Abstract
Previous studies have demonstrated that male hypogonadism is associated with a low level of vitamin D. However, no reports have investigated the effects of vitamin D on testosterone levels in Korean men. Our aim was to investigate whether testosterone levels are associated with serum vitamin D levels and whether seasonal variation exists. This cross-sectional study analyzed serum 25-hydroxyvitamin D [25(OH)D], total testosterone (TT), and free testosterone (FT) in 652 Korean men over 40 years of age who had undergone a comprehensive medical examination. The average age of the subjects was 56.7 ± 7.9 years, and the mean serum 25(OH)D, TT and FT levels were 21.23 ± 7.9 ng ml−1, 4.70 ± 1.6 ng ml−1, and 8.12 ± 3.3 pg ml−1, respectively. In the multiple linear regression model, 25(OH)D showed positive association with TT (β =0.137, P < 0.001) and FT (β =0.103, P = 0.008). 25(OH)D and FT showed similar seasonal or monthly variation after adjustment for age. A vitamin D deficiency [25(OH)D < 20 ng ml−1] was associated with an increased risk of deficiencies of TT (<2.30 ng ml−1) (odds ratio [OR]: 2.65; 95% confidence interval [CI]: 1.21–5.78, P = 0.014) and FT (<6.50 pg ml−1) (OR: 1.44; 95% CI: 1.01–2.06 P = 0.048) after adjusting for age, season, body mass index, body composition, chronic disease, smoking, and alcohol use. In conclusion, we demonstrated a positive correlation between 25(OH)D and testosterone, which showed similar seasonal variation in Korean men.
Keywords: 25-hydroxyvitamin D, hypogonadism, seasons, testosterone, vitamin D
INTRODUCTION
Vitamin D has important functions in physiological processes, and vitamin D deficiency may play a role in pathophysiological processes. In addition to its classic effects on bone homeostasis, many studies have supported that vitamin D receptors (VDRs) are present on various nonmusculoskeletal organs and tissues,1 suggesting that vitamin D levels could be implicated in many clinical conditions. Vitamin D deficiency, the most widespread nutritional disorder in the world, has increasingly been recognized as an important human health problem.2 Currently, vitamin D deficiency is associated with several pathological states, including cardiovascular diseases,3 inflammatory disease,4 and high all-cause mortality in the general population.5 VDR expression has also been observed in reproductive tissues, such as the testes, prostate, and human sperm.6 A recent cross-sectional study, the Health Professionals Follow-up Study, included 1362 males and identified weak positive correlations between 25-hydroxyvitamin D [25(OH)D] and testosterone levels.7 However, the study excluded Asian participants to avoid confounding race/ethnicity bias. In Asia, there is little current data on the association between vitamin D levels and testosterone.
An age-related decrease in total testosterone (TT) has been well documented in middle-aged men, with lower TT levels suggested to be a risk factor for cardiovascular disease, cancer, and all-cause mortality.8 In addition, as the average life expectancy has increased, so has the importance of quality of life after middle-age. Thus, the association between vitamin D and gonadal function is of clinical importance. Nevertheless, as of today, few studies have investigated the effect of vitamin D on testosterone in Korean men. Therefore, the aim of the present study was to investigate the relationship between serum 25(OH)D and testosterone levels in Korean men. We hypothesized that a higher serum vitamin D level is associated with a higher level of testosterone. In addition, 25(OH)D levels differ notably according to season, which is a consequence of variations in sunlight-induced vitamin D synthesis in the skin. Thus, we also hypothesized that levels of sex hormones will be highest in sunnier seasons.
