher than those in other studies, ranging from 133.6 × 106 to 203.2 × 106. The mean values of total motility in our study (68.0%) are similar to the other reports (47.7%–77.2%). Compared semen parameters reported here with earlier studies of American (USA) and European (France, Denmark, Finland, Estonia and Norway) men,35,36,37,38 the mean semen volume in Chinese men was lower by 0.6–1.4 ml. The mean sperm concentration (80.0 × 106 ml−1) in Chinese fertile men is higher than that in young American and Nordic-Baltic men (range: 54.3 × 106–75.5 × 106 ml−1), but lower than that of French men (95 × 106 ml−1). The mean total sperm counts (238 × 106) in our study was higher than was recorded for men in the USA (range: 113.0 × 106–149.7 × 106), but was markedly lower than that of French men (337 × 106). For the mean total motility, our study indicated that motility in Chinese men (68.0%) was similar to that of European men (range: 64%–73%), but was higher than that in the USA (range: 48.2%–56.4%).
Table 4.
Summary of semen parameters of different regions in China
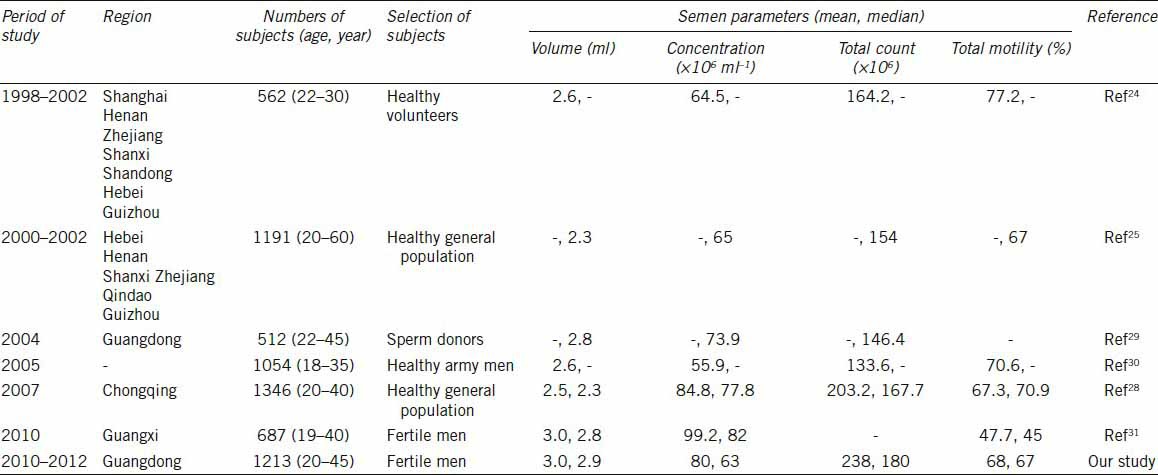
Discrepancies among these studies could result from many factors, such as demographic characteristics, region, lifestyle, environment, and methodology used for semen analyses. For example, semen analysis is a rather subjective technique, and is associated with inter-laboratory variations,36,37 making it difficult to compare assessments performed by different laboratories. We also cannot exclude geographic variations in semen quality, since several studies have shown apparent geographic variations after adjusting for possible confounding factors. Together, we speculate that these variations can result from factors including lifestyle, environmental exposure and genetic variations, or a combination of all those factors.
A lack of significant effect of age on semen parameters in this study can well be due to the narrow age range (20–45 years old) of participants because men of this age group are usually at the peak of reproductive capability. Recent studies suggest that increased age is associated with a decrease not only in semen volume, but also in the percentage of sperm morphology and motility.4,39 Other studies have shown that there is no correlation between sperm concentration and age.40,41 Discrepancies among these studies may be due to the different ages of men examined, or due to other confounding factors.
