Abstract
This study aimed to evaluate the role of serum lipid profiles as novel biomarkers in predicting pathological characteristics of prostate cancer (PCa). We retrospectively analyzed 322 consecutive patients with clinically localized PCa receiving radical prostatectomy (RP) and extended pelvic lymphadenectomy. Unconditional logistic regression was used to estimate the prostatectomy Gleason score (pGS), pathological stage and lymph node involvement (LNI) in RP specimens. Preoperative prostate-specific antigen (PSA) levels, biopsy GS (bGS), and preoperative tumor, node, metastasis staging were used as basic variables to predict postoperative pathological characteristics. Preoperative serum lipid profiles were introduced as potential predictors. A receiver operating characteristic (ROC) curve was used to determine predictive efficacy. Significant differences in pathological characteristics were observed among patients with normal and abnormal total cholesterol (TC), triglyceride (TG), and low-density lipoprotein (LDL) levels, with the exception of pGS in the TG group. Multivariable regression analysis revealed that the odds ratio for high levels of TC for LNI compared with normal TC levels was 6.386 (95% confidence interval [CI] 1.510–27.010), 3.270 (95% CI: 1.470–7.278) for high levels of TG for pT3–4 disease, and 2.670 (95% CI: 1.134–6.287) for high levels of LDL for pGS. The area under the ROC curve of the models with dyslipidemia was larger than that in models without dyslipidemia, in predicting pathological characteristics. Abnormal TC, TG, and LDL levels are significantly associated with postoperative pathological status in PCa patients. Together with preoperative PSA levels, bGS, and clinical stage, dyslipidemia is more accurate in predicting pathological characteristics.
Keywords: biological markers, dyslipidemias, lipids, pathology, prostatic neoplasms
INTRODUCTION
In developed countries, prostate cancer (PCa) is the most common malignancy in men and the second leading cause of cancer-related mortality.1 In China, the incidence of PCa has gradually increased over recent decades. According to the latest Chinese Cancer Registry Annual Report (2012), PCa has become the 6th most prevalent cancer and the 9th leading cause of cancer-related mortality in men, especially in urban areas.2,3 Although the exact mechanisms that underlie PCa carcinogenesis are not well understood, growing evidence suggests that it is partly due to Western lifestyle factors, for example, a high-fat diet.4,5
Many studies have demonstrated that, as a predominant component of metabolic syndrome, dyslipidemia plays an important role in the carcinogenesis of various cancers. An increased risk of colon cancer has been observed in people with high triglyceride (TG) levels,6 and hypercholesterolemia is considered as a risk factor for rectal cancer development.7 A positive association between elevated low-density lipoprotein (LDL) levels and kidney cancer has been observed,8 and low high-density lipoprotein (HDL) levels are associated with breast cancer and non-Hodgkin lymphoma.9,10 To date, a large number of epidemiological studies have revealed an association between dyslipidemia and development of PCa.11,12,13,14 In addition, patients with low total cholesterol (TC) levels are less likely to present with high-grade PCa (Gleason score [GS]≥8),15 and inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase, also called statins and used for dyslipidemia treatment, reduce the risk of advanced PCa.16,17 However, only a few studies have targeted the relationship between abnormal serum lipid levels and postoperative pathological status of PCa.
Together with preoperative prostate-specific antigen (PSA) levels, GS and pathological stage are critical risk factors determining the subsequent interventions after radical prostatectomy (RP); the most effective treatment for PCa patients with organ-confined disease (OCD). However, preoperative imaging currently has limitations with an accurate diagnosis of OCD and micrometastasis to pelvic lymph nodes. Given the intimate relationship between dyslipidemia and PCa, we hypothesized that abnormal levels of serum lipid profiles might be associated with postoperative pathological status and stage. The present investigation was designed to evaluate serum lipid profiles as novel biomarkers to predict pathological characteristics in PCa patients receiving RP.
