Abstract
Objectives
The purpose of this study was to evaluate the efficacy of mineral trioxide aggregate (MTA), Biodentine and Propolis as pulpotomy medicaments in primary dentition, both clinically and radiographically.
Materials and Methods
A total of 75 healthy 3 to 10 yr old children each having at least one carious primary molar tooth were selected. Random assignment of the pulpotomy medicaments was done as follows: Group I, MTA; Group II, Biodentine; Group III, Propolis. All the pulpotomized teeth were evaluated at 3, 6, and 9 mon clinically and radiographically, based on the scoring criteria system.
Results
The clinical success rates were found to be similar among the three groups at 3 and 6 mon where as a significant decrease in success rate was observed in Group III (84%) compared to both Group I (100%) and Group II (100%) at 9 mon. Radiographic success rates over a period of 9 mon in Groups I, II, and III were 92, 80, and 72%, respectively.
Conclusions
Teeth treated with MTA and Biodentine showed more favorable clinical and radiographic success as compared to Propolis at 9 mon follow-up.
Keywords: Biodentine, Mineral trioxide aggregate, Pulpotomy, Vital pulp therapy
Introduction
The preservation of pulp vitality in primary teeth affected by caries or trauma is critically important for maintaining the integrity of the dental arches as well as for preserving esthetics prior to the eruption of the permanent successors.1 Choice of procedure employed in vital pulp therapy depends on the extent of pulpal inflammation. Pulpotomy is the preferred procedure when only the coronal pulp is inflamed due to bacterial penetration following carious, traumatic or iatrogenic causes, and the radicular pulp is free from inflammation.
Historically, a wide variety of medicaments have been suggested to be used as pulpotomy medicaments. Calcium hydroxide was the first agent in this context, but is usually not recommended in primary dentition owing to its increased alkalinity causing necrosis, inflammation and dystrophic calcification in the pulp tissue leading to internal resorption.2 Sweet introduced Formocresol as pulpotomy dressing that has been most commonly used in primary molars during past several decades.3 Potential carcinogenic and mutagenic effects of formocresol led to its limited use as pulpotomy medicament.4,5,6
In recent years, the introduction of new bio-inductive and regenerative dental materials like mineral trioxide aggregate (MTA) has witnessed new innovations in dentistry. MTA has been used as successful medicament for vital pulp therapy procedures, apexification and repair of root perforations.7,8 It produces significantly effective dentinal bridging in a short time period with significantly lesser inflammation and pulpal necrosis than calcium hydroxide.9,10 MTA also has some associated drawbacks related to mechanical properties, handling characteristics, cost, and composition.11,12 To overcome these shortcomings, efforts have led to the development of new calcium silicate based material called Biodentine with active bio-silicate technology. Biodentine consists of powder and liquid components. The powder mainly contains tricalcium and dicalcium silicate as well as calcium carbonate. The liquid contains calcium chloride and water reducing agent. There is ample evidence for positive effects of Biodentine on vital pulp cells, for stimulating tertiary dentin formation, and early formation of reparative dentin.13,14,15,16
Some natural materials from the field of traditional medicine have also been introduced as medicaments in vital pulp therapy as an alternative to commercially available artificial products. Propolis, a resinous substance collected from trees and shrubs by honey bees is known for its claimed beneficial effects on human health, and has also gained popularity in dentistry as an intracanal medicament, as a cariostatic agent, for storage of avulsed teeth and also as a root canal irrigant due to its known anti-inflammatory and immunomodulating properties.17,18,19 In vitro studies have been done to evaluate toxicity of propolis on human cell lines.20 Also, animal studies on the ethanolic extract of propolis has suggested that propolis promotes bone regeneration and induction of hard tissue bridge formation in pulpotomy and pulp capping.21,22 The pulp responses to propolis evaluated in these studies have been found to be comparable to MTA. Hence, the present study was designed to compare the efficacy of MTA, Biodentine and Propolis as pulpotomy medicaments in primary teeth, both clinically and radiographically.
Materials and Methods
The study was conducted at the Department of Pediatric and Preventive Dentistry, Faculty of Dental Sciences, King George's Medical University, UP, Lucknow. The study protocol was approved by the Ethical Committee of King George's Medical University, UP, Lucknow. The clinical procedure and associated risks and benefits were fully explained to the parents or legal guardian of the participants. Written informed consent were obtained from the parents or legal guardian of the participants prior to investigation. All participants were screened by taking a detailed history and performing a thorough clinical and radiographical examination.
