Abstract
Soil organic carbon (SOC) dynamics in agro-ecosystem is largely influenced by cropping. However, quantifying the contributions of various crops has been lacking. Here we employed a stable isotopic approach to evaluate the contributions of wheat and maize residues to SOC at three long-term experimental sites in north China. Soil samples were collected from 0–20, 20–40, 40–60, 60–80 and 80–100 cm after 13 and 20 years of wheat-maize rotation, and SOC and its stable 13C composition were determined. Our data showed that the δ13C value of SOC varied, on average, from −22.1‰ in the 0–20 cm to −21.5‰ in the 80–100 cm. Carbon input through maize residues ranged from 35% to 68% whereas the contribution of maize residues to SOC (0–40 cm) ranged from 28% to 40%. Our analyses suggested that the retention coefficient was in the range of 8.0–13.6% for maize residues and 16.5–28.5% for wheat residues. The two-fold higher retention coefficient of wheat versus maize residues was due to the differences in the quality of residues and probably also in the temperature during the growing season. Our study highlighted the importance of crop management on carbon sequestration in agricultural lands.
Soil organic carbon (SOC), a key index for soil fertility and a major carbon (C) reservoir in terrestrial ecosystems, plays an important role in crop productivity and the global C cycle1,2. Thus, enhancing C sequestration as SOC in cropland is recognized as a win-win strategy2. Soil organic carbon dynamics depends on the balance between C inputs (e.g., via return of crop residues and organic amendments) and C outputs (primarily through SOC decomposition). While organic C input often leads to SOC enhancement, the relationship between these two parameters is not straightforward. For example, a few studies showed a linear relationship between SOC and C input3,4, but there was also evidence of a non-linear relationship5,6.
Decomposition of SOC is largely regulated by microbial activity, which may be influenced by many factors. Among those, the quality of plant residues has direct impacts on SOC decomposition7,8. In general, the decomposition rate of plant residues is negatively related to the C:N ratio and lignin content7,9. In addition, climate and soil conditions have large effects on SOC decomposition3,10,11,12. Optimal temperature and soil moisture for microbial activity can cause high rates of residue decomposition and thus low rates of SOC accumulation3,13,14. On the other hand, SOC in soils with more clay tends to have a longer turnover time than in sandy soils15,16.
Recent studies have shown an increasing trend in SOC stock over the last three decades in the majority of north China’s cropland17,18. For example, Pan et al.18 reported an increase of 12.40 Tg C yr−1 between 1985 and 2006 in north China where wheat-maize rotation has been a dominant cropping system. There have been a few reports of positively linear relationships between SOC stock and C input through the return of wheat and maize residues in this region3,19. However, there has been limited quantification of the relative contributions of maize and wheat residues to SOC in the maize-wheat rotation system.
Because plant residue is a main contributor for SOC pool, crop type regulates the stable carbon isotopic compositions (δ13C) in SOC since the different isotopic signatures in assimilated CO2 form as a result of photosynthetic 13C discrimination. On average, δ13C value is −27‰ and −13‰ in C3 and C4 plants, respectively20. Thus, stable C isotopic techniques have been widely used to quantify the relative contributions of C3 and C4 plants to SOC21,22,23,24.
In this study, we analyzed data generated from three long-term experiments (LTEs) in north China, which had been under wheat-maize rotation since 1990. We obtained archived soil samples (from 0–100 cm profiles) collected in 2002 and 2009, and determined SOC and its stable 13C composition. Our objectives were to quantify the relative contributions of wheat and maize residues to SOC in soil profile under long-term wheat-maize rotation, and to explore the difference (and possible reasons) in the retention coefficient (i.e., the net changes of SOC per unit C input) between wheat and maize residues.
Results
Cumulative C input
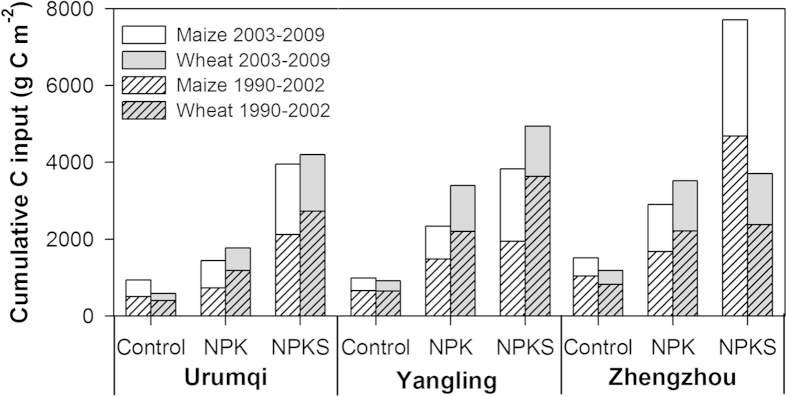
Cumulative C inputs for the periods of 1990–2002 and 2003–2009 are presented in Fig. 1. Clearly, the control treatment yielded the lowest C inputs of both wheat and maize residues. As expected, fertilization treatments led to a greater rate of C input than the control treatment. For example, C input through maize (wheat) residues during the period of 2003–2009 was only 330–475 (188–265) g C m−2 in the control, but 715–1827 (578–1471) g C m−2 at Urumqi with a mono-cropping system and 854–3029 (1200–1329) g C m−2 at Yangling and Zhengzhou with a double cropping system under fertilization treatments. For both maize and wheat residues, C input followed the same order (i.e., control < NPK < NPKS) for both periods at all the sites.
Figure 1. Cumulative carbon inputs by maize and wheat residues under different treatments during the periods of 1990–2002 and 2003–2009.
Under non-fertilization, C input was greater from maize residue compared to wheat residue. However, C input through maize residue was less than that through wheat residue under the NPK treatment. For the NPKS treatment, the C input rate through maize residue was comparable with that through wheat residue at Urumqi, but relatively lower at Yangling and much greater at Zhengzhou, due to the differences of straw return rate between sites.