Several studies have demonstrated that BMI could be associated with semen quality. Jensen et al.38 found a significant association between sperm counts and BMI; both overweight and overly slim men had lower sperm concentrations and lower total sperm counts compared to men with ideal body weights (BMI between 20 and 25 kg m−2). Results reported by Kort et al.42 reveal a significant, negative relationship between BMI and the total number of normal-motile sperm; men presenting with a BMI > 25 kg m−2 have fewer chromatin-intact normal-motile sperm per ejaculate. In our study, BMI do not appear to have an effect on semen quality, which is consistent with the results of the Chinese population reported by Gao et al.25
Our study also suggests that occupation might affect semen quality because semen quality of factory workers is significantly lower than that of farmers and others. We speculate that the differences may be attributable, at least in part, to lifestyle and environmental exposures. It is well-known that long-term chronic alcohol abuse causes erectile dysfunction, reduced libido, and gynecomastia. One mechanism of these effects lies in the reduction in serum testosterone levels due to decreased testicular production and increased metabolic clearance in liver. In addition, the oxidation of alcohol competes with testicular production of testosterone. These mechanisms lead to subsequent decrease in semen volume and sperm concentration, which is consistent with our observation that alcohol intake negatively affects semen quality. Another factor appears to be the elevation of serum estrogen levels due to increased peripheral conversion of testosterone to estrogen through enhanced activity of aromatase, which is present in both liver and peripheral adipose cells. It has reported that progressive deterioration in semen quality correlates with increasing quantity of alcohol intakes and cigarettes smoking,43 and smoking has a significant negative impact on sperm production, motility, and morphology.44 Although the underlying mechanism remains unknown, our study further supports those earlier reports, indicating that alcohol abuse and smoking lower the semen quality mainly by reducing the total sperm counts.
Randomized, controlled trials, and prospective studies that evaluate semen parameters before and after varicocelectomy clearly demonstrate that varicocele repair is associated with a significant improvement in sperm concentration, motility, and normal morphology.45 Our study suggests that normal fertile men with varicocele disease can also affect the semen quality, especially the percentage of normal sperm morphology.
Time-to-pregnancy data have been widely adopted to reflect the fecundity of a couple because these data correlate with sperm quality and quantity as well as sexual activity. However, semen studies often have much lower participation rates than those TTP studies, and TTP studies should, therefore, remain in the toolbox for studies of male fecundity.46 The TTP mean value in our study is relatively higher compared to other published data.47,48 The negative association between sperm vitality and TTP (t = 3.954, P < 0.001) is easy to understand because poor vitality leads to prolonged TTP. However, the positive correlation between sperm concentration and TTP (t = −4.801, P < 0.001) is counterintuitive and puzzling. One possible explanation might be that overcrowding may interfere with sperm swimming and/or the fertilization process. The lack of correlation between sperm morphology and TTP may not be meaningful because the new WHO criteria used for assessing sperm morphology are too loose, e.g. with a threshold as low as 4%–5%, the majority of men are considered to have normal sperm morphology.
CONCLUSION
This report presents a large study on semen quality in the fertility-proven Chinese men whose partners had TTP ≤ 12 months. Our study revealed that a substantial proportion of Chinese fertile males (~37.8%) had at least one of the semen parameters, including semen volume, sperm concentration, total counts, vitality, PR, and normal morphology, below the normal range. For the fifth centiles of semen parameters, our results are similar to the normal threshold values of the WHO guidelines (5th Edition). We did not find any significant associations between the poor semen quality and age or BMI, but did note that occupation, smoking, alcohol abuse, and varicocele may be important factors that influence semen quality. Sperm concentration positively correlates to TTP, whereas vitality is negatively associated with TTP. This study provides important basic data on semen quality of fertile men in Guangdong area, and the large population dataset presented will be useful for comparison of semen quality in different regions of China.
AUTHOR CONTRIBUTIONS
YGT designed the study, drafted the manuscript, and coordinated and participated in every part of the study. LXT participated in the design of the study and drafting of the manuscript. QLW participated in the design and statistical analyses. GS participated in the design of the study. YJJ performed the semen analyses. SMD analyzed the semen quality and interpreted the data. FJ performed semen analyses and interpreted the data. WBQ helped write and revise the manuscript and supplied important comments. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We are grateful to the Guangdong Family Planning Commission, and five local Family Planning Institutions (Heyuan, Jiangmen, Yangjiang, Zhanjiang, Qingyuan) for coordinating the subject recruitment. We also thank all the volunteers for participating in the study. We acknowledge the contributions of Dr. Wei Yan in University of Nevada School of Medicine. This research was supported by the Program of S and T (Guangdong) Project (No. 2010B031600174, No. 2011B031900001) and the Program of Guangdong Province Family Planning Commission (No. 2012101).