MATERIALS AND METHODS
Study population
This was a cross-sectional study based on data extracted from our hospital medical records. We collected data on male subjects over 40 years of age who had undergone a comprehensive medical examination, including TT, free testosterone (FT), and serum 25(OH)D levels (n = 652), from July 2012 to February 2014. The exclusion criteria were as follows: receiving testosterone replacement therapy; presence of other health conditions that can affect the vitamin D level, such as cancer, parathyroid gland disease, liver disease, epilepsy, inflammatory bowel disease, malabsorption, celiac disease, gastric bypass, bowel surgery; and regular administration of vitamin D supplements within 3 months. The study protocol was approved by the Institutional Review Board of Pusan National University Hospital (IRB No. E - 2014014).
Data collection
Subjects were interviewed by a physician about their medical history, smoking status, alcohol intake, and exercise habits. Smoking status was categorized as either nonsmoker or current smoker. The questions regarding alcohol intake involved descriptions of the types of alcohol consumed, the weekly frequency of intake, and the daily consumption amount. The subjects were divided into alcohol drinker (>20 g day−1) and nondrinkers according to the amount of alcohol consumption per day.9 The frequency of exercise was noted as times per week of moderate intensity or more; regular exercise was defined as more than once a week.10 Hypertension (HTN) was defined as receiving current antihypertensive treatment or a blood pressure (BP) >140/90 mmHg. Diabetes mellitus (DM) was defined as receiving insulin or other oral hypoglycemic agents or a fasting plasma glucose (FPG) ≥126 mg dl−1. Dyslipidemia was defined as a fasting total cholesterol (TC) ≥220 mg dl−1 or receiving 3-hydroxy-2-methylglutaryl-CoA reductase inhibitors or fenofibrate. A trained examiner measured the height and body weight of patients wearing a light gown without shoes, to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated by dividing the weight into kilograms by the height in square meters (kg m−2). Waist circumference (WC) was measured as the smallest distance between the lower margin of the rib cage and the iliac crest, at the end of the normal expiration and to the nearest 0.1 cm. BP was measured twice while the men were seated after a 5-min rest using an automated BP measurement device (BP-203RV II, Colin Corp., Aichi, Japan), and the two results were averaged. The percentage of body fat, total muscle mass, and skeletal muscle mass were calculated by bioelectric impedance analysis (Inbody 3.0, Biospace Co., Ltd., Seoul, Korea), with the subjects in a standing position without shoes. For FPG and TC testing, venous blood was drawn from the antecubital vein between 8 and 9 a.m. after a 12-h overnight fasting. The blood samples were subsequently tested at a certified laboratory using an automatic blood analyzer (Hitachi 7600–110 chemical analyzer, Hitachi Co., Ltd., Tokyo, Japan). FPG was measured using the glucose oxidase method with a Synchron L × 20 (Beckman Coulter, Fullerton, CA, USA). TC was assessed using an autoanalyzer with the enzymatic colorimetric method Toshiba TBA200FR (Toshiba Co., Ltd., Tokyo, Japan). The seasonality of the blood draw was categorized into four groups: spring (March to May), summer (June to August), fall (September to November), and winter (December to February).
Measurement of serum 25-hydroxyvitamin D
Vitamin D status is indicated by the serum 25(OH)D level, which reflects vitamin D derived from both dermal production and dietary intake.11 To measure the level of serum 25(OH)D, venous blood samples were collected from the male subjects between 8 and 9 a.m. after a 12-h overnight fasting. The serum 25(OH)D level was evaluated by chemiluminescence immunoassay using the LIAISON®25 OH vitamin D Total Assay (DiaSorin Inc., Stillwater, USA) at the Eone Reference Laboratory (Seoul, Korea), which reported inter-assay and intra-assay coefficients of variation < 10%. According to recent clinical guidelines, vitamin D deficiency was defined as a serum 25(OH)D level < 20 ng ml−1.12
Hormone measurements
To determine the androgen status, venous blood samples were collected between 8 and 9 a.m. after a 12-h overnight fasting and TT and FT were measured using a radioimmunoassay procedure that assessed the Coat-A-Count TT and FT (Siemens Healthcare Diagnostics, Erlangen, Germany). Testosterone deficiency was defined as a serum TT level < 2.30 ng ml−1 or an FT level < 6.50 pg ml−1.13
Statistical analysis
Statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, IL, USA). Unless acknowledged otherwise, the data are presented as means ± standard deviations. Categorical variables are reported as frequencies and proportions. The male subjects were divided into status based on their serum 25(OH)D levels. The chi-square test was used to investigate categorical variables, as appropriate. To compare variables among each group, independent t-test and/or one-way analysis of variance, followed by a Scheffe post hoc test were performed as appropriate. Monthly variations in 25(OH)D, TT and FT levels were assessed by linear regression using the general linear model, adjusting for age. The univariate linear regression analysis was also used to assess correlations among TT, FT and several factors including 25(OH)D levels. The variables with P < 0.05 in univariate analysis were considered for inclusion in multivariate analysis. In addition, a testosterone deficiency was defined as low TT (<2.30 ng ml−1) or FT levels (<6.50 pg ml−1), and the odds ratio (OR) of testosterone deficiency was computed using multiple logistic regression analysis among the serum 25(OH)D level status after adjusting for confounders. P < 0.05 were considered to indicate statistical significance.