Vertical distributions of δ13C, SOC and maize and wheat derived SOC
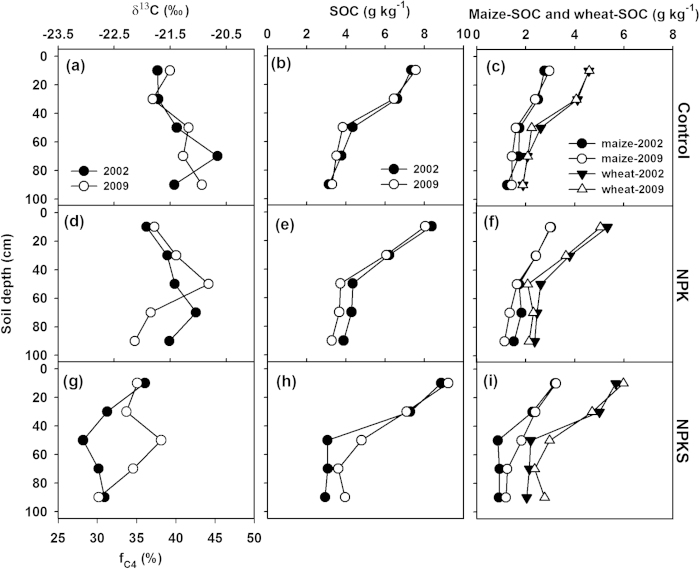
Figure 2 illustrated that there was a small variation in the δ13C value of SOC in the 0–20 cm, but a considerable variation below 20 cm at Urumqi. There was an overall similarity in the magnitude and vertical distribution in the δ13C value of SOC between the control and NPK, with an enrichment of 13C in the subsoil (~60 cm). However, the NPKS treatment showed a depletion of 13C below 20 cm. The estimated fraction of maize derived SOC was 37–45%, 35–44% and 28–38% for the control, NPK and NPKS, respectively. Total SOC content showed a decrease, from 7–9 g kg−1 in the 0–20 cm to ~4 g kg−1 below 40 cm. Maize and wheat derived SOC contents revealed similar vertical distributions. For the 0–40 cm, SOC stock ranged from 1342 to 1428 g m−2 for maize derived SOC, and from 2203 to 2722 g m−2 for wheat derived SOC (Table 1), with the highest found under the NPKS treatment.
Figure 2. Vertical distributions of δ13C in SOC, fraction of maize derived SOC (fC4) (left column), total SOC (middle column), maize and wheat derived SOC (right column) under control (top row), NPK (middle row) and NPKS (bottom row) at Urumqi.
Table 1. Stocks (g C m−2) of maize-, wheat-derived and total SOC in the 0–40 cm depth.
| Treatment | Urumqi |
Yangling |
Zhengzhou |
Mean (S.D.)* |
||||
|---|---|---|---|---|---|---|---|---|
| 2002 | 2009 | 2002 | 2009 | 2002 | 2009 | 2002 | 2009 | |
| Maize-derived SOC | ||||||||
| Control | 1342 | 1363 | 1215 | 1319 | 1128 | 988 | 1228 (107) | 1223 (205) |
| NPK | 1387 | 1381 | 1273 | 1393 | 1007 | 1144 | 1222 (195) | 1306 (140) |
| NPKS | 1387 | 1428 | 1563 | 2021 | 1394 | 1611 | 1448 (99) | 1686 (304) |
| Wheat-derived SOC | ||||||||
| Control | 2215 | 2206 | 1980 | 2102 | 2175 | 1982 | 2123 (125) | 2097 (112) |
| NPK | 2315 | 2203 | 2257 | 2936 | 2570 | 2773 | 2381 (167) | 2637 (385) |
| NPKS | 2722 | 2717 | 2441 | 3008 | 3011 | 3111 | 2725 (285) | 2945 (204) |
| Total SOC | ||||||||
| Control | 3557 | 3569 | 3195 | 3421 | 3303 | 2969 | 3352 (186) | 3320 (312) |
| NPK | 3701 | 3584 | 3531 | 4330 | 3578 | 3917 | 3603 (88) | 3943 (374) |
| NPKS | 4109 | 4145 | 4004 | 5028 | 4405 | 4721 | 4173 (208) | 4631 (448) |
*Standard deviation.
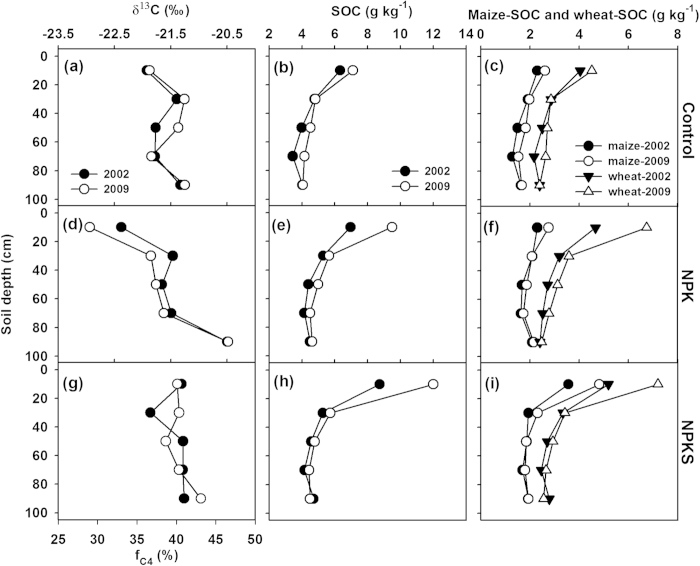
The value of δ13C in SOC at Yangling showed a weaker vertical variability except under the NPK that showed a value of <−22.4‰ in the 0–20 cm and −20.5‰ in the 80–100 cm (Fig. 3). Overall, the estimated fraction of maize derived SOC was in a range of 29–47%. While there was no clear difference in the δ13C of SOC between 2002 and 2009, there was an increasing trend in SOC from 2002 to 2009, particularly in the 0–20 cm under fertilization treatments. Data in table 1 illustrates that in the top 40 cm, maize derived SOC increased from 1215–1563 g m−2 in 2002 to 1319–2021 g m−2 in 2009, and wheat derived SOC from 1980–2441 g m−2 to 2102–3008 g m−2.
