Abstract
Growth hormone (GH) treatment of idiopathic short stature (ISS), defined as height <−2.25 standard deviations (SD), is approved by U.S. FDA. This study determined the gender-specific prevalence of height <−2.25 SD in a pediatric primary care population, and compared it to demographics of U.S. pediatric GH recipients. Data were extracted from health records of all patients age 0.5–20 years with ≥ 1 recorded height measurement in 28 regional primary care practices and from the four U.S. GH registries. Height <−2.25 SD was modeled by multivariable logistic regression against gender and other characteristics. Of the 189,280 subjects, 2073 (1.1%) had height <−2.25 SD. No gender differences in prevalence of height <−2.25 SD or distribution of height Z-scores were found. In contrast, males comprised 74% of GH recipients for ISS and 66% for all indications. Short stature was associated (P < 0.0001) with history of prematurity, race/ethnicity, age and Medicaid insurance, and inversely related (P < 0.0001) with BMI Z-score. In conclusion, males outnumbered females almost 3:1 for ISS and 2:1 for all indications in U.S. pediatric GH registries despite no gender difference in height <−2.25 SD in a large primary care population. Treatment and/or referral bias was the likely cause of male predominance among GH recipients.
In 2003 the U.S. Food and Drug Administration (FDA) approved growth hormone (GH) treatment for children with idiopathic short stature (ISS), defined as height more than 2.25 standard deviations (SD) below mean for age and gender, and without evidence of underlying disease. This represents the shortest 1.2% of the U.S. population, using the 2000 Centers for Disease Control and Prevention [CDC] growth chart data. Prior to the FDA ruling, GH was prescribed primarily for GH deficiency, with an estimated prevalence of 1 in 3500 children1. Now about 1 in 100 children may be eligible for a treatment that requires daily subcutaneous injections and costs about $20,000 annually per patient2. The ISS indication shifted focus from underlying pathophysiology to stature as the criterion for treatment, with ramifications for health expenditures and policy considerations regarding the medicalization of a physical trait.
An international post-marketing registry of pediatric GH recipients showed that the 64% male predominance among U.S. patients exceeded the male:female proportions in other countries, and the greatest gender difference occurred for the familial short stature/constitutional growth delay/ISS group3. Also, males outnumbered females about 2:1 among short stature evaluations at a large U.S. pediatric endocrinology center4. Does this pattern of male predominance in subspecialty care and GH treatment reflect a greater prevalence of growth impairment in males or gender-based referral and/or treatment biases? In an urban, low income, primary care group, the prevalence of growth faltering did not differ by gender5. We now investigated the prevalence of short stature below the ISS threshold by gender in a large, heterogeneous pediatric primary care population. Additionally, this regional cohort was compared with treatment patterns in the GH registries for U.S. patients.
Results
Pediatric primary care population
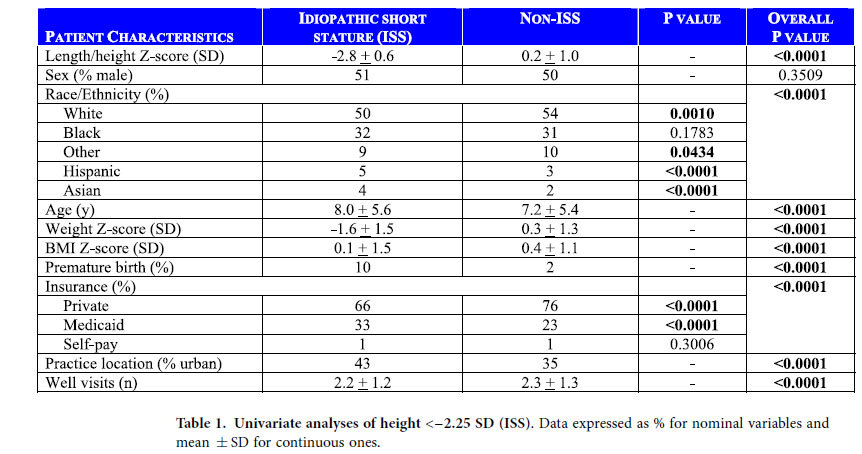
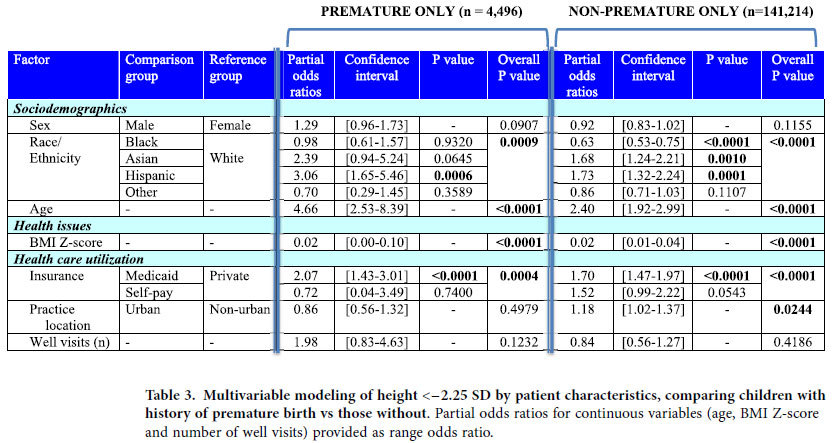
Of the 189,280 subjects evaluated, 2073 (1.1%) had height <−2.25 SD, the FDA height criterion for ISS. Characteristics of the ISS and non-ISS groups were compared in Table 1. Except for gender, differences between the two groups were statistically significant (P < 0.0001) for all patient characteristics. Males comprised 51% of the ISS and 50% of the non-ISS groups (P = 0.35).