RESULTS
Patient characteristics
A total of 652 male subjects aged 40–80 years were included in this study. Their average age was 56.7 ± 7.9 years, and the mean serum 25(OH)D concentration was 21.23 ± 7.9 ng ml−1. Approximately, 48.6% of all subjects were vitamin D deficient [25(OH)D < 20 ng ml−1], with only 15% of subjects being vitamin D sufficient [25(OH)D ≥ 30 ng ml−1]. The mean values of TT and FT levels were 4.70 ± 1.6 ng ml−1 and 8.12 ± 3.3 pg ml−1, respectively. Table 1 presents the clinical characteristics of the study subjects according to the level of 25(OH)D. Men in the highest quartile of serum 25(OH)D had a higher TT level, a lower prevalence of dyslipidemia, and more frequent alcohol consumption than men in the lowest quartile. The FT level was also higher in the higher 25(OH)D quartiles; however, this difference was not significant.
Table 1.
Characteristics of study subjects according to 25(OH)D quartiles
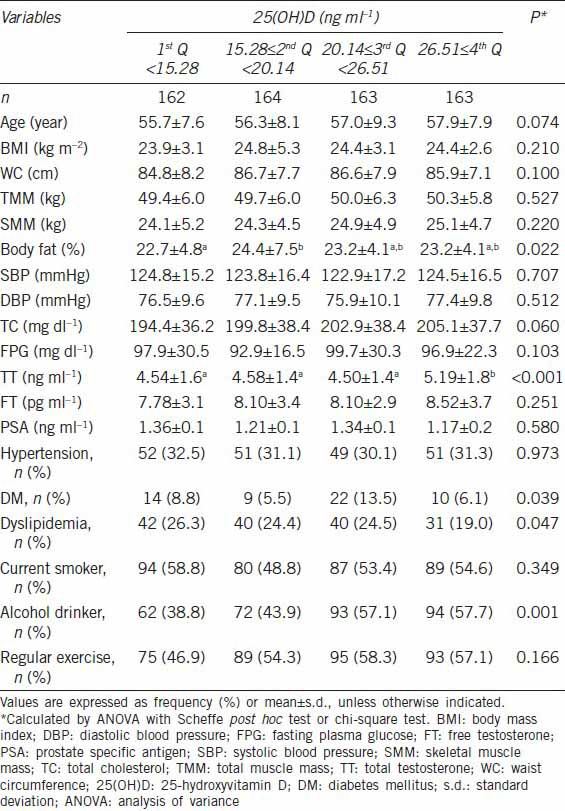
Monthly variability in 25-hydroxyvitamin D, total testosterone and free testosterone levels
In the evaluation of seasonal distribution, the 25(OH)D level was lowest in the spring and highest in the fall (17.76 ± 6.3 ng ml−1 vs 23.01 ± 8.3 ng ml−1, P = 0.016). Similarly, the FT level was lowest in the spring and highest in the summer (7.71 ± 3.2 pg ml−1 vs 8.68 ± 3.9 pg ml−1, P = 0.037). Although not significant, 25(OH)D and FT levels showed similar monthly variations (Figure 1): highest from August to October and lowest from March to April. However, we detected no evidence of seasonal or monthly variation in the TT level.