PATIENTS AND METHODS
Study subjects
This was a retrospective analysis of 322 consecutive patients with clinically localized PCa who underwent RP and extended pelvic lymphadenectomy in Fudan University, Shanghai Cancer Center (FUSCC) from August 2012 to June 2013. None of the patients enrolled received neoadjuvant therapy. Data on age, history of hypertension or diabetes mellitus, family history of PCa, body mass index (BMI), smoking status, lipid profiles, statin usage, preoperative PSA levels, biopsy GS (bGS), histopathology, and stage at diagnosis (tumor, node, metastasis [TNM] classification) were obtained from electronic records and medical charts. Enzymatic methods were used to detect fasting serum lipid profiles by a Hitachi 7600 automatic clinical chemistry analyzer (Boehringer Mannheim, Mannheim, Germany) with reagent kits supplied by the manufacturer. Protocols were approved by the Institutional Research Review Boards of FUSCC, and written informed consent was obtained from all subjects.
Body mass index was defined as weight/height2 (kg m−2), and stratified according to guidelines for prevention and control of overweight and obesity in Chinese adults (<24: normal; ≥ 24: overweight).18 Serum lipid profiles were stratified in accordance with the Chinese Guidelines on Adult Dyslipidemias (2007 version);19 preoperative PSA levels, bGS, and clinical stage were divided into high-, medium-, and low-risk groups, respectively.20
To establish a relationship between preoperative predictive factors and postoperative pathological characteristics, preoperative PSA levels, bGS, and clinical stage were used as basic variables, and preoperative lipid profiles were introduced as potential predictive variables. Age, BMI, hypertension, diabetes, family history of PCa, smoking status, and statin usage were included in the analyses as potential confounders.