A total of 90 primary molar teeth in 90 patients aged from 3 to 10 years were assessed for enrollment in the study, out of which 15 patients were excluded (10, not meeting the inclusion criteria; 5, refusal to participate). Seventy five patients having at least one carious primary molar were finally selected that fulfilled the following inclusion criteria: cooperative; presence of symptom-free deep carious lesions; presence of at least two-thirds of the root length radiographically; restorable tooth. The final selection for inclusion in the study was done intraoperatively, only when hemostasis was adequately achieved within 5 minutes after coronal pulp amputation. The exclusion criteria were as follows: history of systemic diseases; teeth showing clinical and radiographical evidence of pulp degeneration such as history of spontaneous or nocturnal pain, tenderness to percussion or palpation, pathologic mobility, swelling or fistulous tract, periodontal ligament (PDL) space widening, internal root resorption, external root resorption, furcal radiolucency/inter-radicular bone destruction and/or periapical bone destruction; teeth without permanent successor and teeth requiring more than 5 minutes to achieve hemostasis during clinical procedure. Patients eliciting history of known allergy to pollens associated with propolis were also excluded from the study.
All selected primary teeth were randomly divided into three groups with 25 each depending on the type of pulpotomy medicament used. Randomization of the pulpotomy medicament used was done by envelope draw method for all the selected teeth, present either in different patients or in the same patient. After administering local anesthesia, the tooth was isolated with a rubber dam. All caries were removed and coronal access was gained using a sterile No. 330 high speed bur with water spray to expose the pulp chamber. A spoon excavator was used for coronal pulp amputation. One or more sterile cotton pellets moistened with distilled water were placed over the pulp stumps, and light pressure was applied for 2 - 3 minutes for obtaining hemostasis. Depending on the type of pulp medicament, the teeth were treated as follows:
Group I
MTA paste (ProRoot MTA, Dentsply, Tulsa, OK, USA) was prepared as per the manufacturers' instructions to obtain a putty-like consistency. The mixture was delivered to the pulp stumps and condensed lightly with a moistened sterile cotton pellet to ensure a thickness of 2 to 3 mm. A thick mix of zinc oxide eugenol (ZOE) cement base was applied over the MTA followed by glass ionomer cement (GIC) restoration.
Group II
Biodentine (Septodont, Saint-Maur-des-Fossés, France) paste was obtained by mixing premeasured unit dose capsules for 30 seconds at 4,200 rpm in a triturator to obtain putty-like consistency. It was then carried with an amalgam carrier and condensed lightly with a metal condenser on the pulp stumps, in a thickness of 2 - 3 mm. After 12 minutes, allowing Biodentine to set, the access cavity was filled with a thick mix of ZOE cement followed by GIC as final restoration.
Group III
One and half gram of Standardized Propolis Extract powder (HI-Tech Natural Products Ltd., New Delhi, India) at 100% was mixed with 1.75 mL of polyethylene glycol (Continental Chemicals, New Delhi, India) to form a thick consistency on a clean dry glass slab with a metal spatula. The paste was carried to the pulp stumps with a metal carrier and then condensed lightly to a thickness of 2 - 3 mm followed by placement of thick mix of ZOE cement base and GIC to seal the cavity.
After 24 hours, the teeth in all three groups were restored with preformed stainless steel crowns (SSCs). The participants were recalled for clinical and radiographic evaluations after 3, 6, and 9 months as depicted in Figure 1. The teeth were evaluated clinically and radiographically by two observers independently who were blinded to the treatment type. Clinical and radiographic criteria for assessing teeth were explained along with a calibration process to the two observers on three initial cases. The criteria, based on Zurn and Seale has been used for scoring the clinical and radiographical findings as presented in Table 1.23 The scoring system was devised to represent severity of changes but not to define an individual tooth as a 'success' or 'failure', i.e., as the score gets larger, the pathologies get progressively more invasive and require more frequent follow-up. Teeth scored as 1 or 2 were considered successful. Data was analyzed using the Statistical Package for Social Sciences software (Windows version PSAW, IBM Corp., Armonk, NY, USA). Intra- and inter-observer agreement was measured for the radiographic assessment using Cohen's Kappa test.
