Abstract
Direct interspecies electron transfer (DIET) between Geobacter species and Methanosaeta species is an alternative to interspecies hydrogen transfer (IHT) in anaerobic digester, which however has not been established in anaerobic sludge digestion as well as in bioelectrochemical systems yet. In this study, it was found that over 50% of methane production of an electric-anaerobic sludge digester was resulted from unknown pathway. Pyrosequencing analysis revealed that Geobacter species were significantly enriched with electrodes. Fluorescence in situ hybridization (FISH) further confirmed that the dominant Geobacter species enriched belonged to Geobacter metallireducens. Together with Methanosaeta species prevailing in the microbial communities, the direct electron exchange between Geobacter species and Methanosaeta species might be an important reason for the “unknown” increase of methane production. Conductivity of the sludge in this electric-anaerobic digester was about 30% higher than that of the sludge in a control digester without electrodes. This study not only revealed for the first time that DIET might be the important mechanism on the methanogenesis of bioelectrochemical system, but also provided a new method to enhance DIET by means of bioelectric enrichment of Geobacter species.
Anaerobic methanogenesis is an effective way to realize energy recovery from wastes1,2,3. Although this technology has been available for more than 60 years, it is not as widely utilized for solid waste conversion as might be expected. This is due, at least in part, to the widespread belief that anaerobic digestion is a slow process4. For the last decades, the working model for syntrophs and methanogens exchange electrons is regarded as interspecies hydrogen transfer (IHT)5,6,7. H2 is produced from non-methanogenic microorganisms metabolizing the fermentation products and consumed by H2-utilizing methanogens with the reduction of CO2 to CH4. This syntrophic metabolism of fermentation intermediates functions well as long as H2-utilizing methanogens maintain the concentration of H2 low enough that the production of H2 is thermodynamically favorable. Formate is an alternative to H2 and can also act as an electron carrier between syntrophic partners7,8,9. The exchange of H2 between the syntrophs and methanogens is a weak link. Any slight disruption in the rate of H2 consumption will break the balance of syntrophic metabolism, resulting in the accumulative short-chain fatty acids (SCFAs), which further inhibits the activity of H2-consuming methanogens to exacerbate the digester function.
Extracellular electrons are also exchanged via direct interspecies electron transfer (DIET), which is first documented in defined co-cultures of Geobacter metallireducens and Geobacter sulfurreducens10. G. metallireducens can metabolize ethanol, but cannot use fumarate as an electron acceptor11, whereas G. sulfurreducens can reduce fumarate, but cannot metabolize ethanol12. By DIET, G. metallireducens and G. sulfurreducens could grow in a medium with ethanol as the electron donor and fumarate as the electron acceptor. Morita et al.13 reported that the potential for direct electron exchange between Geobacter species and Methanosaeta species could happen in the brewery wastewater digesters for methane production. Methanosaeta species accounted for about 90% of the methanogenic archaea 16S rRNA gene sequences recovered, and H2-utilizing methanogens only accounted for less than 0.6% of the methanogenic archaea 16S rRNA gene sequences recovered, which implied that IHT had only a little contribution to the whole methane production7,13. [14C]-bicarbonate analysis suggested that DIET between Geobacter species and Methanosaetae species contributed 1/3 of methane production7. This discovery that Geobacter species transferred electrons to Methanosaeta species via DIET has challenged the long-held assumption that H2 are the primary interspecies electron carrier in conversion of organic matter into methane.
Commonly, Methanosaeta species are the predominant microbes in most of anaerobic methanogenic environments or anaerobic waste digesters, and the precursor of more than half of methane production14. However, Geobacter species are only frequently abundant in some limited anaerobic methanogenic environments, such as soils and sediments15,16,17. For some important methanogenic environments, such as anaerobic digestion of municipal sludge or of saccharides, the relative abundance of Geobacter species detected are low18,19,20. It meant that DIET from Geobacter species to Methanosaet a species for methane production was weak in these anaerobic system.
It was reported that Geobacter species usually adapt to grow with Fe (III) oxides21,22,23 or electrodes24,25 as electron acceptors. This discovery revealed the reason why Geobacter species could be detected in most bioelectrochemical systems with over 30–40% of 16S rRNA gene sequences recovered in the anodic microbial communities26,27,28. This finding predicted that the additional bioelectrochemical system might create a favorable condition to support the growth of Geobacter species24,29,30. We hereby assumed that a pair of electrodes inserted into an anaerobic digester was likely to enrich Geobacter species, which was expected to increase methane production via potential DIET between Geobacter species and Methanosaeta species. In this study, a single-chamber bioelectrochemical system was operated to treat waste activated sludge (WAS) with the aim to clarify the potential DIET for methane production during sludge digestion. The WAS used as the substrate was because Geobacter species were rare in the waste activated sludge which provided the possibility to better observe the enrichment of Geobacter species and its effects on methane production via DIET.
Results
Potential of DIET for methane production
The accumulative methane production during 51 days experiments is shown in Fig. 1A,B. From this figure, the accumulative methane production of the bioelectrochemical system with applied voltage of 0.6 V (R1) reached 2998.4 ± 89.2 mL (mean ± standard deviation) during the initial 33 days, roughly equal to its final methane production at day 51 (3017.6 ± 107.9 mL). Comparatively, the accumulative methane production of R2 (without electrodes) was only 904.5 ± 45.5 mL at day 33, about 3/10 (or 30.2%) of that of R1. After the 51 days experiments, the accumulative methane production of R2 increased to 2763.5 ± 102.5 mL, as similar as that of R1 at day 33 (2998.4 ± 89.2 mL). Remarkably, there was no significant difference of accumulative methane production between R2 and R3 (with electrodes but without power supply) during the 51 days experiments (P > 0.05), which implied that the electrode materials themselves nearly had no effect on the methane production. These results indicated that the bioelectrochemical system had a significant contribution to the methane production. Similarly, Sasaki et al.19 had operated a cylindrical bioelectrochemical reactor using carbon fiber fabric as electrode and also obtained the enhancement of methane fermentation from thickened sewage sludge.
Figure 1.