Statistical analysis
Differences in categorical variables were compared using χ2 tests. Unconditional multiple logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) of the probability of lymph node involvement (LNI), pT3–4 disease, and prostatectomy GS (pGS) in RP specimens. A receiver operating characteristic (ROC) curve was used to determine the efficacy of the predictive variables. The values of P were two sided, and P < 0.05 was considered statistically significant. SPSS version 20.0 (IBM Corporation, Somers, NY, USA) was used for statistical analyses.
RESULTS
The study included 322 cases of newly diagnosed PCa (age range: 47–79 years, median age: 68 years), whose preoperative PSA levels were in the range of 3.7–143.0 ng ml−1 (median: 14.34 ng ml−1). There were 172, 90, and 60 patients with ≤cT2a, cT2b, and cT2c disease, respectively, according to the American Joint Committee on Cancer TNM staging system (2002). Distribution of PCa cases according to demographic and clinical characteristics is indicated in Table 1.
Table 1.
Demographic and clinical characteristics stratified by serum lipid profiles in PCa patients receiving RP
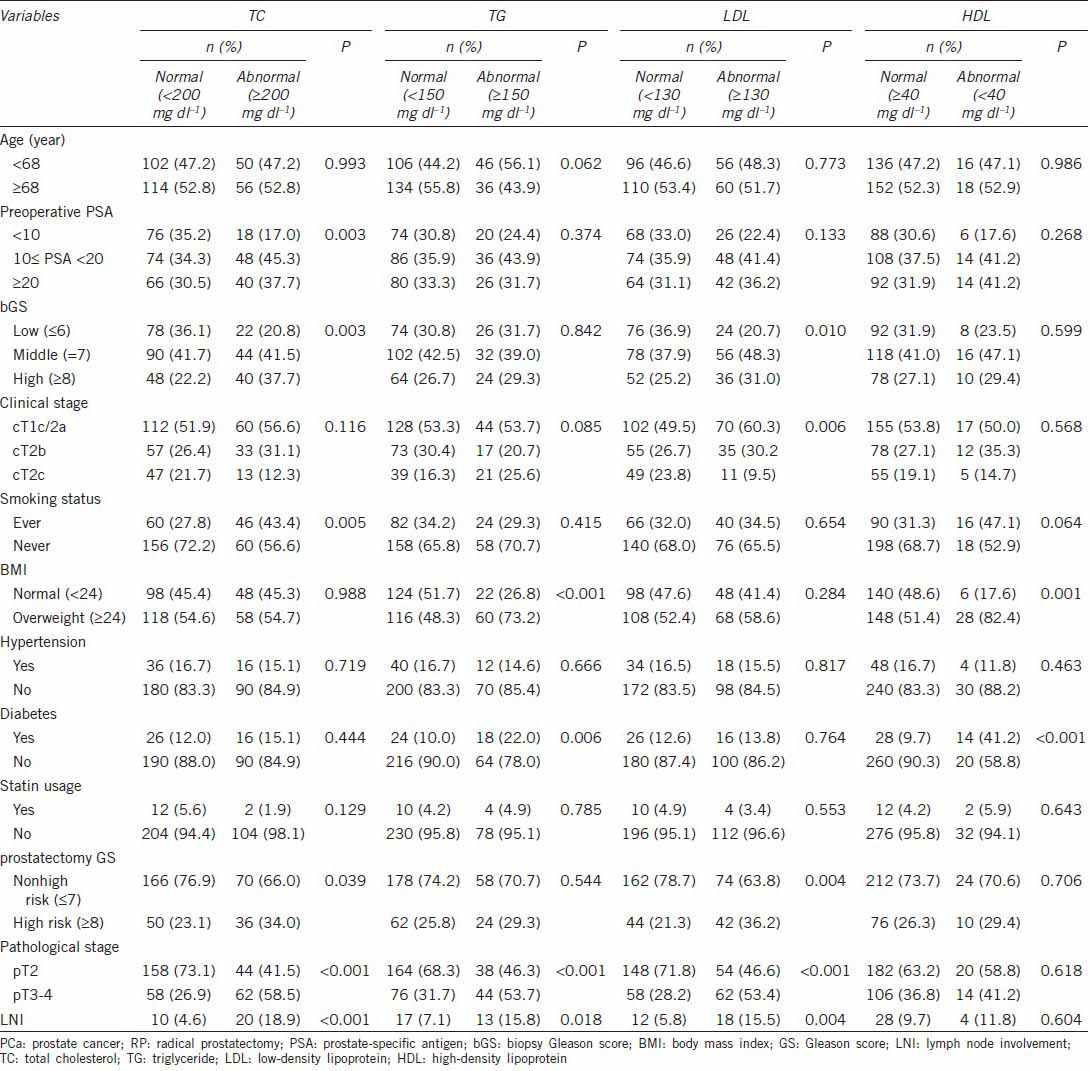
No significant differences in age, hypertension, and statin usage were observed between patients with normal and abnormal levels in TC, TG, LDL and HDL groups. Differences in preoperative PSA levels, bGS, clinical stage, smoking status, BMI, diabetes, pGS, pathological stage, and LNI varied in different groups. As far as postoperative pathological characteristics were concerned, notable differences existed among the TC, TG, and LDL groups, with the exception of pGS in the TG group. Interestingly, there were no differences observed between patients with normal and abnormal HDL levels (Table 1).