Table 1. Univariate analyses of height <−2.25 SD (ISS).
Height below the ISS threshold was modeled by logistic regression against patient characteristics among the 145,710 subjects with complete data (Table 2). Gender was not significant; male gender had an odds ratio (OR) of 0.96 (95% C.I. [0.87–1.06], P = 0.38). The factors significantly (P < 0.0001) associated with ISS-level height were, in descending value of ORs: history of prematurity, age, race/ethnicity (Hispanic and Asian >White >Black), Medicaid insurance, and BMI Z-score. Because prematurity had such a strong effect in the model, yet applies to distinct subjects, the analyses also were performed for the premature subgroup (n = 4,496) and non-premature subgroup (n = 141,214) separately (Table 3). Results were similar, except practice location was significant for the non-premature subgroup only (P = 0.02), and the racial/ethnic profiles varied slightly between the two subgroups. Of note, gender was not significant in either group.
Table 2. Multivariable modeling of height <−2.25 SD by patient characteristics (n = 145,710 with complete data).
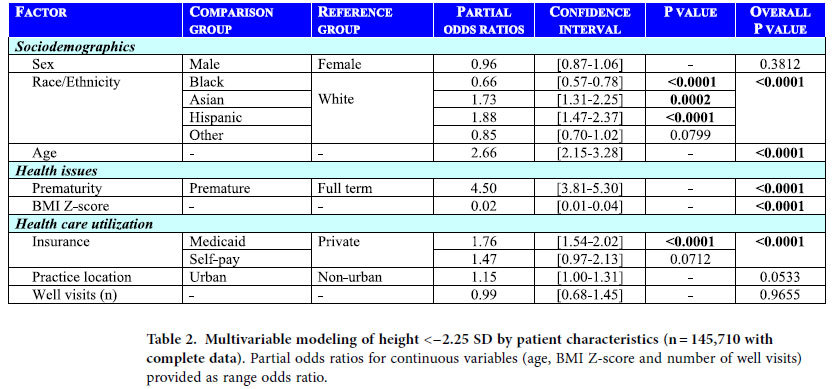
Table 3. Multivariable modeling of height <−2.25 SD by patient characteristics, comparing children with history of premature birth vs those without.
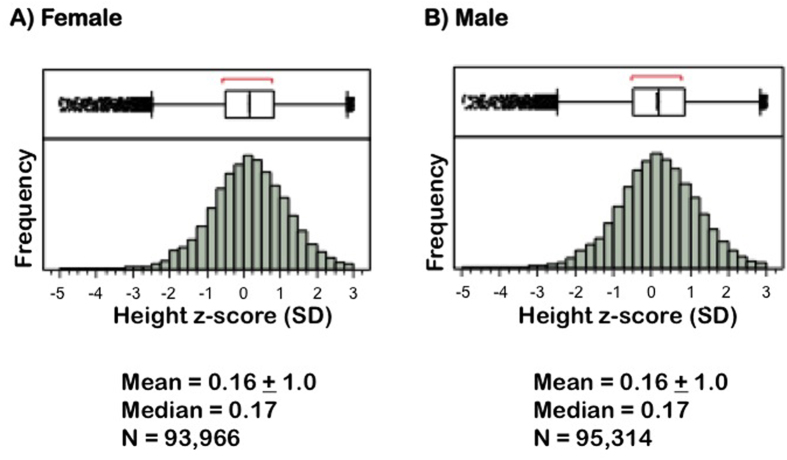
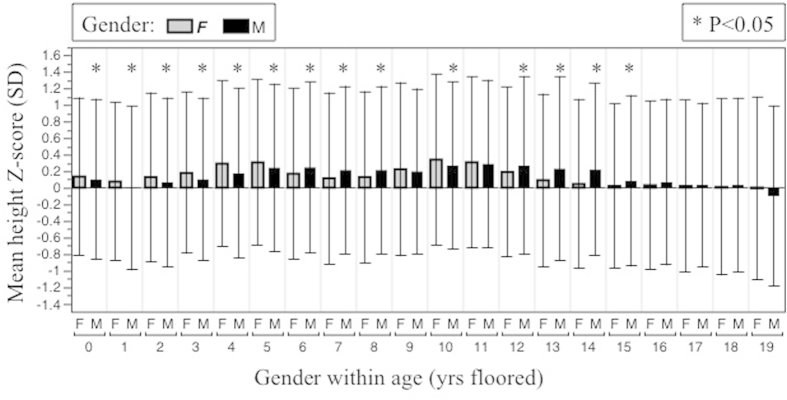
Thus, gender was not a significant factor in the univariate or any of the multivariable analyses of ISS-level height. Further, distributions of height Z-scores by gender in the entire study population were found to be similar (Fig. 1). Comparison of age-specific mean height Z-scores by gender are shown in Fig. 2.