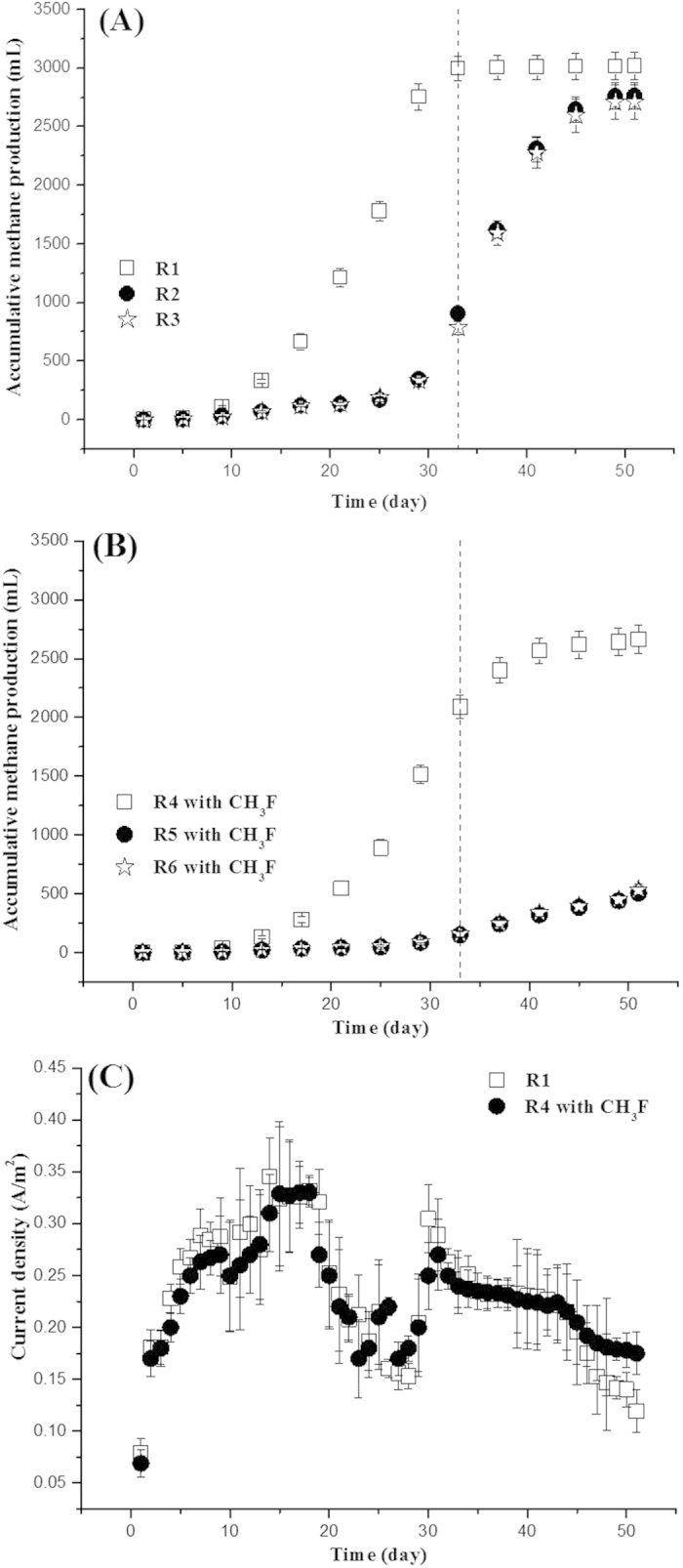
Accumulative methane production of R1, R2 and R3 (A) and R4, R5 and R6 with addition of CH3F (B). Change of current density in R1 and R4 (C). Error bars represent standard deviations of three groups of parallel experiments.
To assess the contribution of bioelectrochemical system to methanogenesis, the change of current density in R1 was recorded during the 51 days experiments (Fig. 1C). It is believed that exoelectrogenic bacteria like Geobacter species in the anodic biofilm are able to transfer the electrons from anodic oxidation of organic matters to electrode, and then hydrogenotrophic methanogens (often Methanobacterium species) as biocathode accept these electrons and reduce CO2 into CH4 according to the reaction: CO2 + 8H+ + 8e− = CH4 + 2H2O31,32. Theoretically, the electrons might be also recovered in the cathode for hydrogen production33. The hydrogen production detected in R1 was only less than 0.1% (<5 mL) of total biogas production during the whole experiments, which could be neglected. The low hydrogen production was likely because ‘electrohydrogenesis’ usually required the precious metal catalysts as catalyst34. During the initial 33 days, the total available electrons of R1 for the reduction of CO2 to CH4 was 0.13 mol calculated by the formula (1) base on current density (Fig. 1C). Even if these electrons were totally used for cathodic methanogenese, the methane production during the initial 33 days would not exceed 403.7 mL (403.7 mL = 0.13 moL / 8 × 24.8 × 103 mL/mol [the molar volume of gas at room temperature]), which only accounted for 13.5% (13.5% = 403.7 mL / 2998.4 mL × 100%) of total methane production of R1. It indicated that the combination of anodic oxidation and cathodic reduction of CO2 into CH4 was not an important mechanism for the increased methane production in R1. Thus, at least 56.4% (56.4% = [2998.4 mL − 904.5 mL − 403.7 mL] / 2998.4 mL × 100%) of the methane production of R1 should be produced from the other pathway.
Commonly, Methanosaeta species is responsible for directly converting acetate to methane. The acetate-utilizing methanogens are the prevailing species for methanogenesis. CH3F (3% [v/v], 99%) as a selective inhibitor of aceticlastic methanogenesis were added into another three parallel reactors (R4 same as R1, R5 same as R2 and R6 same as R3) to further observe the unknown methanogenesis (shown in Fig. 1B). The accumulative methane production in the three reactors all decreased with the addition of CH3F as compared with that of R1, R2 and R3. The accumulative methane production of R5 was only 150.9 ± 16.8 mL during the initial 33 days, which was still similar to that of R6. The methane production of these two reactors (R5 and R6) was mainly ascribed to H2-utilizing methanogenesis since aceticlastic methanogenesis was inhibited by CH3F. The accumulative methane production of R4 was 2089.0 ± 98.5 mL, significantly higher than that of those two reactors (R5 and R6). The available electrons for cathodic methanogenesis of R4 was 0.12 mol during the initial 33 days calculated by the formula (1) according to the current density (Fig. 1C). If these electrons were totally utilized for cathodic reduction of CO2, the accumulative methane production calculated would not excess 374.5 mL (374.5 mL = 0.12 moL / 8 × 24.8 × 103 mL/mol). Thus, the unknown methane production contributed at least 1563.6 mL (1563.6 mL = 2089.0 mL − 374.5 mL − 150.9 mL) of the total methane production in R4 during the initial 33 days, which was equal to 52.1% (52.1% = 1563.6 mL / 2998.4 mL × 100%) of total methane production in R1. This methane production (1563.6 mL) was well in agreement with the unknown methane production of R1 mentioned-above (1691.1 mL, [1691.1 mL = 56.4% × 2998.4 mL]).
Considering that Geobacter species could directly exchange electron with Methanosaeta species via DIET providing another methanogenic pathway to produce methane, it was assumed that DIET might be a reason for the increased methane production in this bioelectrochemical system. If so, why the other two no-electricity reactors (R2 and R3) did not have DIET? To address this question, the microbial communities in the different reactors were analyzed.