Figure 3. Vertical distributions of δ13C in SOC, fraction of maize derived SOC (fC4) (left column), total SOC (middle column), maize and wheat derived SOC (right column) under control (top row), NPK (middle row) and NPKS (bottom row) at Yangling.
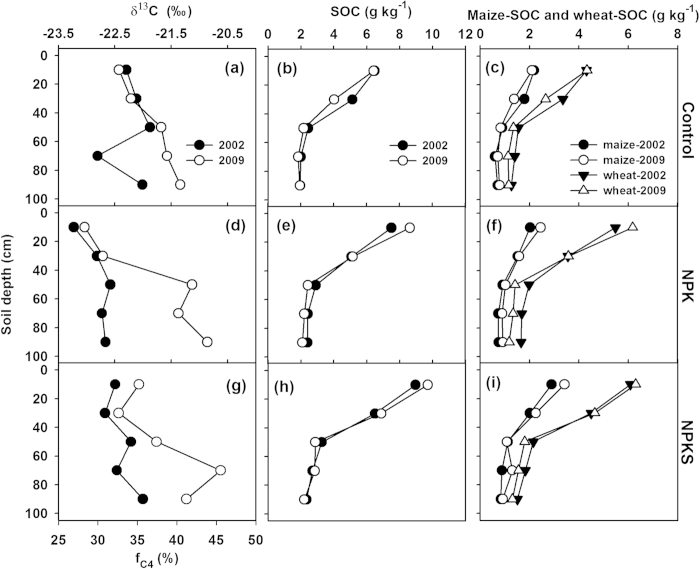
A small difference in the value of δ13C in SOC above 40 cm, but a large difference below 40 cm occurred at Zhengzhou with enrichment of 13C in 2009 (Fig. 4). On average, the estimated fraction of maize derived SOC was 27–37% and 28–46% for year 2002 and 2009, respectively. Overall, there was little change in total SOC stock over time. However, maize derived SOC stock in the 0–40 cm increased by ~15% from 1007–1394 g m−2 in 2002 to 1144–1611 g m−2 in 2009 under fertilization treatments (Table 1).
Figure 4. Vertical distributions of δ13C in SOC, fraction of maize derived SOC (fC4) (left column), total SOC (middle column), maize and wheat derived SOC (right column) under control (top row), NPK (middle row) and NPKS (bottom row) at Zhengzhou.
Statistical analyses showed that the δ13C value of SOC was significantly affected by site, year, and depth (Table S1). In particular, there were significant differences (P < 0.001) between sites and between depths. Table 2 shows that 13C in SOC of the 0–40 cm was more depleted at Zhengzhou (~ −22.5‰) compared with that at Yangling (−21.5 to −22‰) and Urumqi (~ −21.9‰), indicating smaller contribution to SOC by maize at Zhengzhou (~32%) relative to the other two sites (36–39%). 13C in SOC was depleted in the 0–20 cm (−22.1‰), and became enriched below 40 cm (−21.7‰). Accordingly, maize’s contribution to SOC increased from 34.8% to 39.2%.
Table 2. Average values of δ13C in SOC for each layer at the three LTE sites.
| Layer | Urumqi | Yangling | Zhengzhou | Mean |
|---|---|---|---|---|
| 0–20 | −21.83 Aa* | −21.97 Ba | −22.59 Bb | −22.13 (34.8)**C |
| 20–40 | −21.90 Aa | −21.53 Ba | −22.49 Bb | −21.97(35.9) BC |
| 40–60 | −21.58 Aa | −21.57 Ba | −21.87 Aa | −21.68 (38.0) AB |
| 60–80 | −21.63 Aa | −21.56 Ba | −21.93 Aa | −21.71 (37.8) AB |
| 80–100 | −21.91 Ab | −20.96 Aa | −21.68 Ab | −21.52 (39.2) A |
*Values followed by the same letter (lower case letter within a row or upper case letter within a column) are not significantly different at P ≤ 0.05 based on LSD test.
**Percentage of maize derived SOC
Comparison of maize’s contribution to C input and SOC
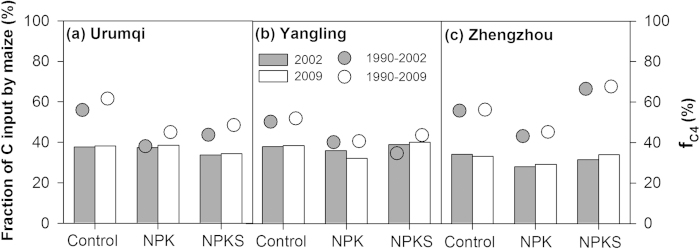
Figure 5 illustrated that the percentage of C input through maize residues varied from 35% to 68%, which was higher than maize’s contribution to SOC (28–40%) over the 0–40 cm across all three sites. There were considerable differences in the percentage of C input through maize residues but relatively small differences in the maize’s contribution to SOC between treatments. For instance, the percentage of C input through maize residues was significantly higher under the control treatment than under the NPK at all three sites. However, maize’s contribution to SOC was slightly higher under the control treatment relative to the NPK treatment only at Zhengzhou and Yangling. Although the percentage of C input through maize residues at Urumqi was greater under the control (56–62%) than under the NPK treatment (38–45%), maize’s contribution to SOC was the same under the two treatments (~38%). Similarly, C input through maize residues was much greater under the NPKS treatment (66–68%) relative to the control treatment (56%) at the Zhengzhou site, while maize derived SOC was similar (~33%) under both treatments. On average, the percentage of C input through maize residues was 48–51% whereas maize’s contribution to SOC was 35% over the 0–40 cm, indicating that the retention coefficient of maize residues was lower than wheat residues under long-term wheat-maize rotation in northern China.