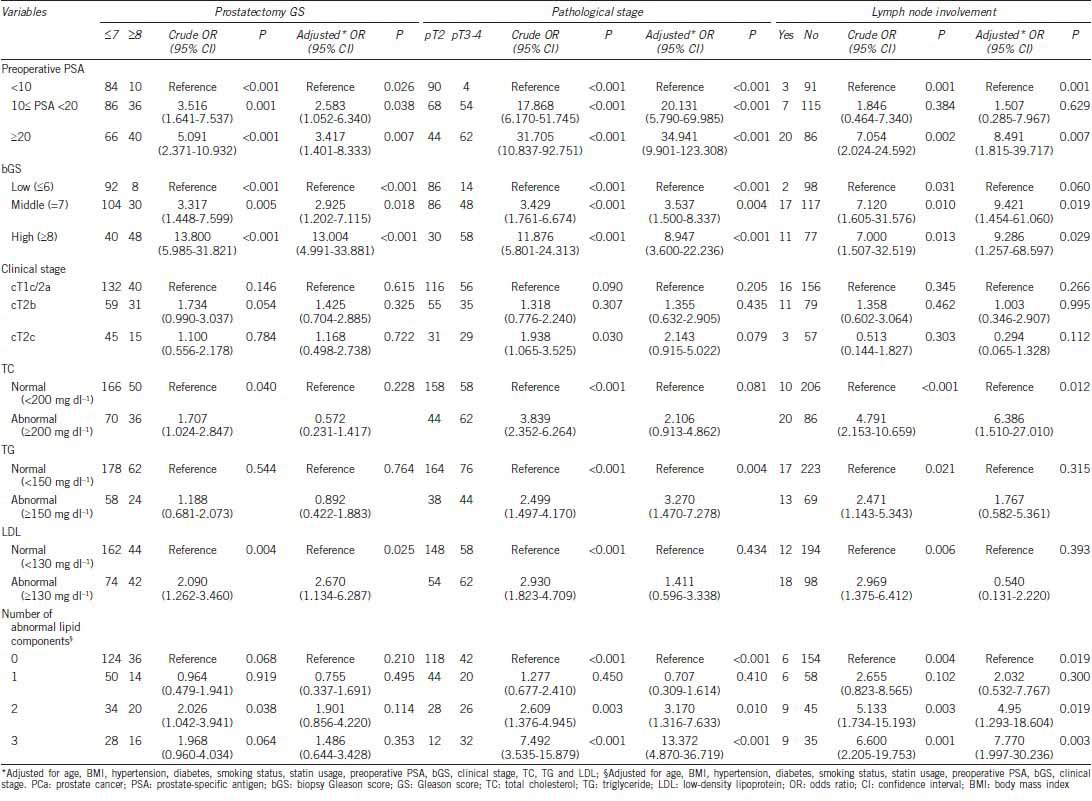
We investigated the association of postoperative pathological characteristics with preoperative PSA levels, bGS, a clinical stage, and TC, TG, and LDL levels, using univariate and multivariable logistic regression models, respectively. As shown in Table 2, after adjusting for potential confounders, high levels of TC were associated with increased risk of LNI (OR: 6.386, 95% CI: 1.510–27.010), and elevated TG levels were associated with a more than two-fold increased risk of pT3–4 disease (OR: 3.270, 95% CI: 1.470–7.278). In addition, high levels of LDL were an independent predictor of pGS ≥8 (OR: 2.670, 95% CI: 1.134–6.287). Further, we examined the OR (95% CI) of postoperative pathological characteristics when a patient harbored one, two or all three of the lipid-related risk factors. With the increase of the number of abnormal lipid components, a higher probability of pT3–4 disease and LNI was observed, yet no significant association was found with respect to pGS, whether in univariate or multivariable logistic regression models (Table 2).
Table 2.
Logistic regression analysis of the association between serum lipid profiles and pathological characteristics in PCa patients
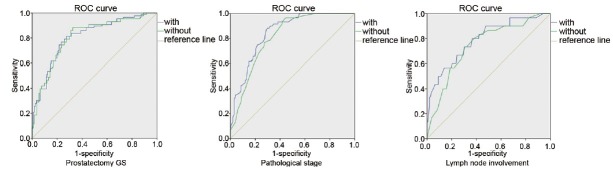
We used ROC curves to detect the efficacy of predictive variables for postoperative pathological characteristics of PCa. The models were constructed using preoperative PSA levels, bGS, and clinical stage, with or without lipid profiles. As shown in Figure 1, area under the ROC curve of the models with dyslipidemia was larger than that without dyslipidemia, with regard to all the pathological status, including pGS, pT3–4 disease, and LNI.
Figure 1.
ROC curve of pathological characteristics and dyslipidemia: area under the ROC curve of the models with dyslipidemia was larger than that without dyslipidemia, with regard to pGS (0.810 vs 0.808), pT3–4 disease (0.848 vs 0.814), and LNI (0.791 vs 0.745). ROC: receiver operating characteristic; pGS: prostatectomy Gleason score; LNI: lymph node involvement.