REFERENCES
- 1.Cooper TG, Noonan E, Eckardstein S, Auger J, Gordon Baker HW, et al. World Health Organization reference values for human semen characteristics. Hum Reprod Update. 2010;16:231–45. doi: 10.1093/humupd/dmp048. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305:609–13. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Younglai EV, Collins JA, Foster WG. Canadian semen quality: an analysis of sperm density among eleven academic fertility centers. Fertil Steril. 1998;70:76–80. doi: 10.1016/s0015-0282(98)00118-6. [DOI] [PubMed] [Google Scholar]
- 4.Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75:237–48. doi: 10.1016/s0015-0282(00)01679-4. [DOI] [PubMed] [Google Scholar]
- 5.Lackner J, Schatzl G, Waldhör T, Resch K, Kratzik C, et al. Constant decline in sperm concentration in infertile males in an urban population: experience over 18 years. Fertil Steril. 2005;84:1657–61. doi: 10.1016/j.fertnstert.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 6.Shine R, Peek J, Birdsall M. Declining sperm quality in New Zealand over 20 years. N Z Med J. 2008;121:50–6. [PubMed] [Google Scholar]
- 7.Geoffroy-Siraudin C, Loundou AD, Romain F, Achard V, Courbière B, et al. Decline of semen quality among 10 932 males consulting for couple infertility over a 20-year period in Marseille, France. Asian J Androl. 2012;14:584–90. doi: 10.1038/aja.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rolland M, Le Moal J, Wagner V, Royère D, De Mouzon J. Decline in semen concentration and morphology in a sample of 26,609 men close to general population between 1989 and 2005 in France. Hum Reprod. 2013;28:462–70. doi: 10.1093/humrep/des415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rasmussen PE, Erb K, Westergaard LG, Laursen SB. No evidence for decreasing semen quality in four birth cohorts of 1,055 Danish men born between 1950 and 1970. Fertil Steril. 1997;68:1059–64. doi: 10.1016/s0015-0282(97)00377-4. [DOI] [PubMed] [Google Scholar]
- 10.Yogev L, Paz G, Kleiman SE, Shabtai E, Gamzu R, et al. Freezability and semen parameters in candidates of sperm bank donors: 1992-2010. J Androl. 2012;33:999–1006. doi: 10.2164/jandrol.111.013045. [DOI] [PubMed] [Google Scholar]
- 11.Dama MS, Rajender S. Secular changes in the semen quality in India during the past 33 years. J Androl. 2012;33:740–4. doi: 10.2164/jandrol.111.015057. [DOI] [PubMed] [Google Scholar]
- 12.Nieschlag E, Lerchl A. Sperm crisis: what crisis? Asian J Androl. 2013;15:184–6. doi: 10.1038/aja.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pacey AA. Are sperm counts declining? Or did we just change our spectacles. Asian J Androl. 2013;15:187–90. doi: 10.1038/aja.2012.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang L, Li Y, Xiong H, Cao J. Changing tendency analysis of Chinese normal male's semen quality in recent 25 years: samples from Chinese documents. J Reprod Contracept. 2010;21:229–41. [Google Scholar]
- 15.Wen R, Ma C, Tang L, Wang Q, Liu M, et al. [No declining semen parameters from Guangdong: an analyses comparing with 18 years ago] Zhongguo Nan Ke Xue Za Zhi. 2006;20:3–5. Article in Chinese. [Google Scholar]
- 16.Nordkap L, Joensen UN, Blomberg Jensen M, Jørgensen N. Regional differences and temporal trends in male reproductive health disorders: semen quality may be a sensitive marker of environmental exposures. Mol Cell Endocrinol. 2012;355:221–30. doi: 10.1016/j.mce.2011.05.048. [DOI] [PubMed] [Google Scholar]
- 17.Lepecka-Klusek C, Wdowiak A, Pilewska-Kozak AB, Syty K, Jakiel G. The role of age, environmental and occupational factors on semen density. Ann Agric Environ Med. 2011;18:437–40. [PubMed] [Google Scholar]