Figure 5. Fractions of carbon input by maize during 1990–2002 (solid circles) and 1990–2009 (hollow circle), and maize’s contribution to SOC (fC4, 0–40 cm) in 2002 (grey bars) and 2009 (white bars) under different fertilization treatments.
Discussion
Our data showed that 13C became enriched in SOC with depth (Fig. 2, 3, 4, Table 2), which was consistent with the typical trends observed in well-drained soils under C3 or C3/C4 mixed vegetation21,24,25,26. The depletion of 13C in the topsoil (with newer crop residues) might partly reflect the trend of δ13C in the atmospheric CO2. There is evidence that the δ13C values of both wheat and maize tissues decreased over the past decades27,28, which would lead to the depletion of 13C in SOC. On the other hand, the enrichment of 13C in the subsoil (with older SOC) might be partly caused by the isotopic fractionation during SOC decomposition25,29. In addition, subsoil was less responsive to changes in land use26,30.
In agricultural ecosystem, management practices, e.g., fertilization and straw retention, could have effects on the δ13C value in SOC19,23,31. Our study demonstrated that fertilization treatments mainly affected the δ13C of SOC in the top layer (Fig. 2, 3, 4). For example, at Yangling and Zhengzhou, NPK treatment resulted in more depletion of 13C in top layer due to its relatively lower C input through maize residues, compared with the control and NPKS treatment. However, at Urumqi, although C input through maize residues was greater without fertilization than the NPK treatment, the δ13C values of SOC were similar. This was probably due to the small amount of crop residues returned into soil under non-fertilization at Urumqi (Fig. 1), which might require a longer period to show significant effects on the δ13C value in SOC.
Based on the linear relationship between SOC stock over the 0–40 cm and cumulative C input, one could estimate the retention coefficient of crop residues, i.e., the slope of the regression line. As illustrated in Fig. 6, the retention coefficient was 24.4% and 11.2% for wheat and maize residues, respectively, and 17.0% for the wheat-maize rotation system.
Figure 6. Relationships between SOC stocks in 0–40 cm for 2002 (2009) and cumulative C inputs during 1990–2002 (1990–2009), using the averages of all sites.
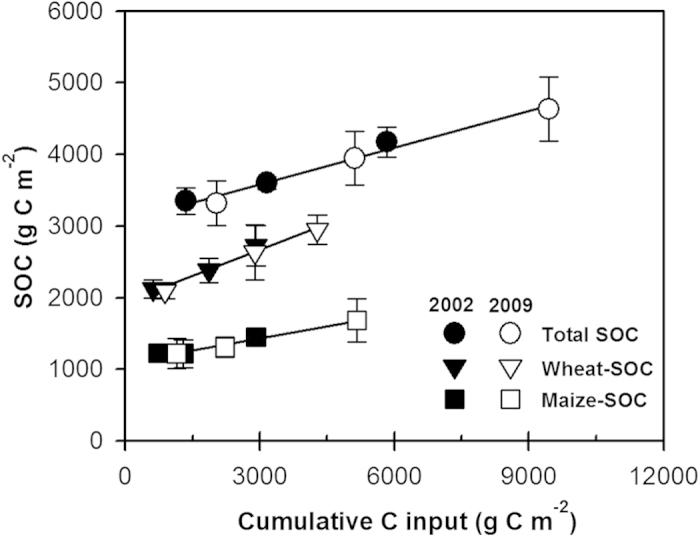
The error bars denote standard deviations. Maize-SOC: y = 0.112x + 1102, R2 = 0.97; Wheat-SOC: y = 0.244x + 1937, R2 = 0.98; Total SOC: y = 0.170x + 3073, R2 = 0.98.
Due to the difficulty of direct measurement of roots and a lack of data for δ13C values in wheat and maize, we estimated root biomass by multiplying aboveground biomass with root:shoot ratios and used mean δ13C values for wheat and maize residues from literature32, which might introduce some uncertainties in our calculations of retention coefficient. As such we conducted uncertainty analyses by testing different values for root:shoot ratio and δ13C in wheat and maize residues. Table 3 summarises comparisons of the reference method based on Bolinder et al.32, with three other commonly used methods by setting the root:shoot ratios to: (I) 0.24 and 0.22 (i.e., IPCC2006)33, (II) 0.43 and 0.35 (i.e., Li1994)34, and (III) 0.50 and 0.33 (i.e., BW1997)35 for wheat and maize, respectively. On average, the maize:wheat ratio in C input was higher in the reference (1.27 ± 0.49) than in the IPCC2006 (1.04 ± 0.44), Li1994 (1.02 ± 0.37) and BW1997 (0.88 ± 0.32). Interestingly, the retention coefficient of maize residues was similar (varying from 10.5% to 12.1%) across all methods whereas the retention coefficient of wheat residues varied considerably, from 16.5% to 24.6%. The uncertainty associated with the chosen δ13C values for wheat and maize was small, in which the retention coefficient was in a range of 8.0–13.6% for maize and 21.4–28.5% for wheat (Table 4).
Table 3. Uncertainty analyses of the maize:wheat ratios in C input and retention coefficient using different root:shoot ratios (RRS)*.
| Method | Maize:wheat in C input** | Retention coefficient |
|||
|---|---|---|---|---|---|
| Maize | Wheat | Maize:wheat | |||
| Reference | 0.112 | 0.244 | 0.46 | ||
| IPCC2006 | 1.27 ± 0.49 | 0.121 | 0.246 | 0.49 | |
| Li1994 | 1.04 ± 0.44 | 0.105 | 0.182 | 0.58 | |
| BW1997 | 1.02 ± 0.37 | 0.107 | 0.165 | 0.65 | |
Table 4. Uncertainty analyses of the retention coefficient using different combinations of δ13C values (‰) for maize and wheat.