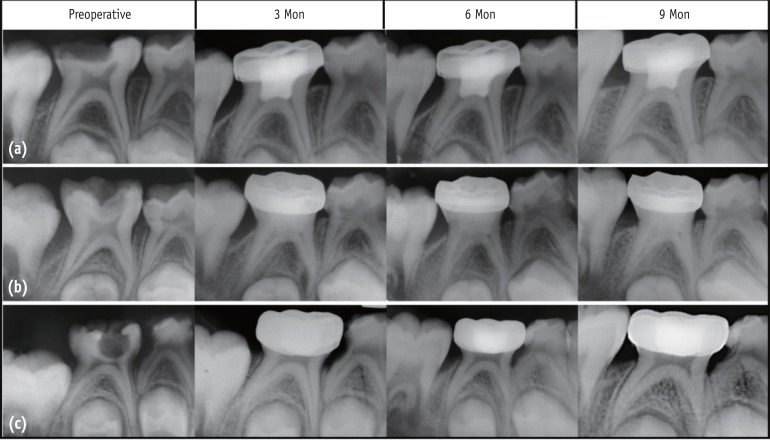
Figure 1. Representative radiographic observations in all the three groups over different follow-up periods. (a) MTA; (b) Biodentine; (c) Propolis pulpotomies in primary molars.
Table 1. Clinical and radiographic scoring criteria.
| Criteria for clinical and radiographic scoring | ||
|---|---|---|
| Clinical score | Clinical symptom | Definition |
| 1 | asymptomatic | •Pathology: absent |
| •Normal functioning | ||
| •Mobility (physiological) ≤ 1 mm | ||
| 2 | slight discomfort, short-lived | •Pathology: questionable |
| •Percussion sensitivity | ||
| •Gingival inflammation (due to poor oral hygiene) | ||
| •Mobility (physiological) > 1 mm, but < 2 mm | ||
| 3 | minor discomfort, short-lived | •Pathology: initial changes present |
| •Gingival swelling (not due to poor oral hygiene) | ||
| •Mobility > 2 mm, but < 3 mm | ||
| 4 | major discomfort, long-lived Extract immediately | •Pathology: late changes present |
| •Spontaneous pain | ||
| •Gingival swelling (not due to poor oral hygiene) | ||
| •Periodontal pocket formation (exudate) | ||
| •Sinus tract present | ||
| •Mobility ≥ 3 mm | ||
| •Premature tooth loss, due to pathology | ||
*time period from the beginning of the treatment to the assigned follow-up
Results
The mean (± SD) age in Groups I, II, and III were 6.48 ± 1.73 years, 6.92 ± 1.78 years, and 7.00 ± 1.63 years, respectively. They were not different statistically. Further, in all three groups, the frequency (%) of females was higher than males except Group II. Comparing the sex proportions (Male/Female) of three groups, χ2 test revealed similar sex proportions among the groups (χ2 = 0.75; p = 0.686), which were not different statistically. The interobserver reliability/variability of clinical and radiographical signs over the periods (baseline 0, 3, 6, and 9 months) was analyzed by using Kappa-Cohen test (κ value) and was found 100.0% (κ = 1.00) at all periods.
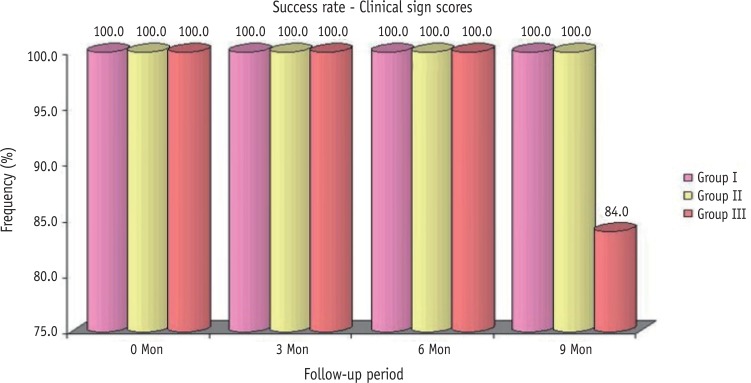
The clinical scores obtained by the individual teeth under observation, are presented in Table 2. At 6 months followup, four cases (16.0%) in Group III had clinical sign score 2 and one case (4.0%) had clinical sign score 3, and the frequency (%) of these were significantly different and higher as compared to 0.0% of both Group I and Group II (χ2 = 10.71, p = 0.030). Further, at 9 months, one case (4.0%) had clinical sign score 2 and four cases (16.0%) had clinical sign score 4 in Group III, and the frequency of these were also significantly higher as compared to 0.0% of both Group I and Group II (χ2 = 10.71, p = 0.030). The success rate for the pulpotomy medicaments calculated on the basis of the clinical scores obtained by the teeth in the respective groups over 9 months follow-up period were 100, 100, and 84%, respectively (Figure 2).