Figure 1. Distributions of height Z-scores by gender in the entire study population.
Above each histogram is an outlier box plot; the boxes, demarcating the interquartile range and median, surround the middle half of the data points, while the tails extend to the farthest value still within 1.5 interquartile ranges from the quartiles. All data points beyond the tails are shown individually as outliers. Means are presented as ± SD.
Figure 2. Age-specific distributions of height Z-scores by gender.
Mean height Z-score ± 1 SD is shown for each gender for each year of age floored. Gender differences for each age were compared by one-way ANOVA.
GH registry data
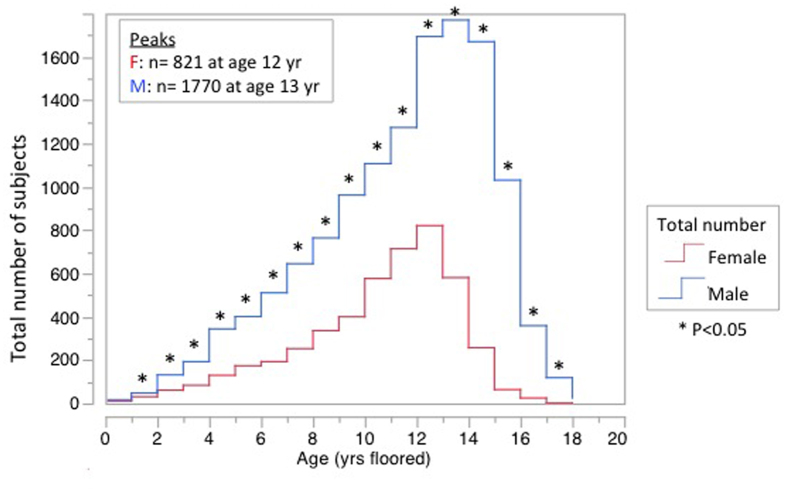
Males comprised 66% of the 93,736 subjects enrolled in the four U.S. GH registries combined, and 74% of those (18%) treated for the ISS indication (Table 4). The ISS subgroup also differed (P < 0.0001) from the other indications regarding racial/ethnic composition, and neither reflected demographics from U.S. Census data (Table 4). At the time of GH initiation for ISS, females were shorter than males in each of the four registries and in all of the registries combined (mean height Z-score −2.8 ± 0.1 SD vs −2.5 ± 0.1 SD, respectively, P < 0.0001). At GH initiation for ISS, males outnumbered females (P < 0.05) at every year of age except the first year, with the greatest numbers prescribed and the greatest gender difference occurring during the peri-pubertal period (Fig. 3). The median age [interquartile range] at GH initiation was 11 [8–12] years for females and 12 [9–13] years for males.
Table 4. Demographics of subjects enrolled in the four U.S. pediatric growth hormone registries, with U.S. Census data for comparison.
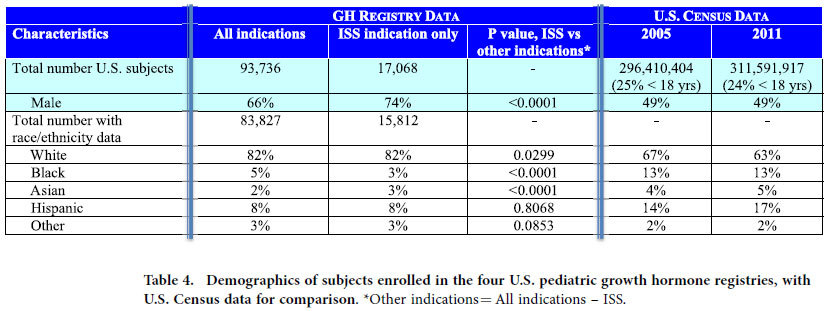
Figure 3. Total number of males and females in the four U.S growth hormone registries by year of age at initiation of treatment for idiopathic short stature.
Gender differences for each age were compared by one sample exact binomial test (one-tail) against the null hypothesis that prescription rates were independent of gender.