| δ13C |
Retention coefficient |
||||
|---|---|---|---|---|---|
| Maize | Wheat | Maize | Wheat | Maize: Wheat | |
| Reference | −13 | −27 | 0.112 | 0.244 | 0.46 |
| S1 | −13 | −29 | 0.136 | 0.214 | 0.64 |
| S2 | −13 | −25 | 0.080 | 0.285 | 0.28 |
| S3 | −11 | −27 | 0.098 | 0.259 | 0.38 |
| S4 | −15 | −27 | 0.130 | 0.224 | 0.58 |
Our estimated retention coefficient of maize residues was close to those (5.3–16.6%) observed in the Corn Belt region of the central USA under 8–35 years of continuous maize cropping21,22. Similarly, Kristiansen et al.31 reported a retention coefficient of 11–15% for maize straw in four Danish arable soils. It seems that our estimated retention coefficients of wheat residues were higher than some earlier reports. For instance, Follett et al.24 reported a value of 5.4% following 84 years of wheat-fallow cultivation at Akron and 10.5% after 20 years of cropping at Sidney in the North American Great Plains. These variations in retention coefficient were probably due to the differences in climate and soil conditions and the duration as well.
Despite some differences in the findings from the limited reports, there were studies showing greater contribution to SOC by wheat residues than maize residues. For example, based on a 16 year continuous cropping experiment (conducted in a semi-arid, subtropical highlands of Central Mexico), Fuentes et al.23 demonstrated that the contribution of maize residues was significantly smaller than that of wheat residues. There was also other evidence of relatively faster decomposition of maize residues than wheat residues, based on a two-year field incubation at Sanborn Field in the Midwest US35.
There were a few field incubation experiments (using buried bag methods) of comparing C remaining or retention between maize and wheat straws and/or roots in China10,36,37. Table 5 illustrates that all studies reveal a decreasing trend in the remaining of crop straw/root with time. Clearly, root has more C remaining thus higher retention coefficient than straw for both maize and wheat37. In addition, studies by Liu et al.36 and Wang et al.37 indicated that the retention coefficient of maize straw:root was significantly lower than that of wheat, which yielded the maize:wheat ratio in retention coefficient ranging from 0.53 to 0.77. On the other hand, Wang et al.10 demonstrated that decomposition of maize straw was more sensitive to environmental changes than that of wheat straw during 1–2 years of incubation, which resulted in a large variation (0.52–1.22) in the maize:wheat ratio of retention coefficient.
Table 5. Remaining fraction of carbon or dry weight from wheat and maize residues after 1-2 years of field incubation.
| Location | Incubation time (yr) | Maize |
Wheat |
Maize:wheat |
|||
|---|---|---|---|---|---|---|---|
| Straw | Root | Straw | Root | Straw | Root | ||
| Beijinga (39°57′N, 116°19′E) | 1 | 0.215 | 0.23 | 0.278 | 0.382 | 0.77 | 0.60 |
| 2 | 0.177 | 0.185 | 0.241 | 0.347 | 0.73 | 0.53 | |
| Hailunb (47°26′N, 126°38′E) | 1 | 0.391 | 0.549 | 0.71 | |||
| 2 | 0.313 | 0.42 | 0.75 | ||||
| Hailunc (47°26′N, 126°38′E) | 1 | 0.537 | 0.441 | 1.22 | |||
| 2 | 0.300 | 0.245 | 1.22 | ||||
| Fengqiuc (35°00′N, 114°24′E) | 1 | 0.235 | 0.353 | 0.67 | |||
| 2 | 0.123 | 0.234 | 0.52 | ||||
| Yingtanc (28°15′N, 116°55′E) | 1 | 0.319 | 0.295 | 1.08 | |||
| 2 | 0.199 | 0.234 | 0.85 | ||||
There may be many driving factors responsible for the differences in the retention between maize and wheat residues. A few studies have demonstrated that the decomposition rate of litter was negatively related to the C:N ratio and lignin content in litter7,8. It appears that crop residues with higher C:N ratio tend to have a greater retention coefficient (see Fig. S1). In general, maize residues have lower lignin contents35,37,38 and smaller C:N ratios39, implying a greater decomposition rate relative to wheat residues.
Apart from the quality of residues, decompositions of maize and wheat residues may have different responses to climate conditions10. In our study wheat and maize have different growing seasons with their own environmental conditions that may also lead to differences in the fates of their residues. For example, the cumulative effective temperature, the sum of daily temperate above 0 °C, is greater during maize’s growing season (2889–3232 °C) than during wheat’s (1865–2603 °C) (see Table 6). Therefore, the organic materials generated from the root system of maize would be subject to a faster decomposition rate than those of wheat, resulting in lower retention coefficient for the former than for the latter.
Table 6. Locations, climate conditionsa and initial surface soil properties (in 1990) at the long-term experiment sites.
| Variables | Urumqi | Yangling | Zhengzhou | |
|---|---|---|---|---|
| Latitude | N | 43°49´ | 34°17´ | 34°46´ |
| Longitude | E | 87°36´ | 108°03´ | 113°39´ |
| Altitude | / m | 600 | 523 | 59 |
| Annual mean temp. | / oC | 7.3 | 13.5 | 14.7 |
| Cum. E.Tb for winter wheat | / oC | 2603 | 2115 | 2400 |
| Cum. E.T for spring wheat | / oC | 1865 | — | — |
| Cum. E.T for maize | / oC | 3232 | 2889 | 3056 |
| Annual precipitation | / mm | 299 | 585 | 641 |
| Precip. for winter wheat | / mm | 248 | 216 | 213 |
| Precip. for spring wheat | / mm | 126 | — | — |
| Precip. for maize | / mm | 172 | 369 | 427 |
| Annual mean irrigationc | / mm | 450 | 270 | 225 |
| Annual open pan evaporation | / mm | 2015 | 1292 | 1808 |
| Cropping system | Mono | Double | Double | |
| Crop rotation | Wheat-maized | Wheat-maize | Wheat-maize | |
| Plot size | / m2 | 468 | 196 | 400 |
| Soil classification (FAO) | Haplic Calcisol | Calcaric Regosol | Calcaric Cambisol | |
| Parent material | Limestone | Loess | River Alluvium | |
| Clay (<0.002 mm) | / % | 20.4 | 21.0 | 10.1 |
| Silt (0.002–0.05 mm) | / % | 44.0 | 73.6 | 57.3 |
| Sand (>0.05 mm) | / % | 35.6 | 5.4 | 32.6 |
| Soil pH | 8.1 | 8.6 | 8.3 | |
| Bulk density (0–20 cm) | / g cm−3 | 1.21 | 1.35 | 1.41 |
| Bulk density (20–40 cm) | / g cm−3 | 1.35 | 1.56 | 1.44 |
| SOC (0–20 cm) | / g kg−1 | 8.8 | 7.44 | 6.7 |
| TN (0–20 cm) | / g kg−1 | 0.87 | 0.93 | 0.67 |
aData are means over 1981–2010 from the China meteorological sharing service system ( http://cdc.cma.gov.cn/).