Table 2. Clinical sign scores of three groups over the periods.
| Time period | Clinical sign score | Group I | Group II | Group III | χ2 value | p value |
|---|---|---|---|---|---|---|
| baseline/0 mon | 1 | 25 (100.0) | 25 (100.0) | 25 (100.0) | NA | - |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 3 mon | 1 | 25 (100.0) | 25 (100.0) | 24 (96.0) | 2.03 | 0.363 |
| 2 | 0 (0.0) | 0 (0.0) | 1 (4.0) | |||
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 6 mon | 1 | 25 (100.0) | 25 (100.0) | 20 (80.0) | 10.71 | 0.030 |
| 2 | 0 (0.0) | 0 (0.0) | 4 (16.0) | |||
| 3 | 0 (0.0) | 0 (0.0) | 1 (4.0) | |||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 9 mon | 1 | 25 (100.0) | 25 (100.0) | 20 (80.0) | 10.71 | 0.030 |
| 2 | 0 (0.0) | 0 (0.0) | 1 (4.0) | |||
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 4 | 0 (0.0) | 0 (0.0) | 4 (16.0) |
The numbers in the parentheses were the percentages of the cases rated as the score.
NA, not applicable.
Figure 2. Comparison of clinical success rates in all the three groups over different follow-up periods.
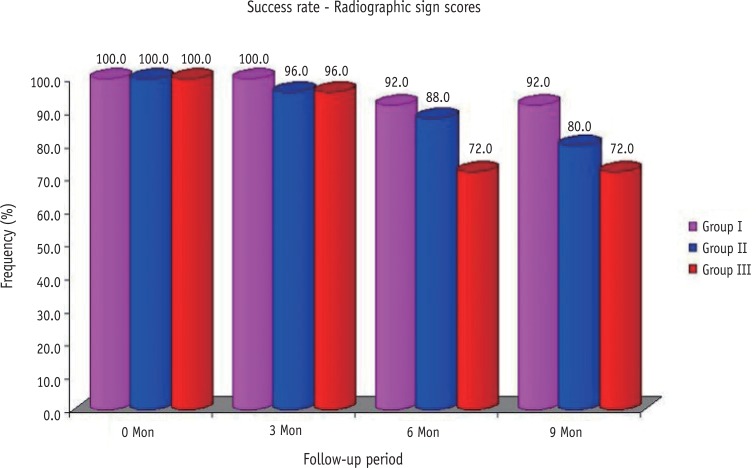
The radiographic sign scores of three groups over the periods are summarized in Table 3. The frequency of proportions of radiographic sign's scores differed significantly among the three groups at both 6 and 9 months follow-up. The success rate for the pulpotomy medicaments calculated on the basis of the radiographic scores obtained by the teeth in the respective groups over 9 months follow-up period were 92, 80, and 72%, respectively (Figure 3).
Table 3. Radiographic sign scores of three groups over the periods.
| Time period | Radiographic sign score | Group I | Group II | Group III | χ2 value | p value |
|---|---|---|---|---|---|---|
| 0 mon | 1 | 25 (100.0) | 25 (100.0) | 25 (100.0) | NA | - |
| 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 3 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 3 mon | 1 | 25 (100.0) | 23 (92.0) | 24 (96.0) | 3.08 | 0.544 |
| 2 | 0 (0.0) | 1 (4.0) | 0 (0.0) | |||
| 3 | 0 (0.0) | 1 (4.0) | 1 (4.0) | |||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |||
| 6 mon | 1 | 20 (80.0) | 16 (64.0) | 18 (72.0) | 13.11 | 0.041 |
| 2 | 3 (12.0) | 6 (24.0) | 0 (0.0) | |||
| 3 | 2 (8.0) | 3 (12.0) | 4 (16.0%) | |||
| 4 | 0 (0.0) | 0 (0.0) | 3 (12.0) | |||
| 9 mon | 1 | 17 (68.0) | 14 (56.0) | 18 (72.0) | 21.78 | 0.001 |
| 2 | 6 (24.0) | 6 (24.0) | 0 (0.0) | |||
| 3 | 2 (8.0) | 5 (20.0) | 1 (4.0) | |||
| 4 | 0 (0.0) | 0 (0.0) | 6 (24.0) |
The numbers in the parentheses were the percentages of the cases rated as the score.
NA, not applicable.
Figure 3. Comparison of radiographic success rates in all the three groups over different follow-up periods.