- 18.Kovac JR, Addai J, Smith RP, Coward RM, Lamb DJ, et al. The effects of advanced paternal age on fertility. Asian J Androl. 2013;15:723–8. doi: 10.1038/aja.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cherry N, Moore H, McNamee R, Pacey A, Burgess G, et al. Occupation and male infertility: glycol ethers and other exposures. Occup Environ Med. 2008;65:708–14. doi: 10.1136/oem.2007.035824. [DOI] [PubMed] [Google Scholar]
- 20.Taha EA, Ez-Aldin AM, Sayed SK, Ghandour NM, Mostafa T. Effect of smoking on sperm vitality, DNA integrity, seminal oxidative stress, zinc in fertile men. Urology. 2012;80:822–5. doi: 10.1016/j.urology.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Yu B, Qi Y, Liu D, Gao X, Chen H, et al. Cigarette smoking is associated with abnormal histone-to-protamine transition in human sperm. Fertil Steril. 2014;101:51–7.e1. doi: 10.1016/j.fertnstert.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 22.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braga DP, Halpern G, Figueira Rde C, Setti AS, Iaconelli A, Jr, et al. Food intake and social habits in male patients and its relationship to intracytoplasmic sperm injection outcomes. Fertil Steril. 2012;97:53–9. doi: 10.1016/j.fertnstert.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Junqing W, Qiuying Y, Jianguo T, Wei Y, Liwei B, et al. Reference value of semen quality in Chinese young men. Contraception. 2002;65:365–8. doi: 10.1016/s0010-7824(02)00281-0. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Gao ES, Yang Q, Walker M, Wu JQ, et al. Semen quality in a residential, geographic and age representative sample of healthy Chinese men. Hum Reprod. 2007;22:477–84. doi: 10.1093/humrep/del383. [DOI] [PubMed] [Google Scholar]
- 26.Chia SE, Tay SK, Lim ST. What constitutes a normal seminal analysis? Semen parameters of 243 fertile men. Hum Reprod. 1998;13:3394–8. doi: 10.1093/humrep/13.12.3394. [DOI] [PubMed] [Google Scholar]
- 27.Fisch H, Goluboff ET, Olson JH, Feldshuh J, Broder SJ, et al. Semen analyses in 1,283 men from the United States over a 25-year period: no decline in quality. Fertil Steril. 1996;65:1009–14. doi: 10.1016/s0015-0282(16)58278-8. [DOI] [PubMed] [Google Scholar]
- 28.Gyllenborg J, Skakkebaek NE, Nielsen NC, Keiding N, Giwercman A. Secular and seasonal changes in semen quality among young Danish men: a statistical analysis of semen samples from 1927 donor candidates during 1977-1995. Int J Androl. 1999;22:28–36. doi: 10.1046/j.1365-2605.1999.00137.x. [DOI] [PubMed] [Google Scholar]
- 29.Meeker JD, Ryan L, Barr DB, Herrick RF, Bennett DH, et al. The relationship of urinary metabolites of carbaryl/naphthalene and chlorpyrifos with human semen quality. Environ Health Perspect. 2004;112:1665–70. doi: 10.1289/ehp.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L, Bao H, Liu F, Zhang J, Shen H. Phthalates exposure of Chinese reproductive age couples and its effect on male semen quality, a primary study. Environ Int. 2012;42:78–83. doi: 10.1016/j.envint.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Lin H, Ma M, Li L, Cai M, et al. Semen quality of 1346 healthy men, results from the Chongqing area of Southwest China. Hum Reprod. 2009;24:459–69. doi: 10.1093/humrep/den399. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Ma C, Tang L, Wen R, Deng S, et al. [Quality analysis of the primary semen samples from 512 donors] Zhonghua Nan Ke Xue. 2004;10:734–6. Article in Chinese. [PubMed] [Google Scholar]