Analysis of microbial community structure
Archaeal microbial community structure after the 51 days experiments (Fig. 2) revealed that the most dominant genus of methanogenic archaea was Methanosaeta accounting for 40.55%, 39.13%, 39.82% and 40.22% of archaea 16S rRNA gene sequences recovered in the suspended sludge of R1, R2 and R3 and anodic biofilm of R1, respectively. Methanobacterium only accounted for 10.89%, 10.65%, 9.66% and 10.38% of archaea 16S rRNA gene sequences recovered in the above four communities, respectively. The OTUs analysis (Fig. S1) also showed no significant difference among the different communities.
Figure 2. Archaeal community structure at genus level of the suspended sludge of R1, R2 and R3 and anodic biofilm of R1 based on high-throughput 16S rRNA pyrosequencing.
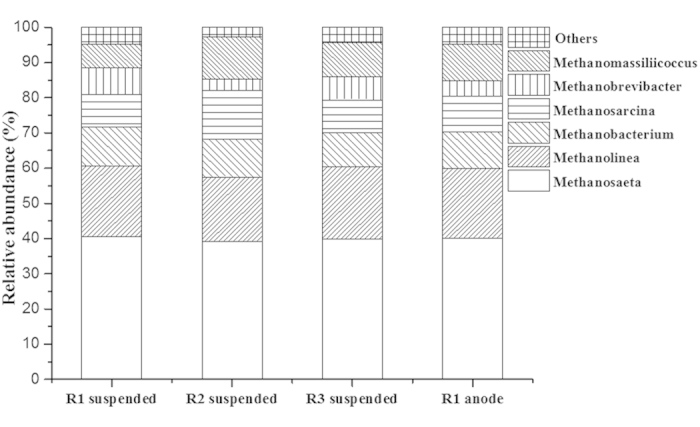
Genera with relative abundance lower than 1.00% was classified into 32 group ‘Others’.
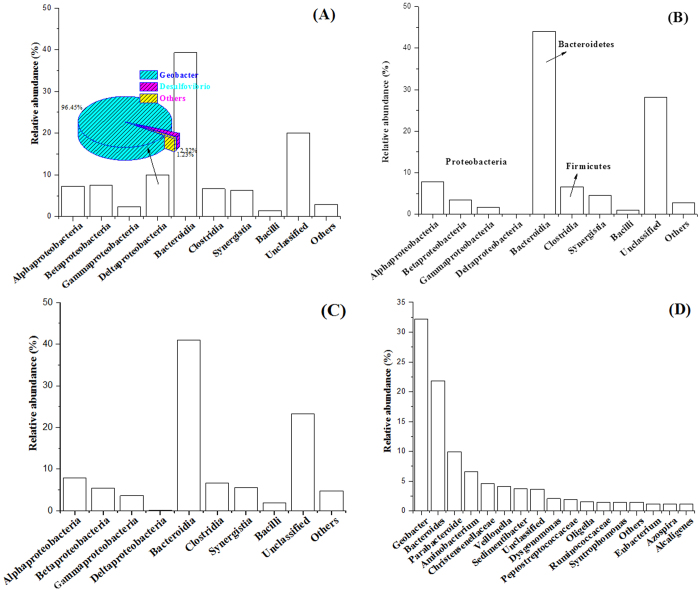
The class level identification of bacterial community structure in R1, R2 and R3 are illustrated in Fig. 3. Remarkably, Deltaproteobacteria species, mainly containing Geobacter species, accounted for 10.01% of the bacteria 16S rRNA gene sequences recovered in the suspended sludge of R1. Further identification at genus level showed that Geobacter species accounted for 96.45% of total sequences recovered of Deltaproteobacteria species in R1 (Fig. 3A). It meant that Geobacter species made up about 10% of bacteria 16S rRNA gene sequences in the suspended sludge of R1. Conversely, Deltaproteobacteria species was almost undetected in the suspended sludge of R2 and R3 (Fig. 3B,C). It indicated that Geobacter species was quite scarce during the anaerobic sludge digestion, making it difficult to directly exchange electrons with Methanosaeta species for methane production. It might be the reason for the lower methane production in R2 and R3. However, Geobacter species could be significantly enriched in the bioelectrochemical system based on the comparison above. It could be further confirmed by analyzing Geobacter species in the anodic biofilm of R1 (Fig. 3D), which accounted for 32.18% of the bacteria 16S rRNA gene sequences recovered in the anodic biofilm, significantly higher than that in the suspended sludge of R1. Based on the enrichment of Geobacter species and the dominant Methanosaeta species in the anodic biofilm and suspended sludge of R1, it was concluded that the potential DIET between these two species might be an important reason for the increased methane production in R1.
Figure 3.
Bacterial community structure at class level in the suspended sludge of R1 (A), R2 (B) and R3 (C) and at genus level of anodic biofilm of R1 (D) based on high-throughput 16S rRNA pyrosequencing. Classes and genera with relative abundance lower than 1.00% was classified into group ‘Others’.
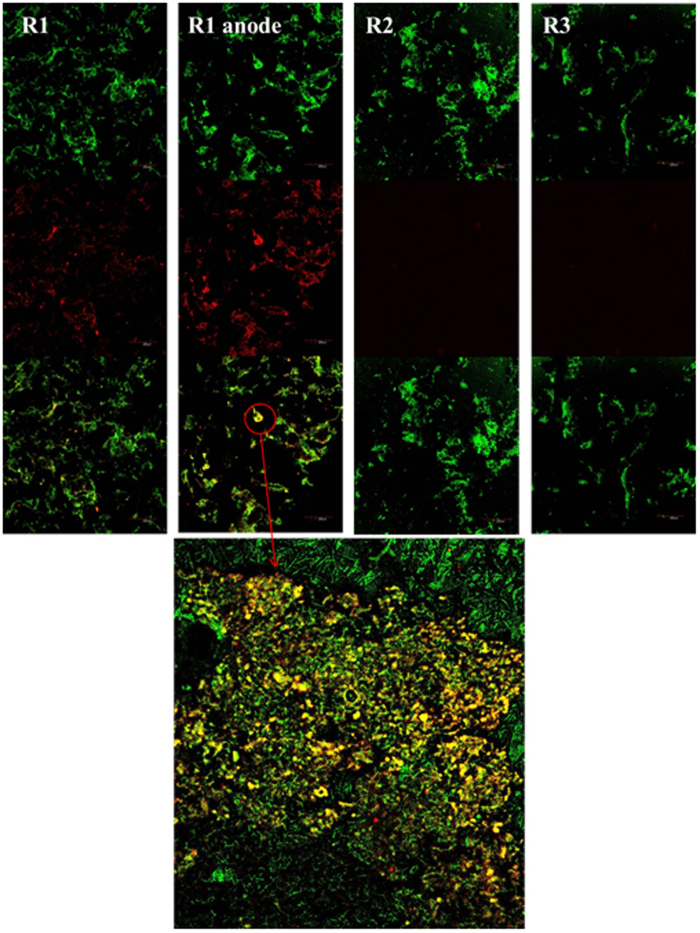
Fluorescence in situ hybridization (FISH) was used to further demonstrate the potential DIET from Geobacter species and Methanosaeta species for methane production. The FISH analysis showed that the dominant Geobacter species enriched in the anodic biofilm and suspended sludge of R1 was just Geobacter metallireducens (GEO1-Cy3, AGAATCCAAGGACTCCGT, red) (Fig. 4), which had a relative abundance of 73.2% and 81.1% of total Geobacter species (GEO825-FITC, TACCCGCRACACCTAGT, green) (Fig. S3), respectively. From FISH images, Geobacter metallireducens (ellipsoid, red) closely gathering with Methanosaeta species (long slender rods, green) implied the potential for biological interspecies electrical connections via DIET especially in the anodic biofilm of R1 (Fig. 4). Remarkably, there was almost no Geobacter species detected in the suspended sludge of R2 and R3 (Fig. S3). This result was well in agreement with the bacterial community analysis via high-throughput 16S rRNA pyrosequencing.