Figure 1.
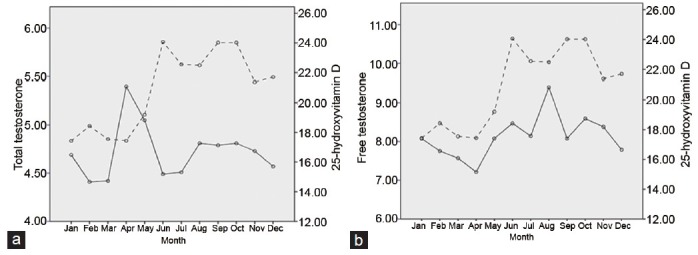
Monthly variation in mean levels of serum 25-hydroxyvitamin D (ng ml−1), total testosterone (ng ml−1) (a) and free testosterone (pg ml−1) (b) by ANCOVA adjusted for age.
Associations of total testosterone and free testosterone with 25-hydroxyvitamin D
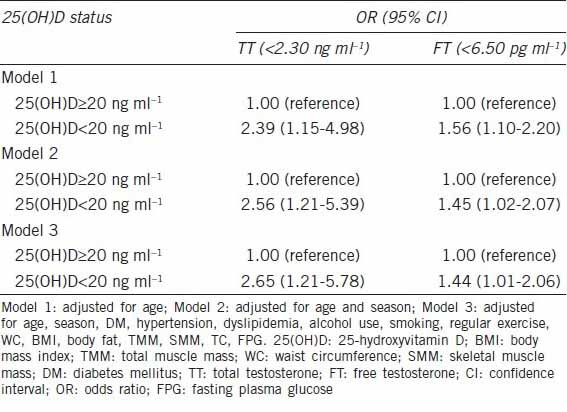
Table 2 presents a univariate and multivariate regression analysis between hormonal parameters and several factors. Percentage of body fat, WC, BMI, FPG, and 25(OH)D showed linear correlations, respectively, with TT. Total muscle mass, skeletal muscle mass, TC and 25(OH)D were positively associated with FT. In the multiple linear regression model, 25(OH)D showed a positive association with TT (P < 0.001) and FT (P = 0.008) (Table 2). After adjusting for age, season, WC, BMI, body fat, total muscle mass, skeletal muscle mass, DM, HTN, dyslipidemia, alcohol use, smoking, regular exercise, TC and FPG, vitamin D deficiency [25(OH)D < 20 ng ml−1] was independently associated with TT deficiency (<2.30 ng ml−1), with an OR of 2.65 (95% confidence interval [CI]: 1.21–5.78), and FT deficiency (<6.50 pg ml−1), with an OR of 1.44 (95% CI: 1.01–2.06) (Table 3).
Table 2.