Discussion
There were no gender differences in the prevalence of height <−2.25 SD, the ISS threshold, in this large, heterogeneous sample of children and adolescents cared for in a primary care setting. In contrast, GH registries documented that more males than females are treated in the U.S. (2M:1F), especially for the ISS indication (3M:1F). While male predominance among U.S. GH recipients has been described since the first published registry report of GH use in 1985–19876, the current study is unique in combining data across all four U.S. pediatric GH registries, which are usually analyzed and reported separately due to the proprietary nature of the data. A detailed analysis of one of the registries, taking into account the accumulation of FDA approved indications, showed that the almost 2 M:1 F ratio was consistent across the first twenty years of recombinant GH use, possibly related to off label prescriptions3.
The greatest numbers of patients started on GH treatment for ISS occurred during the peri-pubertal period for both genders, with the median age at GH initiation of 11 for females and 12 for males. This likely reflects the physiologically earlier onset of puberty in females, and the greater concern about short stature when puberty heralds ultimate growth plate fusion and hence, limited remaining opportunity for potential medical intervention. The maximal gender disparity for GH prescribing occurred in the pubertal age range, raising the possibility that some children were treated with GH for constitutional delay of growth and puberty (CDGP) unnecessarily. However, the number of males prescribed GH for ISS exceeded females for every year of age except the first, suggesting puberty contributes only part of the disparity.
Gender was not a significant predictor of height below the ISS threshold in our primary care population, yet males were 74% of patients treated for ISS and were on average taller at time of GH initiation. What causes this treatment bias (Fig. 4)? GH is prescribed mostly by pediatric endocrinologists7. In 1996 and again in 2010, U.S. pediatric endocrinologists, evaluating hypothetical case scenarios in surveys, were more likely to recommend GH treatment for boys than girls in otherwise identical scenarios8,9. Also, pediatric endocrinologists see about twice as many boys than girls for evaluation of short stature; further, the female patients referred to a pediatric endocrinology center had greater height deficits than the boys and a risk ratio of 2.7 for having an identifiable organic disease4. While primary care physicians (PCP) are responsible for specialist referrals, their decisions are influenced by the level of parental concern7, and sometimes parents seek specialist care directly. Thus, it is difficult to quantify the relative contributions of parents and PCP to the endocrine referral bias. Many children with short stature do not see a pediatric endocrinologist and are managed by their PCP. In a 3-year study of four urban pediatric primary care practices affiliated with a tertiary pediatric hospital, only 2.8% of children with linear growth faltering saw an endocrinologist, and PCP obtained laboratory tests of the GH axis for twice as many boys (1.8%) than girls (0.9%; P < 0.05) with growth faltering10.
Figure 4. Sequence of events determining who receives pediatric growth hormone treatment.
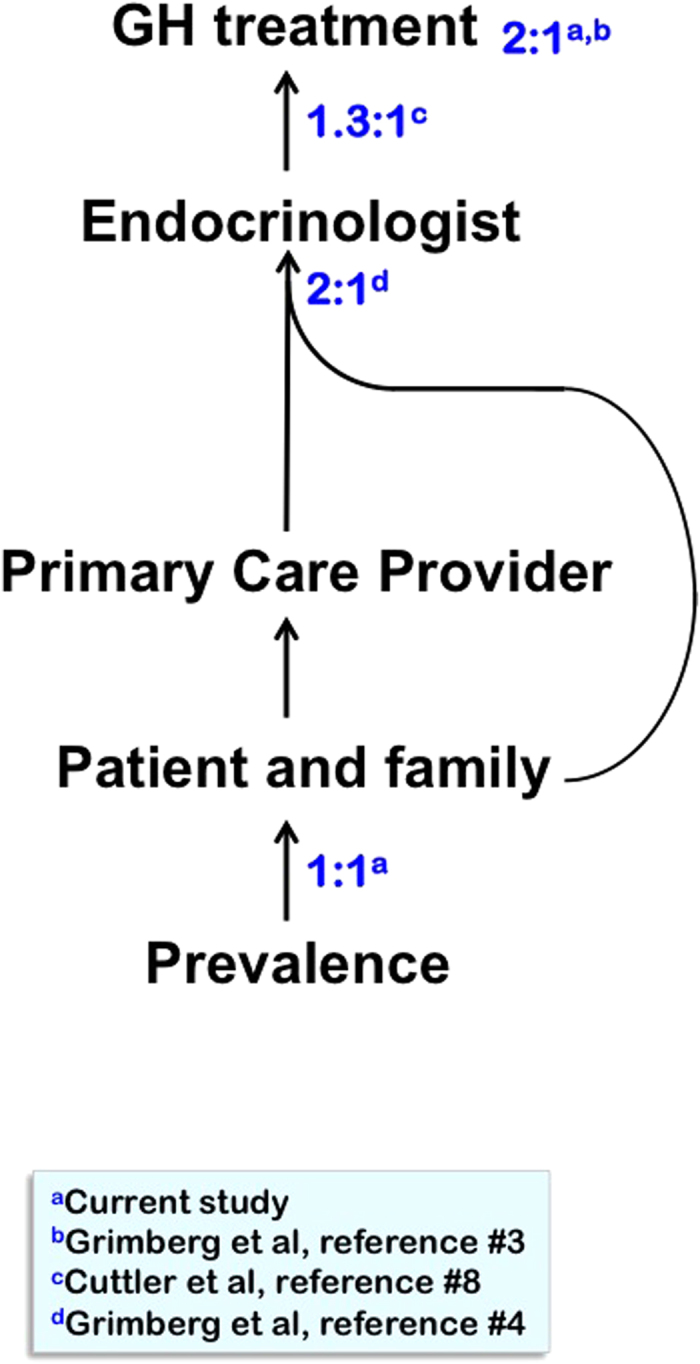
Ratios indicate male:female proportions at the various steps, with their respective references. The 2:1 male predominance among children receiving short stature evaluations at endocrine clinics results from the combination of gender-based referral biases by their primary care providers and gender-based biases of patient-families who directly seek specialist evaluation; the relative contributions of these two sources could not be quantified.