bCum. E.T: cumulative effective temperature above 0 °C.
cBased on Zhao et al.40.
dCrop rotation at Urumqi during 1990–2009 was as follows: maize in 1990, 1993, 1996, 2000, 2003, 2005 and 2008; spring wheat in 1991, 1994, 2002 and 2006; winter wheat in 1992, 1995, 1997, 1998, 2001, 2004, 2007 and 2009; and cotton in 1999.
Materials and methods
Site descriptions and experimental design
The National Long-term Monitoring Network of Soil Fertility and Fertilizer Effects was established around 1990 to study the responses of crop yields and soil fertility to fertilization management in the main agricultural regions of China. We used three experimental sites located in north China with arid and semi-arid climatic conditions (Table 6). The Urumqi site had a mono-cropping system with a rotation of maize - spring wheat - winter wheat whereas the other two sites had a double-cropping system with a winter wheat-summer maize rotation. All three sites had a history of cropping with various C3 and C4 rotations, in which wheat and maize were dominant crops. According to an earlier report19, the δ13C value in SOC of the 0–20 cm was −21.7‰ at Yangling and −22.2‰ at Zhengzhou prior to the experiments, reflecting a mixture of C3 and C4 cropping. Annual average temperature varied from 7.3 °C at Urumqi to 14.7 °C at Zhengzhou. Annual precipitation was generally low (299–641 mm), but open pan evaporation ranged from 1292 mm to 2015 mm. Over 60% of the precipitation occurred during the maize growing season. Irrigation was applied when needed, ranging from 225 mm at the Zhengzhou site to 450 mm at the Urumqi site. Conventional tillage was applied and ploughing depth varied from 20 cm to 30 cm. All three sites had calcareous soils, with initial SOC content in a range of 6.3–8.8 g kg−1, initial TN 0.67–0.87 g kg−1, and soil pH (in 1:2.5 soil/water) 8.1–8.6.
Three common management treatments were selected: (i) control- no fertilizer application, (ii) NPK- mineral nitrogen (N), phosphorus (P) and potassium (K) fertilization, and (iii) NPKS- mineral NPK fertilization with straw incorporation. Mineral N, P and K fertilizers used were urea, calcium superphosphate, and potassium sulphate (or potassium chloride), respectively. Details of fertilizer application were reported previously3,40. Briefly, the application rates of mineral fertilizers ranged from 123 to 242 kg N ha−1, 25 to 60 kg P ha−1, and 39 to 78 kg K ha−1 for each growing season (Table S2).
The aboveground biomass was removed for all the treatments, except the NPKS treatment that had straw incorporation of one crop each year, i.e., either wheat or maize at the Urumqi site and only maize at the Yangling and Zhengzhou sites. On average, annual rates of straw returned were 7, 4 and 6 Mg ha−1 yr−1 (air-dry matter) at Urumqi, Yangling and Zhengzhou, respectively, during the period of 1990–2009.
Estimation of C input from wheat and maize residues
Crop residues included straw, stubble and roots. The amount of straw return in the NPKS treatment was measured annually. Carbon input (g C m−2) of stubble (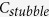 ) and roots (Croots) were determined as:
) and roots (Croots) were determined as:
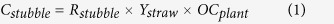 |
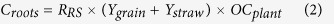 |
where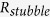 was the ratio of the stubble left in soil to the straw biomass;RRS was the ratio of belowground biomass to aboveground biomass, or the root:shoot ratio; Ygrain and
was the ratio of the stubble left in soil to the straw biomass;RRS was the ratio of belowground biomass to aboveground biomass, or the root:shoot ratio; Ygrain and 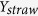 were the yields (g m−2) of crop grain and straw, respectively. Following Zhang et al.3,
were the yields (g m−2) of crop grain and straw, respectively. Following Zhang et al.3, 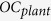 , the C content (g C g−1) of aboveground biomass, was set as the China’s average, i.e., 0.399 and 0.444 g C g−1 (oven-dried basis) for wheat and maize, respectively39. We set Rbg as 0.24 and 0.29 for wheat and maize, respectively, based on Bolinder et al.32. According to Wang et al.41, Rstubble was 0.03 for maize, and 0.26 (0.35) for wheat with (without) fertilization treatments.
, the C content (g C g−1) of aboveground biomass, was set as the China’s average, i.e., 0.399 and 0.444 g C g−1 (oven-dried basis) for wheat and maize, respectively39. We set Rbg as 0.24 and 0.29 for wheat and maize, respectively, based on Bolinder et al.32. According to Wang et al.41, Rstubble was 0.03 for maize, and 0.26 (0.35) for wheat with (without) fertilization treatments.
Soil sampling and analyses
Soil samples were collected after harvest (i.e., during September-October), with 15 soil cores (5-cm-diam) collected as a composite sample from each plot from 0–20, 20–40, 40–60, 60–80 and 80–100 cm layers. Soils from the same layer were air dried and thoroughly mixed. Representative sub-samples were crushed to 0.25 mm. For SOC measurement, 1 g soil was pretreated with 10 ml 1 M HCl for 12 hours to remove carbonate. The pretreated sample was combusted at 1020 °C with a constant helium flow carrying pure oxygen to ensure completed oxidation of organic materials. Production of CO2 was determined by a thermal conductivity detector using a CNHS-O analyzer (Model Euro EA 3000). Stable 13C isotope was determined by measuring the isotopic composition of collected CO2 using a Finnigan MAT Delta Plus XP Isotope Ratio Mass Spectrometer at the Nanjing Institute of Geology and Paleontology, Chinese Academy of Sciences (CAS). We reported isotopic data in delta notation relative to the Vienna Pee Dee Belemnite (VPDB).