Associations of total testosterone and free testosterone with several factors by linear regression analysis
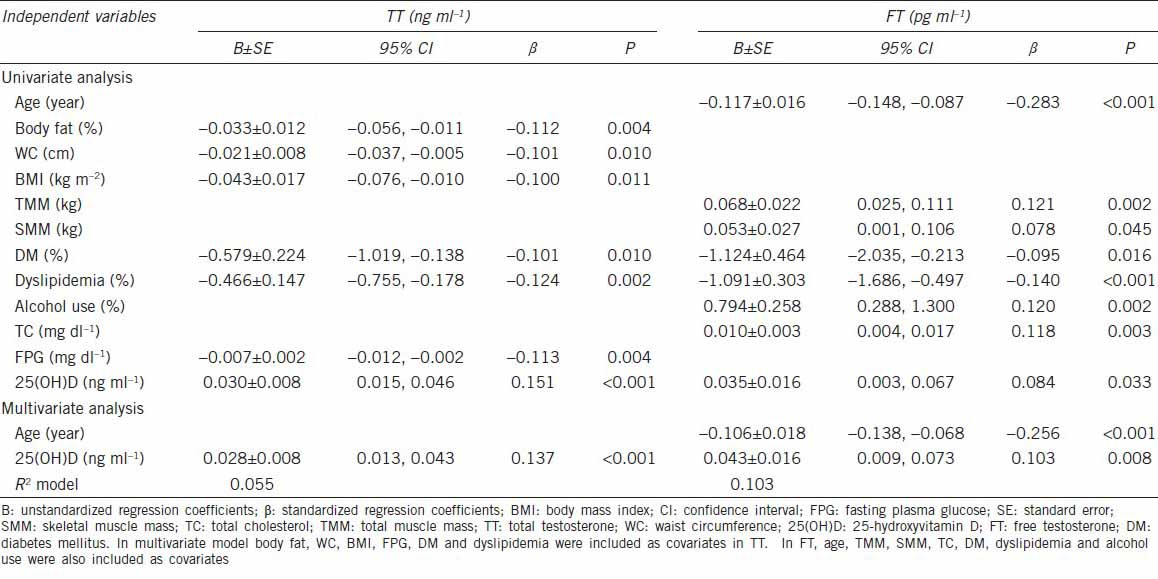
Table 3.
Association between deficiency of total testosterone or free testosterone and vitamin D status
DISCUSSION
The present study demonstrated a relationship between vitamin D levels and sex hormones in Korean men. Men with hypovitaminosis D had low levels of TT and FT. Although not significant, 25(OH)D and FT showed similar monthly variations. Our results support previously observed positive associations between serum 25(OH)D and TT and FT levels.7
Animal and human studies have demonstrated that vitamin D is essential for optimal male reproductive function. The effects of vitamin D are presumably mediated by the existence of VDR and vitamin D metabolizing enzymes in the adult male reproductive tract, male germ cells, and Leydig cells, which are the main sites of testosterone synthesis in males.14,15 In addition, evidence suggests that serum calcium is essential for male reproductive function, especially in spermatogenesis, sperm motility, and the acrosome reaction.16 Because vitamin D is a major regulator of calcium metabolism, vitamin D could influence male sex hormone synthesis. Several studies have supported hypotheses of a positive association between vitamin D and testosterone.7,17 However, it should be noted that the relationship between vitamin D and testosterone also reflects nonbiological associations due to their shared common precursors, such as cholesterol, or similar distributions of confounding factors. In our study, the positive associations between vitamin D and testosterone levels remained after adjusting for confounding factors associated with 25(OH)D levels and TT or FT levels, such as age, body composition, and health habits. Furthermore, adjusting for lipid levels did not change the positive associations between 25(OH)D and TT or FT. In contrast to a multicenter cross-sectional study17 of 3369 European men, the positive association between 25(OH)D and testosterone became nonsignificant following additional adjustments for chronic disease and health-related behaviors. The subjects of each study differed significantly in size, age, median vitamin D level, comorbidities, and relevant confounders such as region of residence, serum calcium level, and time of blood sampling. Thus, a direct comparison between these studies is not suitable.
Recently, Pilz et al.18 reported that daily supplementation of 83 mg vitamin D in overweight healthy men for 12 months increased both serum 25(OH)D and testosterone levels compared with administration of a placebo. However, because the sample size was small (n = 54) and men were participating in a diet program for weight reduction, the findings of the study cannot be extrapolated to other studies. As there is evidence that the prevalence of male hypogonadism differs between ethnic and racial populations,19 further experimental studies are needed to clarify the effect of vitamin D supplementation on testosterone levels in Korean men.