Because the height Z-score references were developed for boys and girls separately, it may seem an inevitability to find no gender difference among children with heights <−2.25 SD. However, two factors may alter the gender-based height distributions in a population. The first possibility involves secular trends or other differences between the specific study sample and the CDC reference population upon which the growth charts were based. The second deviation may result potentially from different frequencies of growth-impairing diseases by gender. Similar to a study of two Australian survey populations in 2006–2007, we found evidence for a secular increase in height (mean and median for both genders were greater than zero relative to CDC reference) but no change in the spread of heights across the population (standard deviations of height Z-score distributions in the studies remained 1 SD)11. In the Australian study, more boys than girls fell below the CDC and study-specific 1st percentiles, but the actual numbers of children were small (<30 of each gender in each survey)11. The authors found no gender difference when the Australian criteria were applied to the National Health and Nutrition Examination Survey (NHANES) of U.S. children in 2005–200611. We likewise found no gender difference in height <−2.25 SD in our population, even though based on the GH registries, one would expect to find more short boys than girls.
The factors we found significantly associated with short stature in our population were consistent with the previous literature. History of premature birth was the strongest predictor of height <−2.25 SD in our logistic regression model even with the analyzed growth data limited to after age 2 years. Increased prevalence of height <−2SD has been reported in Swedish and French cohorts of children born prematurely when measured at age 11 and 5 years, respectively12,13. Age had the second largest effect on height <−2.25 SD in our population, with OR of 2.66 [2.15–3.28] over the age range of 0.5 to 20 years, likely reflecting the greater opportunity for accruing a height deficit with time. Although males with CDGP frequently seek medical attention, CDGP occurs in adolescents of both genders14,15 and in our population, males were not significantly shorter than females after age 10 years. Race/ethnicity differences were also found in our model, with Hispanic and Asian children having greater risk and black children having lower risk of height <−2.25 SD than white children, consistent with other studies16,17,18,19,20,21,22. Medicaid insurance coverage compared to private insurance was a risk factor for height < −2.25 SD in our model. Medicaid coverage is often used as a surrogate for lower socioeconomic status and access to healthcare. The detrimental effect of low socioeconomic status on height has been appreciated since 189223 and confirmed in the various NHANES studies24,25. Finally, BMI Z-score had a strong, but inverse effect in our model of height <−2.25 SD. Poor nutritional intake can stunt statural growth, especially during periods of rapid growth, and nutritional repletion leads to catch-up growth26. Conversely, obesity has been linked to accelerated growth and early pubertal development27. Secular trends in BMI have been associated with the other factors in our model28. Despite complex interactions, the fact that race/ethnicity, Medicaid insurance and BMI Z-score reached significance to P < 0.0001 in our model suggests that they each exerted an independent risk on height <−2.25 SD.
The retrospective nature of this study introduced limitations. Socioeconomic data were not available beyond patient insurance type. To address this, 30,638 subjects were geocoded to extract census-tract data on family structure, maximal household education level, employment and income. The census-tract level variables did not contribute significantly to height <−2.25 SD in our logistic regression model (data not shown), possibly due to variability among residents of a census tract. Socioeconomic factors have been found to influence the medical management of short stature. In an ethnically diverse mid-sized U.S. city, parents seeking evaluation of their child’s short stature by a pediatric endocrinologist were proportionally of higher income and education levels than the surrounding population29. Nonetheless, the limitations of retrospective electronic health records (EHR)-based studies would not be expected to alter the gender-based prevalence of height <−2.25 SD in a primary care population.