Figure 4. FISH images of the suspended sludge in R1, R2 and R3 and anodic biofilm of R1, respectively.
The suspended sludge and anodic biofilm hybridized with specific probes for Geobacter metallireducens (GEO1-CY3, red) and Methanosaeta species (MX825-FITC, green).
Discussion
Recently, DIET from Geobacter species to Methanosaeta species has been confirmed in defined co-culture of G. metallireducens and M. harundinacea7 as well as in brewery wastewater digesters13. Metatranscriptomic analysis revealed that the genes for CO2 reduction pathway in M. harundinacea were highly expressed, which caused that Methanosaeta species had the capacity to directly accept the electrons from Geobacter species for reduction of CO2 to CH435. With the co-existence of Geobacter species and Methanosaeta species in an anaerobic digester, DIET is expected to be another important way to produce methane. Geobacter species is one of the most metabolically active microorganisms in the anaerobic environments, such as soils and sediments17, making DIET potential to contribute a considerable part of methane production in the world. However, the population of Geobacter species is pretty scare in waste activated sludge (Fig. 3B and Fig. 4), which makes DIET difficult to take place. Recently, some reported that the conductive carbon material, such as granular activated carbon (GAC)36, biochar37, carbon cloth38 and carbon nanotube39, were added into the methanogenic digesters to enhance conversion of wastes to methane via DIET. Differently, although a pair of graphite electrodes installed into the reactor (R3) also possibly serving as a similar conductive material to enhance the electron exchange in DIET, the increased methane production was insignificant (P > 0.05) as compared with the reactor with no electrodes (R2) (Fig. 1A). The lack of Geobacter species was the major reason limiting DIET for methane production during anaerobic sludge digestion.
With an electric supply imposed on the electrodes, although the changes of relative abundance of Methanosaeta species was not apparent, the enrichment of Geobacter species was obviously observed in the suspended sludge and especially in the anodic biofilm (Fig. 3). Bond and Lovley24 first revealed that electrode reduction could support the growth of Geobacter species. Further studies reported that Geobacter species usually adapt to grow with electrodes or Fe (III) oxides as electron acceptors21. In agreement, the electrodes with the power supply installed into the anaerobic digester created a favorable condition to enrich Geobacter species. Further FISH analysis showed that the dominant Geobacter species enriched in the anodic biofilm as well as in the suspended sludge of R1 was Geobacter metallireducens, which were well-known as the microorganism capable of DIET for methane production in defined co-cultures7,10 as well as in anaerobic methanogenic digesters13,40. Unlike other Geobacter species, Geobacter metallireducens not only utilize acetate as substrate for extracellular electron transfer but also utilize other SCFAs and alcohols17. It made Geobacter metallireducens more likely to grow in the anode of bioelectrochemical system fed with complex substrates as compared with other Geobacter species41,42,43,44. Another potential evidence to support this was that the current density of R1 dropped from day 18 to 30 (Fig. 1C). This was because acetate as the most favorite substrate for Geobacter species to produce electricity was almost depleted at day 15 (Fig. S2). Afterwards, with enriching Geobacter metallireducens, it began to again utilize propionate or other SCFAs which allowed to recover the electricity production.
The electrically conductive pili produced by Geobacter species for long-range electron exchange is the important mechanism for DIET45,46. If Geobacter species could exchange electron with methanogens through its conductive pili, the conductivity of sludge likely increased due to the participation of conductive pili13,47. The conductivity (μS/cm) in the suspended sludge before and after digestion presented a highly linear growth with the increase of VSS (mg/L) (Fig. 5). The average conductivity (slope of the curve, μS/cm/VSS) in the initial sludge (0.7121 ± 0.0025 μS/cm/VSS) and in the digested sludge of R2 (0.7550 ± 0.0045 μS/cm/VSS) were similar, both about thirty percentage points lower than that in the digested sludge of R1 (0.9614 ± 0.0079 μS/cm/VSS). The higher conductivity of the digested suspended sludge of R1 might be resulted from the direct interspecies electron exchange between the two species.
Figure 5. Conductivity (μS/cm) with the increase of VSS (mg/L) in the initial pretreated sludge and suspended sludge of R1 and R2 after 51 days experiments.
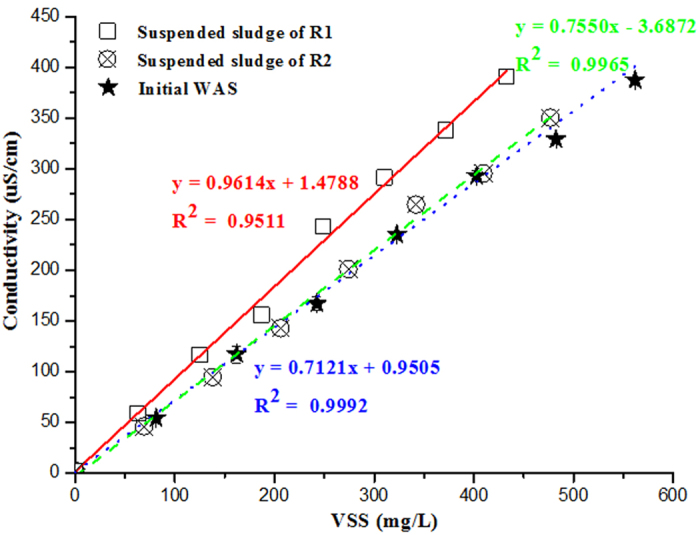
Error bars represent standard deviations of three groups of parallel experiments.
It is worth mentioning that bioelectrochemical methanogenesis in most of recent literatures was ascribed to the anodic oxidation of organics coupled with the cathodic reduction of CO2 into CH431,32,48. Some considered that the more diverse communities formed on electrodes was a result for the increase of producing methane49. All of the present reports on bioelectrochemical methanogenesis have ignored the potential of DIET from Geobacter species to Methanosaeta species for methane production. Actually, the mechanism of anodic oxidation in the bioelectrochemical system was just that exoelectrogenic bacteria like Geobacter species transfer electrons from the oxidation of organic matters to electrodes. This study highly suggested that Methanosaeta species might be another sink to accept electron from Geobacter species in bioelectrochemical system.