Because the 25(OH)D level depends mainly on sunlight exposure,20 it shows seasonal or monthly variation. Previous studies examining the seasonal variation in TT levels have yielded inconsistent results. Tancredi et al.21 reported no seasonal variation in FT levels among 5028 community-dwelling males aged 50–70 years involved in a clinic-based andropause assessment. In contrast, Svartberg et al.22 observed bimodal seasonal variation in TT with the highest levels in October and November and the lowest in June. They also detected a significant monthly variation in FT, with the highest level in December and the lowest in August. In the present study, monthly variation was observed in 25(OH)D levels, with a similar corresponding pattern in FT levels, although this association was not statistically significant. Unlike 25(OH)D and FT, we did not observe seasonal or monthly variation in TT concentrations. Seasonal variation in TT has been investigated in many cross-sectional and longitudinal studies, which have yielded inconsistent results.23 The discrepancy in results among studies is due to differing prevalence of hypovitaminosis D, daily sun exposure, and geographical areas. In the agreement with our results, many studies have found no seasonal variation in TT.23,24 Because FT tends to decrease with age, it may be clinically important to measure FT in older men from winter to spring.
Regarding the association between age and testosterone, it is well established that testicular testosterone synthesis decreases in men by 1%–2% per year after middle-age (>40 years).21 However, due to the age-related escalation in serum sex hormone binding globulin (SHBG) levels were positively associated with age,25 as were serum TT levels. In fact, we found that age was negatively linked to FT, but not TT. The age-related changes may be clinically important and partially responsible for the increased risk of various common diseases and mortality with age.17 Yet, the age-dependent normal range for testosterone has not been determined, and the cut-off value for male hypogonadism remains somewhat controversial. Experimental studies are needed to determine the cut-off age, which appropriately indicates male hypogonadism.
In agreement with previous studies,26,27,28 our study showed that men with dyslipidemia have insufficient vitamin D levels and hypogonadism more often than men without dyslipidemia. As low serum levels of testosterone and dyslipidemia are both associated with increased mortality and cardiovascular risk factors in men,29 the inverse correlation between testosterone and dyslipidemia is clinically important.
Our study has several limitations. First, because we selected men who visited a local university hospital, our findings do not represent the general population. Second, as a cross-sectional study, it was difficult to determine causal relationships among variables. In addition, because we could not obtain data regarding hypogonadism-related symptoms, not all subjects may have had true hypogonadism. TT represents the sum of SHBG-bound, free, and albumin-bound testosterone. The serum level of SHBG is easily affected by various conditions, such that TT measurements can be misleading indicators of hypogonadism. In addition, we were unable to investigate the role of serum PTH, which is a major determinant of vitamin D status.
CONCLUSION
The higher level of 25(OH)D was associated with higher TT and FT levels in Korean men. These associations persisted after adjusting for age, season, body composition, chronic disease, alcohol use, smoking, and exercise. Since people worldwide are living longer, middle-aged males with hypogonadism and a decreased quality-of-life are becoming increasingly more common. Recent guidelines30 suggest that testosterone replacement therapy be recommended for men with TT levels < 2.30 ng ml−1 or FT levels < 6.50 pg ml−1. However, the indications and contraindications for testosterone replacement therapy for male hypogonadism, including the short- and long-term risks and adverse effects, await evidence-based information. Owing to these concerns, it has been reported that only 5% of hypogonadal men actually accept treatment for their condition.30 In contrast, vitamin D supplementation is safe and cheap. If vitamin D deficiency is demonstrated to have effects on male hypogonadism in the prospective study in the future, replacement of vitamin D would be beneficial for Korean males with hypogonadism.