Discussion
The choice of medicament used in pulpotomy procedures is influenced by a number of factors including pulp healing potential, antibacterial properties, mechanical properties, biocompatibility, cytotoxicity, dimensional stability, and handling properties, etc.24 MTA has remained a successful material in this context and was therefore selected as the control pulpotomy medicament for primary teeth in the present study owing to its virtues. The pulpotomy medicaments designated for the experimental groups were Biodentine and Propolis, both of which have shown significant potential for pulpal healing, repair, and also no cytotoxic effects on cells of pulp or PDL.13,25 Biodentine is a restorative based new bioactive material with dentin-like mechanical properties. The common mechanism underlying all three medicaments is the stimulation of transforming growth factor TGF-β1, the key factor which is important for the differentiation of odontoblasts and responsible for the reparative dentinogenesis.
The accurate diagnosis of the status of the pulp and the prediction of response for pulpotomy procedure is dependent on clinicians' judgment and experience, accuracy of eliciting actual period of pulp involvement from the history given, and patient's response to pulp testing. These confounding variables bring uncertainty in determining the extent of bacterial penetration into the pulp from the carious lesion. Therefore, the definitive selection for the screened primary teeth was done intraoperatively in the present study on the basis of time taken for hemostasis after coronal pulp amputation.
Prevention of microleakage is an important factor contributing to the success of any vital pulp therapy procedure. In the study by Nowicka et al., both Biodentine and MTA were found to have excellent sealing properties and potential to prevent microleakage.26 Direct evidence of sealing ability of Propolis is, however, not evidenced in the literature. It is reported that Propolis helps in collagen synthesis and assists in wound healing.27 The role of final restoration over pulpotomized primary molars therefore becomes significant in preventing microleakage. Croll and Killian have recommended SSCs for the treatment of pulpotomized teeth based on the assumption that there is less leakage in crowned teeth than those restored with amalgam.28 It has also been found that protecting the underlying pulp against leakage by covering it completely with a SSC is a necessity for the long-term success of pulp therapy.29 However, Holan et al. reported no statistically significant differences in the success rates of teeth restored with SSCs and amalgam.8 For standardization, all pulpotomized primary molars in the present study were restored with SSCs.
The participants of the study were examined both clinically and radiographically at 3, 6, and 9 months. The objective for scoring assessment was to represent severity of changes, and not to designate any tooth as 'success' or 'failure' based on any isolated signs or symptoms. In a study by Zealand et al., similar scoring based on severity of changes was employed.30 The clinical and radiographic success rates in the study have been calculated by adding number of teeth presenting with clinical scores of 1 or 2 and radiographic scores of 1 or 2, respectively. Similar addition of score categories has been done by Zealand et al. as both clinical and radiographic scores of 2 represented pathological changes of questionable significance. In the present study, the overall success rate on the basis of clinical score evaluated for MTA, Biodentine and Propolis groups were 100, 100, and 84%, respectively, over 9 months period follow-up. Comparable success rates for MTA have been found in the literature. However, there is lack of evidence for clinical success for both Biodentine and Propolis. Their success as potential pulp capping agents is suggested to be based on histological analysis.
The percentages of pulpotomized teeth with no visible radiographic changes over 9 months period of observation in the MTA, Biodentine, and Propolis groups were 68, 56, and 72%, respectively. Presence of minor internal changes observed radiographically within the root canal, such as signs of pulp canal obliteration (PCO) and/or nonperforated internal resorption, were kept under followup observation and not designated as failure. The teeth with presence of definitive radiographic pathological changes including PDL widening, external root resorption, interradicular/periapical bone destruction were considered as failure. The percentage of such pulpotomized primary molars was 28% in the Propolis group, which was significantly higher than the other two groups.
PCO was the most common radiographic findings in both MTA (20.0%) and Biodentine (16.0%) groups over a period of nine months. Erdem et al. has also reported similar high rates of PCO (20%) with MTA.31 PCO or calcific metamorphosis occurs as a result of odontoblastic activity. It is suggestive of retained vitality, and not regarded as failure by many investigators.4,32,33 The frequent observation of PCO in both MTA and Biodentine groups was therefore not considered as failure. The absence of PCO with Propolis as pulpotomy medicament, however, should not be regarded as its limitation, as its pulp healing effect is mainly mediated through the anti-inflammatory properties.34,35,36
Radiographic evidence of non-perforated internal resorption in pulpotomized primary teeth has remained a controversial criterion for determination of success or failure. Holan et al. and many other authors did not consider it as a sign of failure in the absence of clinical signs, symptoms, and suggested to be left for follow-up observations as the pathology may cease and lead to the development of calcific metamorphosis.8 Some researchers on the other hand, have associated non-perforated internal resorption in primary teeth with persistent inflammation and believed that it is usually associated with external perforation due to thinness of primary molar roots.30 In the present study, 4.0% of cases each in MTA and Biodentine groups and 12.0% of cases in Propolis group, respectively, presented with non-perforated internal resorption radiographically over a period of 9 months after pulpotomy. Jabbarifar et al. have reported similar rate of internal resorption with MTA (4%) over 12 to 38 months period.37 There is lack of evidence, however, regarding presentation of internal resorption with Biodentine and Propolis as pulpotomy medicaments.
