Abstract
A recent study published in Cell Research by Li and colleagues reports a detailed biophysical and structural study of AMPK's intra-molecular interactions during activation. By employing subunit tagging and proximity analysis with the aid of AlphaScreen instrumentation, Li et al. add to our understanding of the choreography of activation of AMPK by both nucleotides and phosphorylation.
AMPK activation is triggered by energy depletion resulting in rises in cellular AMP. AMPK phosphorylates many proteins to switch off energy-consuming pathways and switch on energy-generating pathways. As many age-onset diseases involve metabolic disorders, there has been considerable interest in developing AMPK-activating drugs. Understanding the biochemistry and structure-function relationships of AMPK has been challenging due in part to its complex αβγ heterotrimeric structure, multiple ligand-binding sites and extensive posttranslational modifications. Each subunit of the heterotrimer has multiple alternate isoforms (α1-2, β1-2 and γ1-3), with each isoform harboring multiple functional domains. Recent structural information has been a boon to AMPK researchers with two (α2β1γ1 and α1β1γ1) near full-length crystal structures of activated phosphorylated AMPK bound to AMP, with and without AMPK-activating drugs Merck 991 or A-7696621,2. The new findings reported by Li et al.3 focus on an autoinhibitory domain (AID) in the catalytic α-subunit and a carbohydrate-binding module (CBM) in the β-subunit4,5. In the basal state the AID interacts with the kinase domain (KD), but is sequestered to the γ-subunit upon activation6,7. The phosphorylated CBM sits on the N-lobe of the α-subunit KD, forming what we now term the allosteric drug and metabolite (ADaM)-binding pocket. Despite this wealth of structural information, little is known about the inactive form of AMPK, including how nucleotides act to inhibit or activate AMPK.
ATP binds to the AMPK γ-subunit in the inactive conformation, maintaining the α-catalytic subunit in its autoinhibited form via AID interactions. AMP binding to the γ-subunit displaces ATP and triggers AMPK activation. This event is sensed by the α-subunit regulatory subunit-interacting motif (αRIM) and disengages the AID from the KD. Phosphorylation of the activation loop at α-T172/T174 by upstream kinases then locks AMPK in the active conformation.
To examine enzyme transitions from the inactive to the active state Li and colleagues have used tags attached at differing N- and/or C-termini throughout the AMPK heterotrimer, which bind to donor and acceptor beads3. The AlphaScreen luminescence proximity assay can sense the proximity of one domain to another, and in this way the change in proximity between two domains can be assessed under different nucleotide-binding conditions. AMP causes the N-terminal regions of the α-subunit and γ-subunit to move closer together (pAMP), whereas ATP causes these two regions to dissociate (pATP) (Figure 1A and 1B). By comparison, the addition of AMP or ATP does not change the proximity of the α-β and β-γ subunit interactions. Thus, the inactive AMPK (ATP-bound) has an extended conformation, while the AMP-bound form is more compact, in agreement with conclusions drawn from earlier SAXS studies8.
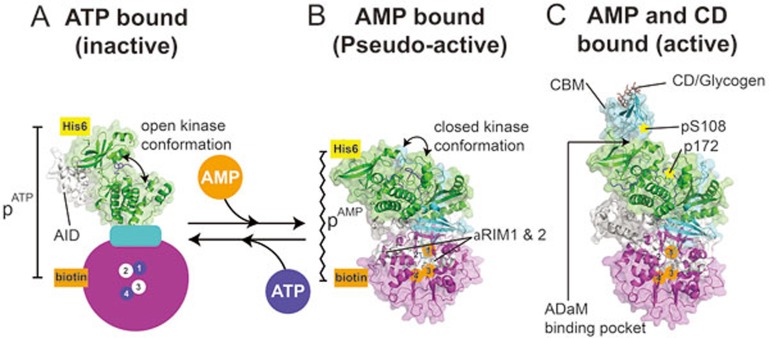
Figure 1.
Nucleotide inhibition and activation of AMPK. Cartoon and surface representations of nucleotide-bound AMPK X-ray crystal structures. α-subunit (green) including the KD and the AID-αRIM (white); β-subunit (cyan) including the CBM; γ-subunit (magenta) with nucleotide-binding (CBS) sites 1-4 labeled. AlphaScreen tags on the N-terminus of the α-subunit (His) and γ-subunit (biotin) are shown. (A) Inactive KD bound to the AID, with an animated γ-subunit showing CBS sites 1 and 4 occupied by ATP (blue), and 2 and 3 unbound. The AID can be seen bound to the hinge region of the KD, maintaining an inactive open conformation. (B) Unphosphorylated AMPK with AMP bound to γ-subunit CBS sites 1, 3 and 4. The heterotrimer has adopted a compact conformation with the proximity between the two AlphaScreen tags (pAMP) closer than that of ATP-bound AMPK (pATP). The KD has adopted a closed pseudo-active conformation. (C) AMPK phosphorylated on αT172 (KD) and βS108 (CBM), with AMP bound to the γ-subunit CBS sites 1, 3 and 4. The CBM is bound to the N-lobe of the KD, forming the ADaM-binding pocket, with CD bound to the opposite face of the CBM.