DISCUSSION
There was a significant association between dyslipidemia and postoperative pathological characteristics, including pGS, pT3–4 disease, and LNI, and the association persisted after adjusting for multiple risk factors for PCa and other lipid parameters. Furthermore, ROC curve analysis suggested that abnormal lipid levels might be efficient predictors of pathological status of PCa.
In recent years, much attention has been focused on the association of lipid profiles with PCa, with conflicting conclusions. As far as TC is concerned, although controversy remains, many researchers have found a positive association between TC levels and total PCa incidence.21,22,23 Some prospective studies did not show increased total PCa risks in populations with high TC levels, whereas increased risk of high-grade or advanced PCa was seen.24,25,26 Apart from this epidemiological evidence, statins are also protective against the development of advanced PCa.16,17 Our study adds to the literature supporting the relationship between dyslipidemia and PCa development. Mondul et al.26 conducted a large cohort study and found that men with normal TC levels were less likely to develop high-grade PCa. Similarly, another study conducted by Platz et al.25 reported that men with < 200 mg dl−1 TC had a lower risk of PCa with GS > 7. Our study focused on the relationship between dyslipidemia and postoperative pathological characteristics of PCa. Although the association of different serum lipid components with pathological status and stage varied, we did observe that dyslipidemia contributed to PCa progression. Several vital prognostic factors, such as pGS, pT3–4 disease, and LNI, were closely related to elevated LDL, TG, and TC levels, respectively. Abnormal HDL levels seemed not to be associated with PCa prognosis in our study, although a close relationship was reported between low HDL levels and PCa risk.23 However, a large-scale external validation of these results is warranted.
At present, the exact molecular mechanisms associated with the role of dyslipidemia in PCa carcinogenesis remain unclear, although several explanations have been proposed. Abnormal regulation of cholesterol metabolism may result in elevated cholesterol levels in PCa cells. Meanwhile, aberrant lipid metabolism can influence signal transduction in PCa, for example, through promoting cancer cell growth and inhibiting apoptosis.27 Androgen receptors located in PCa cells can recruit several transcription factors involved in lipid metabolism,28 of which sterol regulatory element binding protein 2 is notably upregulated in PCa cell xenograft tumors.29 In addition, several important signaling pathways involved in carcinogenesis, such as Akt and sonic hedgehog pathways, are the cholesterol sensitive.30,31 Hence, abnormal serum lipid levels may promote these pro-carcinogenic process in PCa.
Given the important role of dyslipidemia in PCa development and progression, we hypothesized that lipid profiles might be potential biomarkers to predict advanced disease. The present study verified our speculation. Besides preoperative PSA, bGS, and clinical stage, we introduced preoperative TC, TG, and LDL levels into the predictive model, and higher efficiency was observed. Therefore, our results suggest that information about preoperative serum lipid profiles may improve the accuracy of pathological prediction and enable the choice of more appropriate medical intervention.
Furthermore, our results provided another clinical implication. It is well known that dyslipidemia functions as a critical risk factor in the development of coronary artery disease (CAD), which remains a leading cause of health impairment worldwide.32 Intriguingly, some recent studies have found a significant association of CAD with increased PCa diagnosis, and speculated that CAD share etiology with PCa.33,34 Now that dyslipidemia plays an important role in both CAD and PCa development, we believe that better control of dyslipidemia may obtain more benefits than we have expected.
Our study had certain limitations and constraints. First, it was conducted in a single medical center with a small sample, and the results were subject to inherent biases of a retrospective nature. Second, unlike the Partin table,35 we stratified preoperative PSA levels, bGS, and clinical stage in a simple manner. It is uncertain whether more precise stratification might have influenced the predictive efficacy. Finally, although dyslipidemia was suggested as a prognostic factor, different serum lipid components had various relationships with pathological status. A consistent association was not observed between a certain lipid component and pathological characteristics. Clearly, additional prospective studies are necessary to validate our observations in a large population.
CONCLUSIONS
The present study found a significant association between elevated serum TC, TG, and LDL levels and pathological characteristics in PCa patients. Together with preoperative PSA levels, bGS, and clinical stage, dyslipidemia is a novel and useful predictive biomarker for advanced PCa patients. Dyslipidemia is common and preventable; therefore, large prospective, population-based studies are warranted.
