Abstract
A major challenge presently is not only to identify the genetic polymorphisms increasing risk to diseases, but to also find out factors and mechanisms, which can counteract a risk genotype by developing a resilient phenotype. The objective of this study was to examine acquired and innate vagal mechanisms that protect against physical challenges and haemorrhages in 19 athletes and 61 non-athletes. These include examining change in heart rate variability (HF-HRV; an indicator of vagus activity) in response to orthostatic challenge, platelet count (PLT), mean platelet volume (MPV), and single-nucleotide polymorphisms in genes that encode several coagulation factors, PAI-1, and MTHFR. Individual differences in PLT and MPV were significant predictors, with opposite effects, of the profiles of the HF-HRV changes in response to orthostasis. Regular physical training of athletes indirectly (through MPV) modifies the genetic predisposing effects of some haemostatic factors (PAI-1 and MTHFR) on vagal tone and reactivity. Individual differences in vagal tone were also associated with relationships between Factor 12 C46T and Factor 11 C22771T genes polymorphisms. This study showed that genetic predispositions for coagulation are modifiable. Its potential significance is promoting advanced protection against haemorrhages in a variety of traumas and injuries, especially in individuals with coagulation deficits.
‘Resilience’ and ‘adaptation’ are two major concepts used to explain the capacity of a living organism to flourish in the face of adversity and to effectively maintain its health and well-being1,2,3. Resilience contributes to survival and mental and physical fitness by maintaining biological or psychosocial stability in response to potential risks. In contrast, adaptation contributes to survival and fitness by maintaining biological or psychosocial flexibility in response to imposed conditions. Individuals may be genetically or epigenetically predisposed to more flexible adaptation or to more rigid resilience. These mechanisms should be well-balanced in organisms for their final result achieves a good health.
For example, the evaluation of autonomic regulation of the heart rate, which is indicated by its variability changes in response to an orthostatic challenge, has been suggested to predict an individual’s capacity for adaptive responses of the sympathetic nervous system (SNS)4,5,6,7,8,9,10,11. However, withdrawal of these adaptive changes or recovery after an orthostatic challenge should be considered an individual’s capacity for resilience or recovery status of the parasympathetic nervous system (PNS)8,12,13,14,15,16,17. At the same time the impaired adaptive ability to maintain blood pressure with sympathetic control in response to an orthostatic challenge is associated with a reduced ability of the vagus to regulate recovery of the heart at rest18. Thus an increase in baseline vagus or PNS activity increases the SNS capacity to withstand an orthostatic challenge, i.e., higher resilience capacity allows the organism to be more flexible or adaptive in responses to challenges without health impairments.
PNS activity, as a physiological resilience factor against exacerbated sympathetic arousal, is suggested to exert a generalized tonic effect across different physiological (e.g., cardiovascular, metabolic, immune, haemostatic) systems for survival and to provide permanent protection of the organism from impairments associated with physiological over-arousal19. For example, electrical stimulation of the vagus nerve was observed to attenuate the mechanical and metabolic responses of the heart to beta-adrenergic stress by reducing cardiac activity and oxygen, glucose and lactate consumption20. It also lessens peripheral haemorrhaging after severe (hemorrhagic shock) and mild (soft tissue injury) hemorrhage by improving coagulation, reducing bleeding time and total blood loss21,22. In the case of hemorrhage, the protective effect is associated with improved clot formation kinetics, enhanced clot firmness, and increased coagulation factor activity (thrombin-antithrombin complex formation). An enhanced tonic activation of postsynaptic serotonin receptors in response to vagus nerve activation may be the mechanism promoting clot formation at the site of vascular injury23,24. In both cases the loss of vagus regulation should essentially cause physiological over-arousal, that exacerbates damage to the organism. Thus, individuals, whose parasympathetic recovery in the cardiovascular (CV) system is diminished or incomplete after a high arousal challenge may also show a coupled bias in haemostatic variables, indicating a predisposition to a lower coagulatory state.