External root resorption was considered as a radiographic failure in the present study. Previous studies have also followed similar criteria, although a few studies have suggested 1 month follow-up examination prior to the decision of extraction.30 Over 9 months follow-up, 4, 8, and 12% cases of external resorption were observed in teeth treated with MTA, Biodentine and Propolis groups, respectively. Sonmez et al. have found much higher rates for MTA than the reported data in the present study.38 The longer follow-up period may be the possible reason.
In the present study, widening of periodontal ligament space was associated with either external root resorption, inter-radicular and/or periapical bone loss radiographically or with clinical signs and symptoms of pulp degeneration. The association of PDL widening with both radiographic and clinical criteria for failure warranted immediate extraction of such pulpotomized primary teeth. Zealand et al. employed similar criteria and suggested that the PDL widening occurrence all alone should not be considered as a failure.30 Previous studies have been suggested immediate extraction of teeth showing radiographic evidence of interradicular and periapical bone destruction, irrespective of the clinical signs and symptoms. In the present study, 4% of cases in both MTA and Biodentine group each and 48% of cases treated with Propolis were found to be associated with bone destruction over 9 month period. Most of the patients with radiographic evidence of bone destruction presented with clinical evidence of abscesses.
The concept of dentin bridge formation under pulpotomy medicaments has also remained a topic of debate amongst the researchers who suggest it to be either a healing response or the pulp reaction to irritation.35,39 Reactionary dentine formation is regarded as a consequence of repair processes within the pulp tissue by Waterhouse et al.40 This repair process, however, may fail after an initial attempt and ultimately lead to clinical failure. Therefore, dentin bridging cannot be considered as reliable response for determination of success or failure. Caicedo et al. found that the presence of dentin bridge was not evident radiographically in all the pulpotomized teeth, but was clearly seen on histological analysis in those teeth.1 In a clinical setting, determination of dentin bridge formation becomes difficult as radiographs do not clearly indicate their presence. Also, it is not possible to visually inspect the dentin bridge formation after removing the restoration, especially in children. Caicedo et al. also stated that the resultant management of the pulpotomized tooth would not be affected by presence or absence of dentinal bridge when no other signs or symptoms are present.1 In view of these facts and evidence of technical difficulties involved, dentin bridge formation was not included in the radiographic criteria to be observed.
The present study has been designed to overcome potential bias and confounding variables as much as possible. Still we recognize some factors as minor limitations which might be curtailed in future studies. One of them is the consideration of changes in the regenerative ability of dental pulp that varies with age. This variable can be controlled by narrowing the age range of the subjects. However, this might result in practical difficulty of getting an adequate sample size.