In conclusion, the prevalence of height below −2.25 SD, the threshold for ISS, was not different by gender in this large, heterogeneous pediatric primary care population despite a male predominance among U.S pediatric GH recipients (74% for ISS). This is the strongest evidence to date supporting the existence of gender-based referral and treatment biases for short stature in U.S. children. Growth failure is a vital sign of child health and should receive equal import for both genders30. By focusing on the social aspects of height, whose pressures seem to affect males more than females in U.S. society31, the resultant gender-based biases can lead to missed diagnosis of underlying disease in short girls while promoting over-zealous treatment of healthy short boys with an expensive medication.
Methods
Subject selection and patient data
All experiments were performed in accordance with relevant guidelines and regulations. The Children’s Hospital of Philadelphia institutional review board approved this study, and determined that the criteria under 45 CFR 46.116(d) were met for waiver of informed consent. The pediatric primary care population was drawn from the 28 practices in Pennsylvania, New Jersey and Delaware affiliated with a tertiary pediatric hospital. The EHR (EpicCare [Epic Systems Inc, Verona, WI]) of all 190,246 patients aged 0.5–20 years who had at least one recorded height or length measurement in 2006 through 2008 were reviewed. Details of patient data extraction methods have been reported previously5,10 and included: height (or length), weight, BMI, age, gender, race/ethnicity, history of prematurity, insurance provider (private, Medicaid or self-pay), and number of well visits. Practices were categorized as urban or non-urban depending on whether they were located within city population center >100,000.
Height/length, weight and BMI were converted to age- and gender-specific percentiles and Z-scores5,32. Fidelity of the anthropometric data was optimized by exclusions and manual checks as previously described5,10. We excluded growth data for low birth weight or premature infants before age 2 years and all biologically implausible heights, defined by the World Health Organization and CDC as beyond −5 or +3 SDs33,34. Additionally, manual review of the growth charts with outlier BMI Z-scores led to the exclusion of subjects with BMI Z-score below −10 SD or greater than +9 SD as implausible. The resultant study population contained 189,280 subjects.
Statistical analyses of population data
All analyses were performed with JMP software (SAS Institute, Inc, Cary, NC). Categorical variables were presented as percents and compared by the Pearson χ2 test, while continuous variables were presented as mean ± SD and compared by 2-sided t test. Logistic regression analyses with effect Wald tests were performed to quantify the relationships between the potential explanatory variables and height <−2.25 SD; model results were presented throughout as partial odds ratios with 95% confidence intervals (C.I.) and χ2 P values. Odds ratios provided for the continuous variables were range odds ratios.
GH registry data
Demographic data were extracted in summer 2012 from the National Cooperative Growth Study database (NCGS; Genentech, South San Francisco, CA), Pfizer International Growth Study database (KIGS; Pfizer Inc., New York, NY), Genetics and Neuroendocrinology of Short Stature International Study (GeNeSIS; Eli Lilly & Company, Indianapolis, IN) and American Norditropin Studies: Web-Enabled Research Program (ANSWER; Novo Nordisk Inc., Princeton, NJ) by methods and collection previously described35,36,37,38. Subjects treated for ISS and non-ISS indications were compared by Pearson χ2 test. For reference, demographic data from the 2005 and 2011 U.S Census39 were provided, contemporaneous with our observation window of the primary care population and the GH registry queries. Mean height Z-scores of males and females at initiation of GH treatment for ISS were compared by student t-test with both Satterthwaite and Welch’s adjustments for unequal variances. Age (years floored) at GH initiation for ISS was tabulated by gender and compared by one sample exact binomial test (one-tail) against the null hypothesis that prescription rates were independent of gender.
Additional Information
How to cite this article: Grimberg, A. et al. Gender Bias in U.S. Pediatric Growth Hormone Treatment. Sci. Rep. 5, 11099; doi: 10.1038/srep11099 (2015).
Acknowledgments
We want to thank the network of primary care clinicians, their patients and families for their contribution to this project and clinical research facilitated through the Pediatric Research Consortium (PeRC) at the Children’s Hospital of Philadelphia. We also would like to thank Genentech, Inc., Pfizer, Inc., Eli Lilly and Company, and Novo Nordisk, Inc. for generously sharing their data. Funding: This work was supported in part by an investigator-initiated grant from the Genentech Center for Clinical Research in Endocrinology and grant R01 HD057037 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (A.G.). We are grateful to the University of Pennsylvania Clinical and Translational Science Award (National Institutes of Health grant UL1TR000003 from the National Center for Research Resources and the National Center for Advancing Translational Sciences) for providing biostatistical guidance, and to the Pediatric Research Consortium (PeRC) at the Children’s Hospital of Philadelphia, funded in part by the Agency for Healthcare Research and Quality.