AUTHOR CONTRIBUTIONS
YJT, JGL drafted the manuscript and collected the data. YJK, SL carried out data analysis and drafted the manuscript. BMC, NCP performed the statistical analysis and drafted the manuscript. SSK, EHK carried out the hormonal study and manuscript preparation. DWJ, YHY designed the study, participated in its coordination, and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
REFERENCES
- 1.Pérez-López FR, Chedraui P, Fernández-Alonso AM. Vitamin D and aging: beyond calcium and bone metabolism. Maturitas. 2011;69:27–36. doi: 10.1016/j.maturitas.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Choi HS, Oh HJ, Choi H, Choi WH, Kim JG, et al. Vitamin D insufficiency in Korea - a greater threat to younger generation: the Korea national health and nutrition examination survey (KNHANES) 2008. J Clin Endocrinol Metab. 2011;96:643–51. doi: 10.1210/jc.2010-2133. [DOI] [PubMed] [Google Scholar]
- 3.Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, et al. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med. 2010;152:307–14. doi: 10.1059/0003-4819-152-5-201003020-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu PW, Rhew EY, Dyer AR, Dunlop DD, Langman CB, et al. 25-hydroxyvitamin D and cardiovascular risk factors in women with systemic lupus erythematosus. Arthritis Rheum. 2009;61:1387–95. doi: 10.1002/art.24785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aquila S, Guido C, Perrotta I, Tripepi S, Nastro A, et al. Human sperm anatomy: ultrastructural localization of 1alpha, 25-dihydroxyvitamin D receptor and its possible role in the human male gamete. J Anat. 2008;213:555–64. doi: 10.1111/j.1469-7580.2008.00975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nimptsch K, Platz EA, Willett WC, Giovannucci E. Association between plasma 25-OH vitamin D and testosterone levels in men. Clin Endocrinol (Oxf) 2012;77:106–12. doi: 10.1111/j.1365-2265.2012.04332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khaw KT, Dowsett M, Folkerd E, Bingham S, Wareham N, et al. Endogenous testosterone and mortality due to all causes, cardiovascular disease, and cancer in men: European prospective investigation into cancer in Norfolk (EPIC-Norfolk) Prospective Population Study. Circulation. 2007;116:2694–701. doi: 10.1161/CIRCULATIONAHA.107.719005. [DOI] [PubMed] [Google Scholar]
- 9.Corrao G, Bagnardi V, Zambon A, La Vecchia C. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev Med. 2004;38:613–9. doi: 10.1016/j.ypmed.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Haapanen N, Miilunpalo S, Pasanen M, Oja P, Vuori I. Association between leisure time physical activity and 10-year body mass change among working-aged men and women. Int J Obes Relat Metab Disord. 1997;21:288–96. doi: 10.1038/sj.ijo.0800403. [DOI] [PubMed] [Google Scholar]
- 11.Iqbal SJ. Vitamin D metabolism and the clinical aspects of measuring metabolites. Ann Clin Biochem. 1994;31:109–24. doi: 10.1177/000456329403100201. [DOI] [PubMed] [Google Scholar]
- 12.Vieth R, Bischoff-Ferrari H, Boucher BJ, Dawson-Hughes B, Garland CF, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–50. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 13.Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, et al. Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. Eur Urol. 2009;55:121–30. doi: 10.1016/j.eururo.2008.08.033. [DOI] [PubMed] [Google Scholar]
- 14.Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, et al. Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 2000;141:1317–24. doi: 10.1210/endo.141.4.7403. [DOI] [PubMed] [Google Scholar]
- 15.Blomberg Jensen M, Nielsen JE, Jørgensen A, Rajpert-De Meyts E, Kristensen DM, et al. Vitamin D receptor and vitamin D metabolizing enzymes are expressed in the human male reproductive tract. Hum Reprod. 2010;25:1303–11. doi: 10.1093/humrep/deq024. [DOI] [PubMed] [Google Scholar]