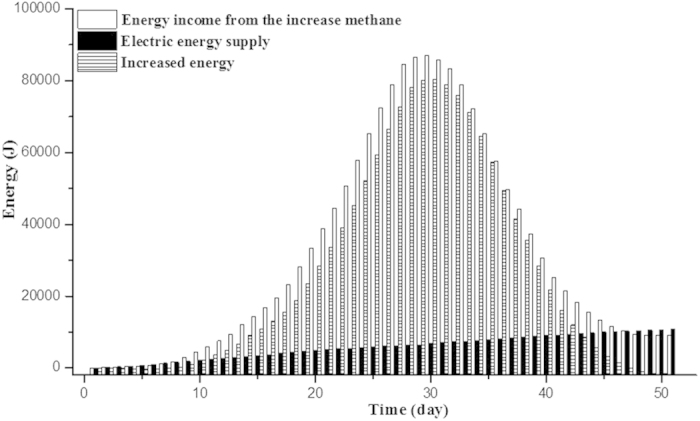
Also, the electric energy supply for the electrodes inserted into is quite lower compared with the increased energy from methane production (Fig. 6). Energy income from the increased methane production (WCH4) of R1 (as compared with R2) reached 7.8 × 104 J during the initial 33 days calculated by the formula (3), while the sum of electricity energy supply (WE) for the electrodes of R1 was only 7487.2 J calculated by the formula (2). It meant that the extra energy income was 10.5 folds (10.5 = 78757.4 J [WCH4] / 7487.2 J [WE]) of the electric energy supply during 33 days. Normally, the disconnection of the voltage supply in the bioelectrochemical system (opened R1) should be operated to further clarify the effects of DIET on methanogenesis. However, with Geobacter species gradually enriched in R1, the available substrates were progressively exhausted in this batch experiment. Assuming in the continuous feed mode, after the Geobacter species was enriched DIET was likely to continuously occur even shifting to the voltage-off state. It might obtain higher energy efficiency.
Figure 6. Change of energy in R1 during 51 days experiments.
After 51 days experiments, the organic matter removal and sludge reduction are illustrated in Table S1 (see supplementary material). From this table, the effluent TSS, VSS and TCOD in R2 was 10320 ± 960 mg/L (mean ± standard deviation), 33500 ± 400 mg/L and 19007.2 ± 165.3 mg /L respectively, which was still similar to that in R3 (103150 ± 850 mg/L, 33600 ± 950 mg/L and 18940.5 ± 428.3 mg/L respectively). While, the effluent TSS, VSS and TCOD in R1 (96100 ± 700 mg/L, 31310 ± 700 mg/L and 16219.7 ± 256.0 mg/L) was lower than that in the other two reactors. It indicated organic matter removal and sludge reduction was enhanced with addition of a pair of electrodes in this study.
Methods
Waste activated sludge and anaerobic inoculum sludge
Waste activated sludge (WAS) used in this study was obtained from a secondary sedimentation tank of a local municipal wastewater treatment plant based on the activated sludge process in Dalian, China. The collected sludge was stored at 4 oC before use. The main characteristics (mean ± standard deviation) of the WAS are as follows: pH 7.14 ± 0.02, total suspended solids (TSS) 100800 ± 200 mg/L, volatile suspended solids (VSS) 43100 ± 434 mg/L, total chemical oxygen demand (TCOD) 51453.5 ± 494.5 mg COD/L, soluble chemical oxygen demand (SCOD) 2903.6 ± 239.1 mg/L, total carbohydrate 1893.3 ± 16.5 mg COD/L, total protein 7217.6 ± 16.4 mg COD/L, total short-chain fatty acids (SCFAs) 1556.5 ± 161.7 mg COD/L.
Anaerobic sludge used as the inoculum for methane production was obtained from a waste sludge treatment plant in Dalian, China. Before the experiments, it was cultured in a batch anaerobic reactor, which had a working volume of 10 L (internal diameter of 200 mm and height of 320 mm). The reactor was operated in a room temperature (22.0 ± 2.0 oC) with a hydrolytic retention time (HRT) of 24 h. Glucose were used as the substrate (COD: 1000 mg/L), and NH4Cl and KH2PO4 were used as nitrogen and phosphorus sources (at ratio of COD: N: P 200: 5: 1), respectively. The trace elements were added according to the following composition: 1 mL/L of a trace element solution containing Zn at 0.37 mmol/L, Mn at 2.5 mmol/L, Cu at 0.14 mmol/L, Co at 8.4 mmol/L, Ni at 0.25 mmol/L, H3BO3 at 0.8 mmol/L and EDTA at 3.4 mmol/L.
Pretreating waste activated sludge at pH 10 for 8 days and mixing with anaerobic inoculum sludge
Before anaerobic inoculum sludge mixed with the initial WAS, the initial WAS was anaerobically pretreated at pH 10 for 8 days according to the method by Zhang et al.50. The experiment of pretreating WAS at pH 10 for 8 days was conducted in a cylindrical anaerobic reactor with a working volume of 5 L (internal diameter of 200 mm and height of 160 mm). The reactor was also operated in a room temperature (22.0 ± 2.0 oC) equipped with mechanical stirrer at a speed of 80 rpm. The fermentation pH was maintained at 10.0 ± 0.2 by 4 M sodium hydroxide (NaOH). After the 8 days pretreatment, the fermentation pH was adjusted to 7.0 using 4 M hydrochloric acid (HCl) and then mixed with the anaerobic inoculum sludge with a ratio of 9:1. The main characteristics of the pretreated WAS are as follows: TSS 104667 ± 580 mg/L, VSS 40667 ± 869 mg/L, TCOD 52307.5 ± 1067.5 mg/L, SCOD 13322.4 ± 512.4 mg/L, total carbohydrates 1893.3 ± 9.0 mg COD/L, solute carbohydrates 1014.8 ± 28.6 mg COD/L, total proteins 1389.3 ± 120.2 mg COD/L, solute proteins 991.2 ± 27.3 mg COD/L and total SCFAs 5446.1 ± 260.5 mg COD/L. The mixed sludge of 400 mL was added to each of reactors for anaerobic digestion.
Batch experimental setup and experimental procedure
Batch experiments of anaerobic sludge digestion were conducted in a cylindrical reactor, each of which had a working volume of 500 mL (internal diameter of 80 mm and height of 100 mm). The graphite-brush anode (external diameter of 25 mm and height of 80 mm, surface areas is 17671 mm2) and the graphite-rob cathode (external diameter of 7 mm and height of 80 mm, surface areas 1759.2 mm2) with a distance of 50 mm were installed into to form a single-chamber bioelectrochemical system (hereafter referred to as R1). The DC power sources (Zhaoxin, RXN-305D, China) connected with the electrodes were used as the electric supply. Two control experiments were operated in this study. One was conducted in a same reactor as R1 but without electrodes (hereafter referred to as R2). The other was also conducted in a same reactor as R1 with the same electrodes but not connected with the DC power (hereafter referred to as R3). Another two groups of parallel experiments as same as R1, R2 and R3 were operated simultaneously. All the reactors equipped with a gas and sludge sampling port placed in a shaker at a speed of 140–150 rpm. All the batch experiments were operated at a temperature of 37.0 ± 2.0 oC.