Calculation of contributions of wheat and maize to SOC
For each layer, SOC stock was calculated from bulk density and C content. Because bulk density was only measured for the 0–20 and 20–40 cm layers (Table 6), we used the bulk density values from the 20–40 cm layer for the calculations of SOC stocks below 40 cm.
Relative contributions (%) of wheat (fC3) and maize (fC4) to SOC were calculated using a two end-member mixing model according to Balesdent et al.42:
 |
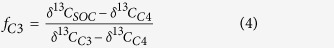 |
where δ13CSOC, δ13CC3 and δ13CC4 were the stable 13C composition in SOC, wheat and maize, respectively. The mean δ13C values for wheat and maize were approximately −27‰ and −13‰, respectively20,43.
Statistical analyses
We carried out analyses of variance (ANOVA) to evaluate the effects of site, year, treatment, depth, and their interactions on the δ13C value of SOC. We applied Fisher’s protected least significant difference (LSD) for the multiple comparisons (i.e., between sites and layers). These analyses were performed by SAS 9.2 (SAS Institute, Cary, NC, USA).
Additional Information
How to cite this article: Wang, J. et al. Contributions of wheat and maize residues to soil organic carbon under long-term rotation in north China. Sci. Rep. 5, 11409; doi: 10.1038/srep11409 (2015).
Supplementary Material
Acknowledgments
This study is financially supported by Natural Science Foundation of China (41171239) and the National Key Basic Research Program (2013CB956602).
Footnotes
Author Contributions X.J.W., M.G.X. and G.F. designed the research. X.Y.Y. and S.M.H. managed the long-term experiments and collected soil samples. W.J.Z. measured soil physical and chemical properties. J.Z.W. and X.J.W. conducted statistical analysis and prepared the manuscript. All other authors contributed to the interpretation of results and/or writing.
References
- Pan G., Smith P. & Pan W. The role of soil organic matter in maintaining the productivity and yield stability of cereals in China. Agr. Ecosyst. Environ. 129, 344–348 (2009). [Google Scholar]
- Lal R. Soil carbon dynamics in cropland and rangeland. Environ. Pollut. 116, 353–362 (2002). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Soil organic carbon dynamics under long-term fertilizations in arable land of northern China. Biogeosciences 7, 409–425 (2010). [Google Scholar]
- Kundu S., Bhattacharyya R., Prakash V., Ghosh B. & Gupta H. Carbon sequestration and relationship between carbon addition and storage under rainfed soybean–wheat rotation in a sandy loam soil of the Indian Himalayas. Soil Till. Res. 92, 87–95 (2007). [Google Scholar]
- Zhang W. et al. Effects of organic amendments on soil carbon sequestration in paddy fields of subtropical China. J. Soils Sediments 12, 457–470 (2012). [Google Scholar]
- Stewart C., Paustian K., Conant R., Plante A. & Six J. Soil carbon saturation: concept, evidence and evaluation. Biogeochemistry 86, 19–31 (2007). [Google Scholar]
- Zhang D., Hui D., Luo Y. & Zhou G. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J. Plant Ecol. 1, 85–93 (2008). [Google Scholar]
- Talbot J. M. & Treseder K. K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry–decay relationships. Ecology 93, 345–354 (2012). [DOI] [PubMed] [Google Scholar]
- Silver W. & Miya R. Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129, 407–419 (2001). [DOI] [PubMed] [Google Scholar]
- Wang X., Sun B., Mao J., Sui Y. & Cao X. Structural convergence of maize and wheat straw during two-year decomposition under different climate conditions. Environ. Sci. Technol. 46, 7159–7165 (2012). [DOI] [PubMed] [Google Scholar]
- Li J., Ziegler S. E., Lane C. S. & Billings S. A. Legacies of native climate regime govern responses of boreal soil microbes to litter stoichiometry and temperature. Soil Biol. Biochem. 66, 204–213 (2013). [Google Scholar]
- Ziegler S. E., Billings S. A., Lane C. S., Li J. & Fogel M. L. Warming alters routing of labile and slower-turnover carbon through distinct microbial groups in boreal forest organic soils. Soil Biol. Biochem. 60, 23–32 (2013). [Google Scholar]
- Kätterer T., Reichstein M., Andrén O. & Lomander A. Temperature dependence of organic matter decomposition: a critical review using literature data analyzed with different models. Biol. Fert. Soils. 27, 258–262 (1998). [Google Scholar]
- Li J., Ziegler S., Lane C. S. & Billings S. A. Warming-enhanced preferential microbial mineralization of humified boreal forest soil organic matter: Interpretation of soil profiles along a climate transect using laboratory incubations. Journal of Geophysical Research: Biogeosciences 117, G02008, 02010.01029/02011JG001769 (2012). [Google Scholar]
- Parton W. J., Schimel D. S., Cole C. V. & Ojima D. S. Analysis of factors controlling soil organic matter levels in Great Plains grasslands. Soil Sci. Soc. Am. J. 51, 1173–1179 (1987). [Google Scholar]
- Müller T. & Höper H. Soil organic matter turnover as a function of the soil clay content: consequences for model applications. Soil Biol. Biochem. 36, 877–888 (2004). [Google Scholar]
- Sun W., Huang Y., Zhang W. & Yu Y. Carbon sequestration and its potential in agricultural soils of China. Global. Biogeochem. Cy 24, GB3001, 3010.1029/2009gb003484 (2010). [Google Scholar]