Footnotes
The Division of Endocrinology and Diabetes of the Children's Hospital of Philadelphia has been participating in the GH registries of all the major GH manufacturers. The authors have no competing financial interests to disclose. The funding organizations had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the paper for publication.
Author Contributions A.G. conceptualized and designed the study, obtained grant funding for the study, oversaw data management, and performed data analyses. L.H.S. performed the manual geocoding for extracting census-tract level data. R.G. designed the electronic health record (EHR) query programming and data quality testing. M.J.R. worked on the E.H.R. query programming and created the patient-level study database. S.P. assisted with study conceptualization and trained L.H.S. in geocoding. A.J.C. created the key outcomes database and performed biostatistical analyses. V.A.S. assisted with study conceptualization and with data management. A.G. wrote the manuscript, which was revised by V.A.S. and critically reviewed by all other authors. All authors approved the final manuscript as submitted.
References
- Lindsay R., Feldkamp M., Harris D., Robertson J. & Rallison M. Utah Growth Study: growth standards and the prevalence of growth hormone deficiency. J. Pediatr. 125, 29–35 (1994). [DOI] [PubMed] [Google Scholar]
- Lee J. M., Davis M. M., Clark S. J., Hofer T. P. & Kemper A. R. Estimated cost-effectiveness of growth hormone therapy for idiopathic short stature. Arch. Pediatr. Adolesc. Med. 160, 263–269 (2006). [DOI] [PubMed] [Google Scholar]
- Grimberg A., Stewart E. & Wajnrajch M. P. Gender of pediatric recombinant human growth hormone recipients in the United States and globally. J. Clin. Endocrinol. Metab. 93, 2050–2056 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg A., Kutikov J. K. & Cucchiara A. J. Gender differences in patients referred for evaluation of poor growth. J. Pediatr. 146, 212–216 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimberg A. et al. Gender-based prevalence of growth faltering in an urban pediatric population. J. Pediatr. 154, 567–572.e2 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- August G. P. et al. Growth hormone treatment in the United States: Demographic and diagnostic features of 2331 children. J. Pediatr. 116, 899–903 (1990). [DOI] [PubMed] [Google Scholar]
- Cuttler L. et al. Patient, physician, and consumer drivers: referrals for short stature and access to specialty drugs. Med. Care. 47, 858–865 (2009). [DOI] [PubMed] [Google Scholar]
- Cuttler L. et al. Short stature and growth hormone therapy. A national study of physician recommendation patterns. J.A.M.A. 276, 531–537 (1996). [PubMed] [Google Scholar]
- Silvers J. B., Marinova D., Mercer M. B., Connors A. & Cuttler, L. A. national study of physician recommendations to initiate and discontinue growth hormone for short stature. Pediatrics. 126, 468–476 (2010). [DOI] [PubMed] [Google Scholar]
- Grimberg A. et al. Medically underserved girls receive less evaluation for short stature. Pediatrics. 127, 696–702 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes I. P., Choong C. S., Cotterill A., Harris M. & Davies P. S. Australasian Paediatric Endocrine Group. The influence of secular trend for height on ascertainment and eligibility for growth hormone treatment. Clin. Endocrinol. (Oxf). 73, 760–768 (2010). [DOI] [PubMed] [Google Scholar]
- Farooqi A., Hägglöf B., Sedin G., Gothefors L. & Serenius F. Growth in 10- to 12-year-old children born at 23 to 25 weeks’ gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 118, e1452–1465 (2006). [DOI] [PubMed] [Google Scholar]
- Pierrat V. et al. Epipage Study Group. Height at 2 and 5 years of age in children born very preterm: the EPIPAGE study. Arch. Dis. Child. Fetal Neonatal. Ed. 96, F348–354 (2011). [DOI] [PubMed] [Google Scholar]
- Wehkalampi K., Widén E., Laine T., Palotie A. & Dunkel L. Patterns of inheritance of constitutional delay of growth and puberty in families of adolescent girls and boys referred to specialist pediatric care. J. Clin. Endocrinol. Metab. 93, 723–728 (2008). [DOI] [PubMed] [Google Scholar]
- Sedlmeyer I. L., Hirschhorn J. N. & Palmert M. R. Pedigree analysis of constitutional delay of growth and maturation: determination of familial aggregation and inheritance patterns. J. Clin. Endocrinol. Metab. 87, 5581–5586 (2002). [DOI] [PubMed] [Google Scholar]
- Winham D. M. Growth status among low-income Mexican and Mexican-American elementary school children. Am. J. Hum. Biol. 24, 690–695 (2012). [DOI] [PubMed] [Google Scholar]