In order to assess the potential of DIET for methane production, the single-chamber bioelectrochemical reactor with applied voltage of 0.6 V (R1) and the two control reactors (R2 and R3) were operated continuously for 51 days. At the same time, three parallel control experiments were operated in the reactors as same as R1, R2 and R3 respectively (hereafter referred to as R4, R5 and R6) with addition of CH3F (3% [v/v], 99%) to inhibit aceticlastic methanogenesis. Remarkably, CH3F, as a specific inhibitor only for the metabolism of aceticlastic methanogenesis but allowing the operation of H2/CO2-dependent CH4 production, has been widely used to monitor the changes in carbon flow in methanogenic systems51,52,53,54. Also, another two groups of parallel experiments as same as R4, R5 and R6 were operated simultaneously. After 33 days experiments, the suspended sludge collected from the bottom of R1, R2 and R3 and anodic biofilm collected from the anodic surface of R1 were used to analyze the microbial community structure. Then, the suspended sludge of R1 and R2 was collected to measure the conductivity.
Chemical analysis
Analysis of total suspended solid (TSS), volatile suspended solid (VSS) and chemical oxygen demand (COD) (include total COD and solute COD) were conducted in accordance with Standard Methods for the Examination of Water and Wastewater. Proteins (include total proteins and solute proteins) were measured with Lowry’s method55 using bovine serum albumin as a standard solution. Carbohydrates (include total carbohydrates and solute carbohydrates) were measured by phenol-sulfuric acid method56 using glucose as a standard solution. The concentrations of SCFAs were analyzed using a gas chromatograph (Tianmei, GC-7900P/FID, China) according to the report by Jiang et al.57. The equivalent relationship between COD and substrates are as follows: 1.5 g-COD/g protein, 1.06 g-COD/g carbohydrate, 1.07 g-COD/g acetate, 1.51 g-COD/g propionate, 1.82 g-COD/g butyrate and 2.04 g-COD/g valerate. Gas collected from all the reactors was used a gas collecting bag and the component was analyzed by another gas chromatograph (Tianmei, GC-7900P/TCD, China). The pH was recorded using a pH analyzer (Sartorius PB-20, Germany). The current was determined by measuring the voltage across a high-precision resistor (10Ω) using a multimeter/data acquisition system (Hongge, PCI-821H, China)58. The conductivity was measured by using a conductivity meter (Leici, DDS-307, China). Before the conductivity measured, the initial pretreated sludge and the suspended sludge of R1 and R2 were diluted according to the dilution ratio of 1:500, 2:500, 3:500, 4:500, 5:500, 6:500 and 7:500 respectively. Then the results measured were analyzed via linear fitting of Origin 8.0 and the slope (μS/cm/VSS) of the curve was the conductivity of the suspended sludge at room temperature (22.0 ± 2.0 oC).
DNA extraction, PCR amplification and high-throughput 16S rRNA pyrosequencing
After 51 days experiments, two types of sludge samples were collected to analyze the microbial community via high-throughput 16S rRNA gene pyrosequencing according to the method by Lu et al.18. One was the suspended sludge with the same volume (10 mL) taken from the bottom of R1, R2 and R3 and then harvested by centrifugation (110 × 100 g for 15 min at 4 °C). The other was the anodic biofilm collected from the anodic surface of R1 which was rinsed twice by phosphate-buffered saline (PBS; 0.13 M NaCl and 10 mM Na2HPO4 at pH 7.2) and then harvested by centrifugation (110 × 100 g for 15 min at 4 °C). The genomic DNA of the sludge samples were extracted using an extraction kit (Bioteke Corporation, Beijing, China) according to the manufacturer’s instructions. The quality of the extracted DNA was checked by determining its absorbance at 260 and 280 nm.
V1–V3 region (length of 455 bp) of the bacterial 16S rRNA gene was amplified using the universal primers 8F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 533R (5’-TTACCGC GGCTGCTGGCA C-3’), as well as the 454 adapter A and B, barcode and linker sequence. After being purified and quantified, pyrosequencing was carried out by a Roche 454 FLX Titanium sequencer (Roche 454 Life Sciences, Branford, CT, USA) according to the standard protocols.
To analyze the microbial community of the two types of sludge samples, the sequences obtained were phylogenetically allocated down to the phylum, class and genus level using the MOTHUR program (http://www.mothur.org/wiki/Main) at a 0.03 distance level (97% similarity), and a confidence threshold of 95% was set for the phylogenetic classification. After phylogenetic allocation of the sequences down to the phylum, class and genus level, relative abundance of a given phylogenetic group was set as the number of sequences affiliated with that group divided by the total number of sequences per sample.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was used to further demonstrate the potential DIET from Geobacter species to Methanosaeta species after 51 days experiments. The suspended sludge samples were taken from the bottom of R1, R2 and R3 with the same volume (5 mL), respectively and harvested by centrifugation (110 × 100 g for 15 min at 4 °C). The anodic biofilm of R1 were rinsed twice by phosphate-buffered saline (PBS; 0.13 M NaCl and 10 mM Na2HPO4 at pH 7.2) and harvested by centrifugation (110 × 100 g for 15 min at 4 °C). Three genus-specific probes for Geobacter metallireducens (GEO1-Cy3, AGAATCCAAGGACTCCGT, red)10, Geobacter species (GEO825-FITC, TACCCGCRACACCTAGT, green)59 and Methanosaeta species (MX825-FITC, TCGCACCGTGGCCGACACCTAGC, green)7 were used in this study. All the samples were rinsed another thrice by phosphate-buffered saline (PBS; 0.13 M NaCl and 10 mM Na2HPO4 at pH 7.2), and then fixed with 4% paraformaldehyde for 2 h at 4 °C. Hybridizations were performed at 46 °C for 1.5 h with buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.2], 0.01 sodium dodecyl sulfate and 35% formamide) containing 50 ng probe per microliter and then washed with buffer (15 min at 48 °C)31. The samples were observed under a confocal laser scanning microscope (Leica SP2, Heidelberger, Germany). The FISH images were imported to Image-Pro Plus 6.0 for analysis of the relative abundance of microorganisms.