- 16.Blomberg Jensen M, Bjerrum PJ, Jessen TE, Nielsen JE, Joensen UN, et al. Vitamin D is positively associated with sperm motility and increases intracellular calcium in human spermatozoa. Hum Reprod. 2011;26:1307–17. doi: 10.1093/humrep/der059. [DOI] [PubMed] [Google Scholar]
- 17.Lee DM, Tajar A, Pye SR, Boonen S, Vanderschueren D, et al. Association of hypogonadism with vitamin D status: the European male ageing study. Eur J Endocrinol. 2012;166:77–85. doi: 10.1530/EJE-11-0743. [DOI] [PubMed] [Google Scholar]
- 18.Pilz S, Frisch S, Koertke H, Kuhn J, Dreier J, et al. Effect of vitamin D supplementation on testosterone levels in men. Horm Metab Res. 2011;43:223–5. doi: 10.1055/s-0030-1269854. [DOI] [PubMed] [Google Scholar]
- 19.Araujo AB, Travison TG, Leder BZ, McKinlay JB. Correlations between serum testosterone, estradiol, and sex hormone-binding globulin and bone mineral density in a diverse sample of men. J Clin Endocrinol Metab. 2008;93:2135–41. doi: 10.1210/jc.2007-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 21.Tancredi A, Reginster JY, Luyckx F, Legros JJ. No major month to month variation in free testosterone levels in aging males. Minor impact on the biological diagnosis of ‘andropause’. Psychoneuroendocrinology. 2005;30:638–46. doi: 10.1016/j.psyneuen.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Svartberg J, Jorde R, Sundsfjord J, Bønaa KH, Barrett-Connor E. Seasonal variation of testosterone and waist to hip ratio in men: the Tromsø study. J Clin Endocrinol Metab. 2003;88:3099–104. doi: 10.1210/jc.2002-021878. [DOI] [PubMed] [Google Scholar]
- 23.Brambilla DJ, O’Donnell AB, Matsumoto AM, McKinlay JB. Intraindividual variation in levels of serum testosterone and other reproductive and adrenal hormones in men. Clin Endocrinol (Oxf) 2007;67:853–62. doi: 10.1111/j.1365-2265.2007.02976.x. [DOI] [PubMed] [Google Scholar]
- 24.Maes M, Mommen K, Hendrickx D, Peeters D, D’Hondt P, et al. Components of biological variation, including seasonality, in blood concentrations of TSH, TT3, FT4, PRL, cortisol and testosterone in healthy volunteers. Clin Endocrinol (Oxf) 1997;46:587–98. doi: 10.1046/j.1365-2265.1997.1881002.x. [DOI] [PubMed] [Google Scholar]
- 25.Huhtaniemi I. Late-onset hypogonadism: current concepts and controversies of pathogenesis, diagnosis and treatment. Asian J Androl. 2014;16:192–202. doi: 10.4103/1008-682X.122336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cutillas-Marco E, Prosper AF, Grant WB, Morales-Suárez-Varela MM. Vitamin D status and hypercholesterolemia in Spanish general population. Dermatoendocrinol. 2013;5:358–62. doi: 10.4161/derm.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Cruz E, Piqueras M, Huguet J, Perez-Marquez M, Gosalbez D, et al. Hypertension, dyslipidemia and overweight are related to lower testosterone levels in a cohort of men undergoing prostate biopsy. Int J Impot Res. 2012;24:110–3. doi: 10.1038/ijir.2011.55. [DOI] [PubMed] [Google Scholar]
- 28.Haring R, Baumeister SE, Völzke H, Dörr M, Felix SB, et al. Prospective association of low total testosterone concentrations with an adverse lipid profile and increased incident dyslipidemia. Eur J Cardiovasc Prev Rehabil. 2011;18:86–96. doi: 10.1097/HJR.0b013e32833c1a8d. [DOI] [PubMed] [Google Scholar]
- 29.Fui MN, Dupuis P, Grossmann M. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J Androl. 2014;16:223–31. doi: 10.4103/1008-682X.122365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gooren LJ, Behre HM, Saad F, Frank A, Schwerdt S. Diagnosing and treating testosterone deficiency in different parts of the world. Results from global market research. Aging Male. 2007;10:173–81. doi: 10.1080/13685530701600885. [DOI] [PubMed] [Google Scholar]