- Martorell R., Mendoza F. S. & Castillo R. O. Genetic and environmental determinants of growth in Mexican-Americans. Pediatrics. 84, 864–871 (1989). [PubMed] [Google Scholar]
- Hyslop A. E., Deinard A. S., Dahlberg-Luby E. & Himes J. H. Growth patterns of first-generation Southeast Asian Americans from birth to 5 years of age. J. Am. Board Fam. Pract. 9, 328–335 (1996). [PubMed] [Google Scholar]
- Gjerdingen D. K., Ireland M. & Chaloner K. M. Growth of Hmong children. Arch. Pediatr. Adolesc. Med. 150, 1295–1298 (1996). [DOI] [PubMed] [Google Scholar]
- Komlos J. & Breitfelder A. Height of US-born non-Hispanic children and adolescents ages 2-19, born 1942-2002 in the NHANES samples. Am. J. Hum. Biol. 20, 66–71 (2008). [DOI] [PubMed] [Google Scholar]
- Komlos J. & Breitfelder A. Differences in the physical growth of U.S.-born black and white children and adolescents ages 2–19, born 1942–2002. Ann. Hum. Biol. 35, 11–21 (2008). [DOI] [PubMed] [Google Scholar]
- Sun S. S. et al. National estimates of the timing of sexual maturation and racial differences among U.S. children. Pediatrics. 110, 911–919 (2002). [DOI] [PubMed] [Google Scholar]
- Roberts C. On the uses and limits of anthropometry. Bulletin de l’Institut International de Statistic. 6, 13–18 (1892). [Google Scholar]
- Finch B. K. & Beck A. N. Socio-economic status and z-score standardized height-for-age of US-born children (ages 2–6). Econ. Hum. Biol. 9, 272–276 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murasko J. E. Trends in the associations between family income, height and body mass index in U.S. children and adolescents: 1971-1980 and 1999-2008. Ann. Hum. Biol. 38, 290–306 (2011). [DOI] [PubMed] [Google Scholar]
- Hizli S., Abaci A., Büyükgebiz B. & Büyükgebiz A. Nutritional stunting. Pediatr. Endocrinol. Rev. 4, 186–195 (2007). [PubMed] [Google Scholar]
- Dunger D. B., Ahmed M. L. & Ong K. K. Effects of obesity on growth and puberty. Best Pract. Res. Clin. Endocrinol. Metab. 19, 375–390 (2005). [DOI] [PubMed] [Google Scholar]
- Ogden C. L., Carroll M. D., Kit B. K. & Flegal K. M. Prevalence of obesity and trends in body mass index among U.S. children and adolescents, 1999-2010. J.A.M.A. 307, 483–490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelstein B. S. et al. Patient attitudes and preferences regarding treatment: GH therapy for childhood short stature. Horm. Res. 51 Suppl 1, 67–72 (1999). [DOI] [PubMed] [Google Scholar]
- Grimberg A. & Lifshitz F. Worrisome growth. Pediatric Endocrinology, 5th Edition, Volume 2 [ Lifshitz F. (ed)] [1–50] Informa Healthcare USA, Inc., New York (2007). [Google Scholar]
- Hall S.S. Size Matters: How height affects the health, happiness, and success of boys – and the men they become. Houghton Mifflin Co., Boston (2006). [Google Scholar]
- National Center for Health Statistics. CDC growth charts: United States. Percentile data files with LMS values. Available at: http://www.cdc.gov/nchs/about/major/nhanes/growthcharts/datafiles.html. (Accessed November 2005).
- World Health Organization. Physical status: The Use and interpretation of anthropometry. Geneva, Switzerland: World Health Organization; WHO technical report series: 854 (1995). [PubMed]
- Centers for Disease Control and Prevention. Cut-offs to define outliers in the 2000 CDC growth charts. Available at: www.cdc.gov/ nccdphp/dnpa/growthcharts/00binaries/ BIV-cutoffs.pdf. (Accessed September 2006).
- Bell J. et al. Long-term safety of recombinant human growth hormone in children. J. Clin. Endocrinol. Metab. 95, 167–177 (2010). [DOI] [PubMed] [Google Scholar]
- Lindberg A. & Ranke M. B. Data analyses within KIGS. Growth Hormone Therapy in Pediatrics – 20 Years of KIGS [ Ranke M. B., Price D. A., Reiter E. O. (eds)]. [23–28] Karger, Basel (2007). [Google Scholar]
- Child C. J. et al. on behalf of the GeNeSIS International Advisory Board. Prevalence and incidence of diabetes mellitus in GH-treated children and adolescents: Analysis from the GeNeSIS observational research program. J. Clin. Endocrinol. Metab. 96, E1025–1034 (2011). [DOI] [PubMed] [Google Scholar]
- Lee P. A., Germak J., Gut R., Khutoryansky N. & Ross J. Identification of factors associated with good response to growth hormone therapy in children with short stature: results from the ANSWER Program. Int. J. Pediatr. Endocrinol. 2011, 6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. State and County QuickFacts. Available at: http://quickfacts.census.gov/qfd/states/42000.html. (Accessed April 2007 for 2005 Census data and October 2012 for 2011 Census data).