Calculation
The amount of available electrons for cathodic reduction of CO2 to CH4 in R1 and R4 was evaluated by the following formula (1):
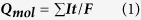 |
Where I is the average current (A/per day) calculated by current density (Fig. 1C), t is the time (24 × 3600 s/hr per day) and F is faradays constant (96485 C/mol). The amount of electric energy supply (WE [J]) in R1 added to the circuit by DC power source, adjusted for loss across the resistor was calculated by the following equation (2)34:
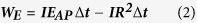 |
Where I is the average current (A/per day) based on current density (Fig. 1C), EAP (V) is the applied voltage of R1 (0.6 V), ∆t (s) is the time of experiments (24 × 3600 s/hr per day), and R is the external resistor (10 Ω). The energy income of R1 from the increased methane production (WCH4 [J]) was calculated by the following equation (2):
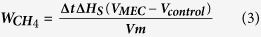 |
Where ∆Hs is the energy content of methane based on the heat of combustion (upper heating value, 890.31 × 103 J/mol), ∆t (s) is the time of experiments (24 × 3600 s/hr per day), VMEC is the volume of methane in R1 (L), VControl is the volume of methane in R2 (L) and Vm is molar volume of the gas at room temperature and atmosphere pressure (24.8 L/mol).
Additional Information
How to cite this article: Zhao, Z. et al. Potential for direct interspecies electron transfer in an electric-anaerobic system to increase methane production from sludge digestion. Sci. Rep. 5, 11094; doi: 10.1038/srep11094 (2015).
Supplementary Material
Acknowledgments
The authors acknowledge the financial support from the National Basic Research Program of China (51378087, 21177015).
Footnotes
Author Contributions Z.Q.Z. and Y.B.Z. conceived and designed the experiments; Z.Q.Z. and L.Y.W. performed the experiments; Z.Q.Z., Y.B.Z. and X.Q. analyzed data; Z.Q.Z. and Y.B.Z. wrote the manuscript; Y.B.Z. and X.Q. contributed reagents and materials, and all authors reviewed the manuscript.
References
- Appels Lise et al. Anaerobic digestion in global bio-energy production: Potential and research challenges. Renew Sustain Energ Rev 15, 4295 (2011). [Google Scholar]
- Batstone D. J. & Virdis B. The role of anaerobic digestion in the emerging energy economy. Curr Opin Biotechnol 27, 142 (2014). [DOI] [PubMed] [Google Scholar]
- Holm-Nielsen J. B., Al , Seadi T. & Oleskowicz-Popiel , P. The future of anaerobic digestion and biogas utilization. Bioresource Technol 100, 5478 (2009). [DOI] [PubMed] [Google Scholar]
- Chen Ye, Cheng Jay J. & Creamer Kurt S. Inhibition of anaerobic digestion process: A review. Bioresource Technol 99, 4044 (2008). [DOI] [PubMed] [Google Scholar]
- Sieber Jessica R., McInerney Michael J. & Gunsalus Robert P. Genomic Insights into Syntrophy: The Paradigm for Anaerobic Metabolic Cooperation. Annu Rev Microbiol 66, 429 (2012). [DOI] [PubMed] [Google Scholar]
- Stams Alfons J. M. & Plugge Caroline M. Electron transfer in syntrophic communities of anaerobic bacteria and archaea. Nat Rev Microbiol 7, 568 (2009). [DOI] [PubMed] [Google Scholar]
- Rotaru Amelia-Elena et al. A new model for electron flow during anaerobic digestion: direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energ Environ Sci 7, 408 (2013). [Google Scholar]
- Dolfing J. et al. Syntrophic Growth on Formate: a New Microbial Niche in Anoxic Environments. Appl Environ Microb 74, 6126 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stams Alfons J. M. et al. Exocellular electron transfer in anaerobic microbial communities. Environ Microbiol 8, 371 (2006). [DOI] [PubMed] [Google Scholar]
- Summers Z. M. et al. Direct exchange of electrons within aggregates of an evolved syntrophic coculture of anaerobic bacteria. Science 330, 1413 (2010). [DOI] [PubMed] [Google Scholar]
- Lovley D. R. et al. Geobacter metallireducens gen. nov. sp. nov., a microorganism capable of coupling the complete oxidation of organic compounds to the reduction of iron and other metals. Arch Microbiol 159, 336 (1993). [DOI] [PubMed] [Google Scholar]
- Caccavo F. Jr et al. Geobacter sulfurreducens sp. nov., a hydrogen- and acetate-oxidizing dissimilatory metal-reducing microorganism. Appl Environ Microb 60, 3752 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M. et al. Potential for direct interspecies electron transfer in methanogenic wastewater digester aggregates. MBio 2, e111 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. S. & Ingram-Smith C. Methanosaeta, the forgotten methanogen? Trends Microbiol 15, 150 (2007). [DOI] [PubMed] [Google Scholar]
- Snoeyenbos-West O. L., Nevin K. P., Anderson R. T. & Lovley D. R. Enrichment of Geobacter Species in Response to Stimulation of Fe(III) Reduction in Sandy Aquifer Sediments. Microbial Ecol 39, 153 (2000). [DOI] [PubMed] [Google Scholar]
- Zhang Tian et al. Stimulating the anaerobic degradation of aromatic hydrocarbons in contaminated sediments by providing an electrode as the electron acceptor. Environ Microbiol 12, 1011 (2010). [DOI] [PubMed] [Google Scholar]
- Lovley D. R. et al. Geobacter: the microbe electric’s physiology, ecology, and practical applications. Adv Microb Physiol 59, 1 (2011). [DOI] [PubMed] [Google Scholar]
- Lu L., Xing D. & Ren N. Pyrosequencing reveals highly diverse microbial communities in microbial electrolysis cells involved in enhanced H2 production from waste activated sludge. Water Res 46, 2425 (2012). [DOI] [PubMed] [Google Scholar]
- Sasaki D. et al. Operation of a cylindrical bioelectrochemical reactor containing carbon fiber fabric for efficient methane fermentation from thickened sewage sludge. Bioresource Technol 129, 366 (2013). [DOI] [PubMed] [Google Scholar]
- Yu Hongguang et al. Start-Up of an Anaerobic Dynamic Membrane Digester for Waste Activated Sludge Digestion: Temporal Variations in Microbial Communities. PLoS ONE 9, e93710 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird L. J., Bonnefoy V. & Newman D. K. Bioenergetic challenges of microbial iron metabolisms. Trends Microbiol 19, 330 (2011). [DOI] [PubMed] [Google Scholar]
- Nevin K. P. & Lovley D. R. Lack of production of electron-shuttling compounds or solubilization of Fe(III) during reduction of insoluble Fe(III) oxide by Geobacter metallireducens. Appl Environ Microb 66, 2248 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber Karrie A., Achenbach, Laurie A. & Coates, John D. Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4, 752 (2006). [DOI] [PubMed] [Google Scholar]