Conclusions
On the basis of the results of the present study, it can be concluded that the clinical outcome of Biodentine is comparable to that of MTA at both 6 and 9 months follow-up period. Furthermore, the radiographic outcome of Biodentine was statistically not different, compared to MTA. These findings suggest the potential of Biodentine for being used as a pulpotomy medicament in primary teeth. On the other hand, Propolis showed greater deterioration in both clinical and radiographic outcomes when compared to MTA as well as Biodentine.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Caicedo R, Abbott PV, Alongi DJ, Alarcon MY. Clinical, radiographic and histological analysis of the effects of mineral trioxide aggregate used in direct pulp capping and pulpotomies of primary teeth. Aust Dent J. 2006;51:297–305. doi: 10.1111/j.1834-7819.2006.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 2.Magnusson B. Attempts to predict prognosis of pulpotomy in primary molars. Scand J Dent Res. 1970;78:232–240. doi: 10.1111/j.1600-0722.1970.tb02069.x. [DOI] [PubMed] [Google Scholar]
- 3.Ranly DM. Pulpotomy therapy in primary teeth: new modalities for old rationales. Pediatr Dent. 1994;16:403–409. [PubMed] [Google Scholar]
- 4.Eidelman E, Holan G, Fuks AB. Mineral trioxide aggregate vs. formocresol in pulpotomized primary molars: a preliminary report. Pediatr Dent. 2001;23:15–18. [PubMed] [Google Scholar]
- 5.Myers DR, Shoaf HK, Dirksen TR, Pashley DH, Whitford GM, Reynolds KE. Distribution of 14C-formaldehyde after pulpotomy with formocresol. J Am Dent Assoc. 1978;96:805–813. doi: 10.14219/jada.archive.1978.0187. [DOI] [PubMed] [Google Scholar]
- 6.Primosch RE, Glomb TA, Jerrell RG. Primary tooth pulp therapy as taught in Predoctoral pediatric dental programs in the United States. Pediatr Dent. 1997;19:118–122. [PubMed] [Google Scholar]
- 7.Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197–205. doi: 10.1016/S0099-2399(99)80142-3. [DOI] [PubMed] [Google Scholar]
- 8.Holan G, Eidelman E, Fuks AB. Long-term evaluation of pulpotomy in primary molars using mineral trioxide aggregate or formocresol. Pediatr Dent. 2005;27:129–136. [PubMed] [Google Scholar]
- 9.Oguntebi BR, Heaven T, Clark AE, Pink FE. Quantitative assessment of dentin bridge formation following pulpcapping in miniature swine. J Endod. 1995;21:79–82. doi: 10.1016/S0099-2399(06)81100-3. [DOI] [PubMed] [Google Scholar]
- 10.Ford TR, Torabinejad M, Abedi HR, Bakland LK, Kariyawasam SP. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127:1491–1494. doi: 10.14219/jada.archive.1996.0058. [DOI] [PubMed] [Google Scholar]
- 11.Damamaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005;21:731–738. doi: 10.1016/j.dental.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 12.Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part I: chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Laurent P, Camps J, About I. Biodentine induces TGF-β1 release from human pulp cells and early dental pulp mineralization. Int Endod J. 2012;45:439–448. doi: 10.1111/j.1365-2591.2011.01995.x. [DOI] [PubMed] [Google Scholar]
- 14.Peng W, Liu W, Zhai W, Jiang L, Li L, Chang J, Zhu Y. Effect of tricalcium silicate on the proliferation and odontogenic differentiation of human dental pulp cells. J Endod. 2011;37:1240–1246. doi: 10.1016/j.joen.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 15.Tran XV, Gorin C, Willing C, Baroukh B, Pellat B, Decup F, Opsahl Vital S, Chaussain C, Boukpessi T. Effect of a calicum-silicate-based restorative cement on pulp repair. J Dent Res. 2012;91:1166–1171. doi: 10.1177/0022034512460833. [DOI] [PubMed] [Google Scholar]
- 16.Zanini M, Sautier JM, Berdal A, Simon S. Biodentine induces immortalized murine pulp cell differentiation into odontoblast-like cells and stimulates biomineralization. J Endod. 2012;38:1220–1226. doi: 10.1016/j.joen.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15:561–571. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 18.Burdock GA. Review of the biological properties and toxicity of bee propolis (propolis) Food Chem Toxicol. 1998;36:347–363. doi: 10.1016/s0278-6915(97)00145-2. [DOI] [PubMed] [Google Scholar]
- 19.Marcucci MC. Propolis: chemical composition, biological properties and therapeutic activity. Apidologie. 1995;26:83–99. [Google Scholar]
- 20.Al-Haj Ali SN. In vitro toxicity of propolis in comparison with other primary teeth pulpotomy agents on human fibroblasts. J Investig Clin Dent. 2015 Apr 27; doi: 10.1111/jicd.12157. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Ozório JE, Carvalho LF, de Oliveira DA, de Sousa-Neto MD, Perez DE. Standardized propolis extract and calcium hydroxide as pulpotomy agents in primary pig teeth. J Dent Child (Chic) 2012;79:53–58. [PubMed] [Google Scholar]