AUTHOR CONTRIBUTIONS
GMZ and XJQ designed the study, collected, analyzed and interpreted the clinical data, and wrote the manuscript. HLZ, WJX, YZ and CYG collected part of the patients’ clinical data. BD and GHS analyzed part of the data. DWY supervised the project and revised the manuscript. All authors vouch for the respective data and analysis, approved the final version and agreed to publish the manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interest.
ACKNOWLEDGMENTS
This study was supported in part by grants from National Natural Science Foundation of China (No. 81272837) and Advanced Technology Foundation of Shanghai’ Hospital (SHDC12013122).
REFERENCES
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.He J, Chen WQ. Beijing: Military Medical Science Press; 2012. Chinese Cancer Registry Annual Report. [Google Scholar]
- 3.Zhu Y, Wang HK, Qu YY, Ye DW. Prostate cancer in East Asia: evolving trend over the last decade. Asian J Androl. 2014 doi: 10.4103/1008-682X.132780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pelser C, Mondul AM, Hollenbeck AR, Park Y. Dietary fat, fatty acids, and risk of prostate cancer in the NIH-AARP diet and health study. Cancer Epidemiol Biomarkers Prev. 2013;22:697–707. doi: 10.1158/1055-9965.EPI-12-1196-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meyer F, Bairati I, Shadmani R, Fradet Y, Moore L. Dietary fat and prostate cancer survival. Cancer Causes Control. 1999;10:245–51. doi: 10.1023/a:1008913307947. [DOI] [PubMed] [Google Scholar]
- 6.Inoue M, Noda M, Kurahashi N, Iwasaki M, Sasazuki S, et al. Impact of metabolic factors on subsequent cancer risk: results from a large-scale population-based cohort study in Japan. Eur J Cancer Prev. 2009;18:240–7. doi: 10.1097/CEJ.0b013e3283240460. [DOI] [PubMed] [Google Scholar]
- 7.Wulaningsih W, Garmo H, Holmberg L, Hammar N, Jungner I, et al. Serum lipids and the risk of gastrointestinal malignancies in the Swedish AMORIS study. J Cancer Epidemiol 2012. 2012 doi: 10.1155/2012/792034. 792034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang GM, Zhu Y, Luo L, Zhang HL, Gu CY, et al. Prevalence of dyslipidaemia in patients with renal cell carcinoma: a case-control study in China. BJU Int. 2014;113:E75–81. doi: 10.1111/bju.12581. [DOI] [PubMed] [Google Scholar]
- 9.Kucharska-Newton AM, Rosamond WD, Mink PJ, Alberg AJ, Shahar E, et al. HDL-cholesterol and incidence of breast cancer in the ARIC cohort study. Ann Epidemiol. 2008;18:671–7. doi: 10.1016/j.annepidem.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim U, Gayles T, Katki HA, Stolzenberg-Solomon R, Weinstein SJ, et al. Serum high-density lipoprotein cholesterol and risk of non-Hodgkin lymphoma. Cancer Res. 2007;67:5569–74. doi: 10.1158/0008-5472.CAN-07-0212. [DOI] [PubMed] [Google Scholar]
- 11.Solomon KR, Freeman MR. The complex interplay between cholesterol and prostate malignancy. Urol Clin North Am. 2011;38:243–59. doi: 10.1016/j.ucl.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi N, Matsushima M, Yamamoto T, Sasaki H, Takahashi H, et al. The impact of hypertriglyceridemia on prostate cancer development in patients aged≥60 years. BJU Int. 2012;109:515–9. doi: 10.1111/j.1464-410X.2011.10358.x. [DOI] [PubMed] [Google Scholar]
- 13.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21:61–8. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bravi F, Scotti L, Bosetti C, Talamini R, Negri E, et al. Self-reported history of hypercholesterolaemia and gallstones and the risk of prostate cancer. Ann Oncol. 2006;17:1014–7. doi: 10.1093/annonc/mdl080. [DOI] [PubMed] [Google Scholar]