- 33.Yan SW, Tang J, Zhang N, Wang XL, Lu HO, et al. [Investigation of semen quality of 18-35 year old Chinese army men] Zhonghua Nan Ke Xue. 2007;13:134–7. Article in Chinese. [PubMed] [Google Scholar]
- 34.Tan D, Mo Y, Xie D, Zhang S, Lv F, et al. [The research on semen quality from males with normal fertility in Guangxi region] Zhongguo Ji Hua Sheng Yu He Fu Chan Ke. 2012;4:9–12. Article in Chinese. [Google Scholar]
- 35.Auger J, Jouannet P. Evidence for regional differences of semen quality among fertile French men. Hum Reprod. 1997;12:740–5. doi: 10.1093/humrep/12.4.740. [DOI] [PubMed] [Google Scholar]
- 36.Jørgensen N, Carlsen E, Nermoen I, Punab M, Suominen J, et al. East-West gradient in semen quality in the Nordic-Baltic area: a study of men from the general population in Denmark, Norway, Estonia and Finland. Hum Reprod. 2002;17:2199–208. doi: 10.1093/humrep/17.8.2199. [DOI] [PubMed] [Google Scholar]
- 37.Swan SH, Brazil C, Drobnis EZ, Liu F, Kruse RL, et al. Geographic differences in semen quality of fertile U.S. males. Environ Health Perspect. 2003;111:414–20. doi: 10.1289/ehp.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen TK, Andersson AM, Jørgensen N, Andersen AG, Carlsen E, et al. Body mass index in relation to semen quality and reproductive hormones among 1,558 Danish men. Fertil Steril. 2004;82:863–70. doi: 10.1016/j.fertnstert.2004.03.056. [DOI] [PubMed] [Google Scholar]
- 39.Levitas E, Lunenfeld E, Weisz N, Friger M, Potashnik G. Relationship between age and semen parameters in men with normal sperm concentration: analysis of 6022 semen samples. Andrologia. 2007;39:45–50. doi: 10.1111/j.1439-0272.2007.00761.x. [DOI] [PubMed] [Google Scholar]
- 40.Eskenazi B, Wyrobek AJ, Sloter E, Kidd SA, Moore L, et al. The association of age and semen quality in healthy men. Hum Reprod. 2003;18:447–54. doi: 10.1093/humrep/deg107. [DOI] [PubMed] [Google Scholar]
- 41.Carlsen E, Swan SH, Petersen JH, Skakkebaek NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod. 2005;20:942–9. doi: 10.1093/humrep/deh704. [DOI] [PubMed] [Google Scholar]
- 42.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, et al. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27:450–2. doi: 10.2164/jandrol.05124. [DOI] [PubMed] [Google Scholar]
- 43.Gaur DS, Talekar MS, Pathak VP. Alcohol intake and cigarette smoking: impact of two major lifestyle factors on male fertility. Indian J Pathol Microbiol. 2010;53:35–40. doi: 10.4103/0377-4929.59180. [DOI] [PubMed] [Google Scholar]
- 44.Pasqualotto FF, Lucon AM, Sobreiro BP, Pasqualotto EB, Arap S. Effects of medical therapy, alcohol, smoking, and endocrine disruptors on male infertility. Rev Hosp Clin Fac Med Sao Paulo. 2004;59:375–82. doi: 10.1590/s0041-87812004000600011. [DOI] [PubMed] [Google Scholar]
- 45.Ficarra V, Crestani A, Novara G, Mirone V. Varicocele repair for infertility: what is the evidence? Curr Opin Urol. 2012;22:489–94. doi: 10.1097/MOU.0b013e328358e115. [DOI] [PubMed] [Google Scholar]
- 46.Olsen J, Ramlau-Hansen CH. Epidemiologic methods for investigating male fecundity. Asian J Androl. 2014;16:17–22. doi: 10.4103/1008-682X.122198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wise LA, Rothman KJ, Mikkelsen EM, Sørensen HT, Riis AH, et al. A prospective cohort study of physical activity and time to pregnancy. Fertil Steril. 2012;97:1136–42.e1. doi: 10.1016/j.fertnstert.2012.02.025. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Joffe M, Key J, Best N, Keiding N, Scheike T, et al. Studying time to pregnancy by use of a retrospective design. Am J Epidemiol. 2005;162:115–24. doi: 10.1093/aje/kwi172. [DOI] [PubMed] [Google Scholar]