- 22.Parolia A, Kundabala M, Rao NN, Acharya SR, Agrawal P, Mohan M, Thomas M. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal. Aust Dent J. 2010;55:59–64. doi: 10.1111/j.1834-7819.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 23.Zurn D, Seale NS. Light-cured calcium hydroxide vs formocresol in human primary molar pulpotomies: a randomized controlled trial. Pediatr Dent. 2008;30:34–41. [PubMed] [Google Scholar]
- 24.Sushynski JM, Zealand CM, Botero TM, Boynton JR, Majewski RF, Shelburne CE, Hu JC. Comparison of gray mineral trioxide aggregate and diluted formocresol in pulpotomized primary molars: a 6- to 24-month observation. Pediatr Dent. 2012;34:120–128. [PMC free article] [PubMed] [Google Scholar]
- 25.Scheller S, Ilewicz L, Luciak M, Skrobidurska D, Stojko A, Matuga W. Biological properties and chemical application of propolis. IX. Experimental observation on the influence of ethanol extract of propolis (EEP) on dental pulp regeneration. Arzneimittelforschung. 1978;28:289–291. [PubMed] [Google Scholar]
- 26.Nowicka A, Lipski M, Parafiniuk M, Sporniak-Tutak K, Lichota D, Kosierkiewicz A, Kaczmarek W, Buczkowska-Radlińska J. Response of human dental pulp capped with biodentine and mineral trioxide aggregate. J Endod. 2013;39:743–747. doi: 10.1016/j.joen.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Park YK, Alencar SM, Aguiar CL. Botanical origin and chemical composition of Brazilian propolis. J Agric Food Chem. 2002;50:2502–2506. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- 28.Croll TP, Killian CM. Zinc oxide-eugenol pulpotomy and stainless steel crown restoration of a primary molar. Quintessence Int. 1992;23:383–388. [PubMed] [Google Scholar]
- 29.Gruythuysen RJ, Weerheijm KL. Calcium hydroxide pulpotomy with a light-cured cavity-sealing material after two years. ASDC J Dent Child. 1997;64:251–253. [PubMed] [Google Scholar]
- 30.Zealand CM, Briskie DM, Botero TM, Boynton JR, Hu JC. Comparing gray mineral trioxide aggregate and diluted formocresol in pulpotomized human primary molars. Pediatr Dent. 2010;32:393–399. [PMC free article] [PubMed] [Google Scholar]
- 31.Erdem AP, Guven Y, Balli B, Ilhan B, Sepet E, Ulukapi I, Aktoren O. Success rates of mineral trioxide aggregate, ferric sulfate, and formocresol pulpotomies: a 24-month study. Pediatr Dent. 2011;33:165–170. [PubMed] [Google Scholar]
- 32.Willard RM. Radiographic changes following formocresol pulpotomy in primary molars. ASDC J Dent Child. 1976;43:414–415. [PubMed] [Google Scholar]
- 33.Fuks AB, Holan G, Davis JM, Eidelman E. Ferric sulfate versus dilute formocresol in pulpotomized primary molars: long-term follow up. Pediatr Dent. 1997;19:327–330. [PubMed] [Google Scholar]
- 34.Koo H, Gomes BP, Rosalen PL, Ambrosano GM, Park YK, Cury JA. In vitro antimicrobial activity of propolis and arnica montana against oral pathogens. Arch Oral Biol. 2000;45:141–148. doi: 10.1016/s0003-9969(99)00117-x. [DOI] [PubMed] [Google Scholar]
- 35.Silva FB, Almeida JM, Sousa SM. Natural medicaments in endodontics - a comparative study of the antiinflammatory action. Braz Oral Res. 2004;18:174–179. doi: 10.1590/s1806-83242004000200015. [DOI] [PubMed] [Google Scholar]
- 36.Tan-No K, Nakajima T, Shoji T, Nakagawasai O, Niijima F, Ishikawa M, Endo Y, Sato T, Satoh S, Tadano T. Antiinflammatory effect of propolis through inhibition of nitric oxide production on carrageenin-induced mouse paw edema. Biol Pharm Bull. 2006;29:96–99. doi: 10.1248/bpb.29.96. [DOI] [PubMed] [Google Scholar]
- 37.Jabbarifar SE, Khademi AA, Ghasemi D. Success rate of formocresol pulpotomy versus mineral trioxide aggregate in human primary molar tooth. J Res Med Sci. 2004;9:304–307. [Google Scholar]
- 38.Sonmez D, Sari S, Cetinbaş T. A Comparison of four pulpotomy techniques in primary molars: a long-term follow-up. J Endod. 2008;34:950–955. doi: 10.1016/j.joen.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 39.del Carmen González Rodríguez W, Carpio MHC, Ramos MRM, Milanés MG, Antúnez LN. Pulpotomies of dead pulps in temporal molars using 10% propolis tinction. Rev Cubana Estomatol [online] 2007;44(3) Available from: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0034-75072007000300006&lng=en&nrm=iso&tlng=en (updated 2015 Sep 3) [Google Scholar]
- 40.Waterhouse PJ, Nunn JH, Whitworth JM. Primary molar vital pulp theory. Br Dent J. 2000;188:417. [PubMed] [Google Scholar]