- Pan G., Xu X., Smith P., Pan W. & Lal R. An increase in topsoil SOC stock of China’s croplands between 1985 and 2006 revealed by soil monitoring. Agr. Ecosyst. Environ. 136, 133–138 (2010). [Google Scholar]
- Tang X. et al. Temporal changes in soil organic carbon contents and δ13C values under long-term maize–wheat rotation systems with various soil and climate conditions. Geoderma 183–184, 67–73 (2012). [Google Scholar]
- Boutton T. W. Stable carbon isotope ratios of natural materials: II. Atmospheric, terrestrial, marine, and freshwater environments. In: Coleman D. C., Fry B. (eds.). Carbon isotope techniques, Academic Press, New York, pp. 173–185 (1991). [Google Scholar]
- Collins H. P. et al. Soil carbon dynamics in corn-based agroecosystems: results from carbon-13 natural abundance. Soil Sci. Soc. Am. J. 63, 584–591 (1999). [Google Scholar]
- Wilts A., Reicosky D., Allmaras R. & Clapp C. Long-term corn residue effects: harvest alternatives, soil carbon turn over, and root-derived carbon. Soil Sci. Soc. Am. J. 68, 1342–1351 (2004). [Google Scholar]
- Fuentes M. et al. Organic carbon and stable 13C isotope in conservation agriculture and conventional systems. Soil Biol. Biochem. 42, 551–557 (2010). [Google Scholar]
- Follett R. F. et al. Carbon isotope ratios of Great Plains soils and in wheat-fallow systems. Soil Sci. Soc. Am. J. 61, 1068–1077 (1997). [Google Scholar]
- Wynn J. G., Bird M. I. & Wong V. N. L. Rayleigh distillation and the depth profile of 13C/12C ratios of soil organic carbon from soils of disparate texture in Iron Range National Park, Far North Queensland, Australia. Geochim. Cosmochim. Ac 69, 1961–1973 (2005). [Google Scholar]
- Gregorich E. G., Monreal C. M. & Ellert B. H. Turnover of soil organic matter and storage of corn residue carbon estimated from natural 13C abundance. Can. J. Soil Sci. 75, 161–167 (1995). [Google Scholar]
- Zhao F.-J., Spiro B. & McGrath S. P. Trends in 13C/12C ratios and C isotope discrimination of wheat since 1845. Oecologia 128, 336–342 (2001). [DOI] [PubMed] [Google Scholar]
- Christensen B. T., Olesen J. E., Hansen E. M. & Thomsen I. K. Annual variation in δ13C values of maize and wheat: Effect on estimates of decadal scale soil carbon turnover. Soil Biol. Biochem. 43, 1961–1967 (2011). [Google Scholar]
- Ågren G. I., Bosatta E. & Balesdent J. Isotope discrimination during decomposition of organic matter: a theoretical analysis. Soil Sci. Soc. Am. J. 60, 1121–1126 (1996). [Google Scholar]
- Flessa H., Ludwig B., Heil B. & Merbach W. The origin of soil organic C, dissolved organic C and respiration in a long-term maize experiment in Halle, Germany, determined by 13C natural abundance. J. Plant Nutr. Soil Sc 163, 157–163 (2000). [Google Scholar]
- Kristiansen S. M., Hansen E. M., Jensen L. S. & Christensen B. T. Natural 13C abundance and carbon storage in Danish soils under continuous silage maize. Eur. J. Agron. 22, 107–117 (2005). [Google Scholar]
- Bolinder M. A., Janzen H. H., Gregorich E. G., Angers D. A. & VandenBygaart A. J. An approach for estimating net primary productivity and annual carbon inputs to soil for common agricultural crops in Canada. Agr. Ecosyst. Environ. 118, 29–42 (2007). [Google Scholar]
- IPCC. IPCC guidelines for national greenhouse gas inventories, volume 4. Agriculture, forestry and other land use. Intergovernmental Panel of Climate Change. (2006). [Google Scholar]
- Li C., Frolking S. & Harriss R. Modeling carbon biogeochemistry in agricultural soils. Global. Biogeochem. Cy 8, 237–254 (1994). [Google Scholar]
- Buyanovsky G. & Wagner G. Crop residue input to soil organic matter on Sanborn Field. In: Paul E. A., Paustian K., Elliott E. T., Cole C. V. (Eds.), Soil Organic Matter in Temperate Ecosystems : Long-Term Experiments in North America. CRC Press, Boca Raton, Florida, pp. 73–83 (1997). [Google Scholar]
- Liu M., zhang L., Yu W. & shen s. Decomposition process and residual rate of organic materials C and N in soils. Chinese J. Appl. Ecol. 18, 2503–2506 (in Chinese with English abstract) (2007). [PubMed] [Google Scholar]
- Wang W., Wang W., Zhang J., Cai D. & Zhang M. Decomposition of crop residues in the cropland soil of Beijing. Chinese J. Soil. Sci. 20, 113–115 (1989). [Google Scholar]
- Dignac M. F. et al. Carbon-13 natural abundance as a tool to study the dynamics of lignin monomers in soil: an appraisal at the Closeaux experimental field (France). Geoderma 128, 3–17 (2005). [Google Scholar]
- NCATS. Chinese organic fertilizer handbook, National Center for Agricultural Technology Service, Chinese Agricultural Publisher, Beijing. (1994). [Google Scholar]
- Zhao B. et al. Long-term fertilizer experiment network in China: crop yields and soil nutrient trends. Agron. J. 102, 216–230 (2010). [Google Scholar]
- Wang J. et al. Soil organic carbon sequestration under different fertilizer regimes in north and northeast China: RothC simulation. Soil Use. Manage. 29, 182–190 (2013). [Google Scholar]
- Balesdent J., Mariotti A. & Guillet B. Natural 13C abundance as a tracer for studies of soil organic matter dynamics. Soil Biol. Biochem. 19, 25–30 (1987). [Google Scholar]
- John B., Yamashita T., Ludwig B. & Flessa H. Storage of organic carbon in aggregate and density fractions of silty soils under different types of land use. Geoderma 128, 63–79 (2005). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.