- Bond D. R. & Lovley D. R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl Environ Microb 69, 1548 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley Derek R. Live wires: direct extracellular electron exchange for bioenergy and the bioremediation of energy-related contamination. Energ Environ Sci 4, 4896 (2011). [Google Scholar]
- Logan Bruce E. Exoelectrogenic bacteria that power microbial fuel cells. Nat Rev Microbiol 7, 375 (2009). [DOI] [PubMed] [Google Scholar]
- Logan Bruce E. & Regan John M. Electricity-producing bacterial communities in microbial fuel cells. Trends Microbiol 14, 512 (2006). [DOI] [PubMed] [Google Scholar]
- Jia Jianna et al. Electricity generation from food wastes and microbial community structure in microbial fuel cells. Bioresource Technol 144, 94 (2013). [DOI] [PubMed] [Google Scholar]
- Marsili E. et al. Microbial biofilm voltammetry: direct electrochemical characterization of catalytic electrode-attached biofilms. Appl Environ Microb 74, 7329 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter Hanno et al. Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energ Environ Sci 2, 506 (2009). [Google Scholar]
- Cheng Shaoan, Xing Defeng, Call Douglas F. & Logan Bruce E. Direct Biological Conversion of Electrical Current into Methane by Electromethanogenesis. Environ Sci Technol 43, 3953 (2009). [DOI] [PubMed] [Google Scholar]
- Villano Marianna et al. Bioelectrochemical reduction of CO2 to CH4 via direct and indirect extracellular electron transfer by a hydrogenophilic methanogenic culture. Bioresource Technol 101, 3085 (2010). [DOI] [PubMed] [Google Scholar]
- Call Douglas & Logan Bruce E. Hydrogen Production in a Single Chamber Microbial Electrolysis Cell Lacking a Membrane. Environ Sci Technol 42, 3401 (2008). [DOI] [PubMed] [Google Scholar]
- Logan Bruce E. et al. Microbial Electrolysis Cells for High Yield Hydrogen Gas Production from Organic Matter. Environ Sci Technol 42, 8630 (2008). [DOI] [PubMed] [Google Scholar]
- Shrestha P. M. & Rotaru A. E. Plugging in or going wireless: strategies for interspecies electron transfer. Front Microbiol 5, 237 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Fanghua et al. Promoting direct interspecies electron transfer with activated carbon. Energ Environ Sci 5, 8982 (2012). [Google Scholar]
- Luo Chenghao, Lü Fan, Shao Liming & He Pinjing Application of eco-compatible biochar in anaerobic digestion to relieve acid stress and promote the selective colonization of functional microbes. Water Res 68, 710 (2015). [DOI] [PubMed] [Google Scholar]
- Chen Shanshan et al. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresource Technol 173, 82 (2014). [DOI] [PubMed] [Google Scholar]
- Li Ling-Li et al. Response of anaerobic granular sludge to single-wall carbon nanotube exposure. Water Res 70, 1 (2015). [DOI] [PubMed] [Google Scholar]
- Liu Fanghua et al. Promoting direct interspecies electron transfer with activated carbon. Energ Environ Sci 5, 8982 (2012). [Google Scholar]
- Kouzuma Atsushi et al. Comparative Metagenomics of Anode-Associated Microbiomes Developed in Rice Paddy-Field Microbial Fuel Cells. PLoS ONE 8, e77443 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire K. P. & Becker J. G. Design and characterization of a microbial fuel cell for the conversion of a lignocellulosic crop residue to electricity. Bioresource Technol 119, 208 (2012). [DOI] [PubMed] [Google Scholar]
- Kiely Patrick D. et al. Anode microbial communities produced by changing from microbial fuel cell to microbial electrolysis cell operation using two different wastewaters. Bioresource Technol 102, 388 (2011). [DOI] [PubMed] [Google Scholar]
- Qu Youpeng et al. Salt removal using multiple microbial desalination cells under continuous flow conditions. Desalination 317, 17 (2013). [Google Scholar]
- Reguera G., Pollina R. B., Nicoll J. S. & Lovley D. R. Possible nonconductive role of Geobacter sulfurreducens pilus nanowires in biofilm formation. J Bacteriol 189, 2125 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera G. et al. Extracellular electron transfer via microbial nanowires. Nature 435, 1098 (2005). [DOI] [PubMed] [Google Scholar]
- Malvankar Nikhil S., Tuominen Mark T. & Lovley Derek R. Biofilm conductivity is a decisive variable for high-current-density Geobacter sulfurreducens microbial fuel cells. Energ Environ Sci 5, 5790 (2012). [Google Scholar]
- Tartakovsky B., Mehta P., Bourque J. S. & Guiot S. R. Electrolysis-enhanced anaerobic digestion of wastewater. Bioresource Technol 102, 5685 (2011). [DOI] [PubMed] [Google Scholar]
- De Vrieze J. et al. Biomass retention on electrodes rather than electrical current enhances stability in anaerobic digestion. Water Res 54, 211 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang Peng et al. Understanding Short-Chain Fatty Acids Accumulation Enhanced in Waste Activated Sludge Alkaline Fermentation: Kinetics and Microbiology. Environ Sci Technol 44, 9343 (2010). [DOI] [PubMed] [Google Scholar]
- Liu He, Wang Jin, Wang Aijie & Chen Jian. Chemical inhibitors of methanogenesis and putative applications. Appl Microbiol Biot 89, 1333 (2011). [DOI] [PubMed] [Google Scholar]
- Hao Liping et al. Self-adaption of methane-producing communities to pH disturbance at different acetate concentrations by shifting pathways and population interaction. Bioresource Technol 140, 319 (2013). [DOI] [PubMed] [Google Scholar]
- Hao Li-Ping et al. Predominant Contribution of Syntrophic Acetate Oxidation to Thermophilic Methane Formation at High Acetate Concentrations. Environ Sci Technol 45, 508 (2011). [DOI] [PubMed] [Google Scholar]
- Daebeler Anne, Gansen Martina & Frenzel Peter. Methyl Fluoride Affects Methanogenesis Rather than Community Composition of Methanogenic Archaea in a Rice Field Soil. PLoS ONE 8, e53656 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frolund B., Griebe T. & Nielsen P. H. Enzymatic activity in the activated-sludge floc matrix. Appl Microbiol Biotechnol 43, 755 (1995). [DOI] [PubMed] [Google Scholar]
- Masuko Tatsuya et al. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem 339, 69 (2005). [DOI] [PubMed] [Google Scholar]
- Jiang Su, Chen Yinguang & Zhou Qi. Effect of sodium dodecyl sulfate on waste activated sludge hydrolysis and acidification. Chem Eng J 132, 311 (2007). [Google Scholar]
- Wang Aijie et al. A rapid selection strategy for an anodophilic consortium for microbial fuel cells. Bioresource Technol 101, 5733 (2010). [DOI] [PubMed] [Google Scholar]
- Anderson Robert T., Rooney-Varga Juliette N., Gaw Catherine V. & Lovley Derek R. Anaerobic Benzene Oxidation in the Fe(III) Reduction Zone of Petroleum-Contaminated Aquifers. Environ Sci Technol 32, 1222 (1998). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.