- 15.Shafique K, McLoone P, Qureshi K, Leung H, Hart C, et al. Cholesterol and the risk of grade-specific prostate cancer incidence: evidence from two large prospective cohort studies with up to 37 years’ follow up. BMC Cancer. 2012;12:25. doi: 10.1186/1471-2407-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-lowering drugs and prostate cancer risk: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 2007;16:2226–32. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 17.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 18.Chen C, Lu FC. Department of Disease Control Ministry of Health P R C. The guidelines for prevention and control of overweight and obesity in Chinese adults. Biomed Environ Sci. 2004;17(Suppl):1. [PubMed] [Google Scholar]
- 19.Joint Committee for Developing Chinese guidelines on Prevention and Treatment of Dyslipidemia in Adults. Chinese guidelines on prevention and treatment of dyslipidemia in adults. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:390–419. [PubMed] [Google Scholar]
- 20.D’Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–74. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 21.Iso H, Ikeda A, Inoue M, Sato S, Tsugane S, et al. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer. 2009;125:2679–86. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 22.Kitahara CM, Berrington de González A, Freedman ND, Huxley R, Mok Y, et al. Total cholesterol and cancer risk in a large prospective study in Korea. J Clin Oncol. 2011;29:1592–8. doi: 10.1200/JCO.2010.31.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magura L, Blanchard R, Hope B, Beal JR, Schwartz GG, et al. Hypercholesterolemia and prostate cancer: a hospital-based case-control study. Cancer Causes Control. 2008;19:1259–66. doi: 10.1007/s10552-008-9197-7. [DOI] [PubMed] [Google Scholar]
- 24.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123:1693–8. doi: 10.1002/ijc.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, et al. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18:2807–13. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mondul AM, Weinstein SJ, Virtamo J, Albanes D. Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control. 2011;22:1545–52. doi: 10.1007/s10552-011-9831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hager MH, Solomon KR, Freeman MR. The role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab Care. 2006;9:379–85. doi: 10.1097/01.mco.0000232896.66791.62. [DOI] [PubMed] [Google Scholar]
- 28.Heemers H, Vanderhoydonc F, Roskams T, Shechter I, Heyns W, et al. Androgens stimulate coordinated lipogenic gene expression in normal target tissues in vivo. Mol Cell Endocrinol. 2003;205:21–31. doi: 10.1016/s0303-7207(03)00205-3. [DOI] [PubMed] [Google Scholar]
- 29.Ettinger SL, Sobel R, Whitmore TG, Akbari M, Bradley DR, et al. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 2004;64:2212–21. doi: 10.1158/0008-5472.can-2148-2. [DOI] [PubMed] [Google Scholar]
- 30.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate Akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62:2227–31. [PubMed] [Google Scholar]
- 31.Oh HY, Lee EJ, Yoon S, Chung BH, Cho KS, et al. Cholesterol level of lipid raft microdomains regulates apoptotic cell death in prostate cancer cells through EGFR-mediated Akt and ERK signal transduction. Prostate. 2007;67:1061–9. doi: 10.1002/pros.20593. [DOI] [PubMed] [Google Scholar]
- 32.Okrainec K, Banerjee DK, Eisenberg MJ. Coronary artery disease in the developing world. Am Heart J. 2004;148:7–15. doi: 10.1016/j.ahj.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 33.Neugut AI, Rosenberg DJ, Ahsan H, Jacobson JS, Wahid N, et al. Association between coronary heart disease and cancers of the breast, prostate, and colon. Cancer Epidemiol Biomarkers Prev. 1998;7:869–73. [PubMed] [Google Scholar]
- 34.Thomas JA, 2nd, Gerber L, Bañez LL, Moreira DM, Rittmaster RS, et al. Prostate cancer risk in men with baseline history of coronary artery disease: results from the REDUCE Study. Cancer Epidemiol Biomarkers Prev. 2012;21:576–81. doi: 10.1158/1055-9965.EPI-11-1017. [DOI] [PubMed] [Google Scholar]
- 35.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, et al. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58:843–8. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]