Li et al. solved the X-ray crystal structure of the previously recalcitrant human KD-AID enzyme fragment by introducing the entropy reduction mutation K43A1. The human KD-AID structure showed that the AID motif bound to the KD hinge region, holding KD in an inactive open conformation (Figure 1A). This observation was confirmed by a series of mutations, which abolished the inhibitory effect of the AID on the KD. These mutations in full-length AMPK also eliminate the AMP regulation of kinase activity, strongly supporting previous results4.
Remarkably the nucleotide regulation of AMPK by AMP and ATP could be recapitulated with fragments of the enzyme including the AMPK core complex (α1 [395-550]-β2 [187-272]-γ1) and an AID-αRIM fragment (α1 [282-392]). Upon AMP binding to the core complex, the proximal distance of the AID-αRIM motif to the core decreased whereas upon ATP binding the proximal distance increased. These results were supported by previously reported mutations that cause AMPK to become AMP-independent, which inhibited the core complex from interacting with the AID7.
The CBM of the β-subunit (Figure 1C), which is critical for the AMPK allosteric activation by several drugs2, is known to bind glycogen and cyclodextran (CD). However, a physiological role for the glycogen interaction has not been conclusively shown. Li et al. reveal that the interaction between isolated CBM and KD can be reduced upon glycogen or CD binding to the CBM3. Blocking CD/glycogen binding by mutating residue W99 in the CBM partially reversed the reduction in CBM-KD interaction caused by glycogen/CD binding. As originally reported by Polekhina et al.9, Li et al. found that glycogen had no effect on AMPK activity and failed to reproduce the claims by Hardie and colleagues that glycogen inhibits AMPK activity10.
As stated earlier, AMPK is an important drug target. While some drugs show β-isoform selectivity, others can activate either β1 or β2 isoforms. While previous structures have revealed the drug-binding pocket of β1-containing AMPK1,2, this report by Li et al.3 represents the first time that the β2 allosteric binding pocket has been visualized. Interestingly, the allosteric binding pockets of the two isoforms superimpose closely, suggesting a shared mechanism. The ADaM-binding pocket was formed in the absence of any allosteric activating drugs, such as Merck 991 or A-769662. This is in agreement with a recently reported rat α1β1γ1 structure2.
Collectively, the study by Li and colleagues extends our understanding of how AMPK is physiologically activated and inactivated. This study demonstrated that the AID-αRIM element is responsible for sustaining either the active or inactive state as had previously been inferred from structural studies1,2,4,6,11.
References
- 1Xiao B, Sanders MJ, Carmena D, et al. Nat Commun 2013; 4:3017. [DOI] [PMC free article] [PubMed]
- 2Calabrese MF, Rajamohan F, Harris MS, et al. Structure 2014; 22:1161–1172. [DOI] [PubMed]
- 3Li X, Wang L, Zhou XE, et al. Cell Res 2015; 25:50–66. [DOI] [PMC free article] [PubMed]
- 4Chen L, Jiao ZH, Zheng LS, et al. Nature 2009; 459:1146–1149. [DOI] [PubMed]
- 5Amodeo GA, Rudolph MJ, Tong L. Nature 2007; 449:492–495. [DOI] [PubMed]
- 6Xiao B, Sanders MJ, Underwood E, et al. Nature 2011; 472:230–233. [DOI] [PMC free article] [PubMed]
- 7Chen L, Wang J, Zhang YY, et al. Nat Struct Mol Biol 2012; 19:716–718. [DOI] [PubMed]
- 8Riek U, Scholz R, Konarev P, et al. J Biol Chem 2008; 283:18331–18343. [DOI] [PubMed]
- 9Polekhina G, Gupta A, Michell BJ, et al. Curr Biol 2003; 13:867–871. [DOI] [PubMed]
- 10McBride A, Ghilagaber S, Nikolaev A, et al. Cell Metab 2009; 9:23–34. [DOI] [PMC free article] [PubMed]
- 11Chen L, Xin FJ, Wang J, et al. Nature 2013; 498:E8–E10. [DOI] [PubMed]