Previously relationships between CV activity and haemostatic factors have been mostly estimated with respect to only blood pressure changes, which do not correlate with heart rate variability changes as an indicator of parasympathetic activity25. Other studies have investigated the relationship between CV activity and haemostatic variables in patients. For example, a recent study observed a lower resting heart rate variability in patients with acute coronary syndrome who also showed increased levels of von Willebrand factor, soluble P-selectin and D-dimer compared to healthy subjects, but did not detect correlations between different indexes of heart rate variability and these thrombotic/haemostatic markers26. Other studies have found that the power of a high frequency component of heart rate variability (HF-HRV) obtained during 24-hour monitoring was inversely associated with both a composite prothrombotic index and individual levels of haemostatic factors, including von Willebrand factor antigen (VWF:Ag), activated clotting factor VII, fibrinogen, plasminogen activator inhibitor-1 (PAI-1), and mean platelet volume (MPV)27,28. In healthy subjects VWF:Ag was inversely correlated with the platelet count (PLT) and positively correlated with MPV29. Platelet activation was found to cause bradycardia and hypotension by a general increase in vagal tone and a decrease in sympathetic tone30. This is caused by serotonin released from platelets. Diminished serotonergic activity in carriers of short/short genotype of the serotonin transporter-linked polymorphic region causes diminished cardiac vagal activity under basal conditions and blunted autonomic reactivity as assessed by heart rate variability (HRV)31. Serotonin activates megakaryocyte apoptosis, platelet activation and potentiates their overall procoagulant activity24,32. Thus the serotonergic system may be responsible for transmitting a general physiological resilience effect from the PNS to specific systems for withstanding different challenges (Fig. 1a).
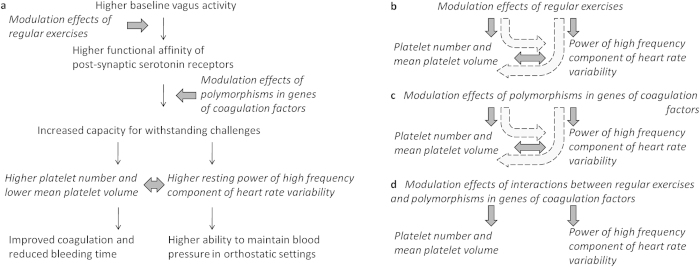
Figure 1.
Hypothetical physiological resilience framework (a) and schemes of planned statistical analyses (b,c,d). Elements in a regular font depict some hypothetical pathways. Elements in an italic font highlight factors and variables used in general linear (regression and variance) analyses to explore some of these hypothetical mechanisms and possible regulatory effects on them in the present study. The same elements were assessed with confirmation bootstrapping procedures. Two-side bold arrow is related to regression analysis of the main hypothesis. One-side bold arrows are related to the analyses of variance of three other hypotheses. Dashed bold arrows show mediation analyses of indirect effects between variables.
The present study was conducted to confirm the main assumption about vagus activity (phenotyped by HF-HRV) as a protective (resilience) factor in response to regular everyday actions such as physical activity (e.g., orthostatic challenge or change in posture), which should also predict a vulnerability to haemostasis or haemorrhages (phenotyped by the platelet index [PLT and MPV])33,34,35,36. Another objective of the study was to observe whether the protective effects of vagal activity could be related to innate mechanisms (an innate resilience determined by single-nucleotide polymorphisms (SNP) in genes of coagulation factors II, V, XI, XII [F2, F5, F11, F12], plasminogen activator inhibitor-1 (PAI-1), glycoprotein 3a [GP3a], methylenetetrahydrofolate reductase [MTHFR]) or could be acquired due to conditioning mechanisms (an acquired resilience determined by aerobic fitness) that differ between athletes (higher fit adults) and nonathletes (lower fit adults). Elucidation of the mechanisms of vagus action could reveal novel therapeutic and pre-conditioning approaches for a variety of combined stress-, trauma-, and haemorrhage-related applications.
The research was planned as pilot study and therefore was limited to the genotyping of mutations in haemostatic factor genes that control either (i) extrinsic and intrinsic clotting paths conferring an inherited thrombophilia tendency in normal and patho-physiological conditions (FII, FV, FXI, FXII, GP3a, and MTHFR) or (ii) fibrinolytic activity conferring an inherited tendency for greater blood loss during injuries (PAI-1). Haemostatic phenotype was restricted to two platelet indices: first (MPV) that previously showed evidence of significant relationship with selected physiological marker of vagus activity (i.e., HRV)28 and second (PLT) that was not found to be studied yet with respect to HRV. As shown previously both haemostatic indices have significant relationships with some other important haemostatic factors (e.g., alpha(2)-plasmin inhibitor-plasmin complex, interleukin-6, thrombin-antithrombin III complex and VWF:Ag)29,37
Results
Sample characteristics
No deviation from the Hardy–Weinberg equilibrium was observed in the athlete and non-athlete samples either separately or combined. Demographic and cardiovascular characteristics and genotype frequencies of athlete and non-athlete samples are shown in Tables 1 and 2. Non-adjusted scores of interbeat intervals (IBI) and the power of a high frequency component of the HRV (HF-HRV) were significantly higher in athletes compared to non-athletes during the baseline and recovery periods, but athletes had significantly lower scores of MPV (Table 1). The distribution of women and men and Factor 5 genotype variants were significantly different between athletes and non-athletes (Tables 1 and 2). All other presented results are controlled for gender and age.
Table 1. Demographic and heart rate characteristics of the samples1.
| Groups: | Athletes | Non-athletes |
|---|---|---|
| Characteristics | Mean (SD) | Mean (SD) |
| Sex, M/F*** | 17/2 | 23/38 |
| Age (year) | 22.8 (11.9) | 26.4 (14.2) |
| BMI | 22.0 (3.2) | 21.8 (3.7) |
| Caucasian (%) | 100 | 100 |
| Lying 1 | ||
| HF-HRV** (ms2) | 1685.7 (2210.8) | 686.6 (759.7) |
| IBI*** (s) | 1.022 (.164) | 0.881 (.134) |
| Standing | ||
| HF-HRV (ms2) | 422.1 (595.8) | 343.4 (442.4) |
| IBI (s) | 0.706 (.081) | 0.717 (.117) |
| Lying 2 | ||
| HF-HRV** (ms2) | 1677.6 (1108.0) | 892.4 (879.0) |
| IBI*** (s) | 1.024 (.180) | 0.872 (.134) |
| PLT | 220.9 (38.2) | 218.2 (46.1) |
| MPV* | 8.0 (0.9) | 8.7 (0.7) |
1means and comparisons (Chi-square test, Mann-Whitney test, and one-way analysis of variance) presented for raw (not transformed and not adjusted) data.
*- p < .05.
**- p < .005.
***- p < .001.
Abbreviations: BMI, body mass index; M, male; F, female; PLT, platelet count; MPV, mean platelet volume; HF-HRV - a power of a high frequency band of heart rate variability; IBI – interbeat intervals.
Table 2. Genotype frequencies of studied loci in athletes and non-athletesa.
| Genetic loci | Groups | Athletes | Non-athletes | |
|---|---|---|---|---|
| % (N) | % (N) | |||
| Factor 2 (prothrombin; Factor II) gene G20210A polymorphism (rs1799963) | ||||
| G/G | 100 (10) | 100 (33) | ||
| G/A | 0 (0) | 0 (0) | ||
| A/A | 0 (0) | 0 (0) | ||
| Factor 5 (proaccelerin; Factor V) gene G1691A polymorphism (rs6025)* | ||||
| G/G | 86 (12) | 100 (50) | ||
| G/A | 14 (2) | 0 (0) | ||
| A/A | 0 (0) | 0 (0) | ||
| Factor 11 (plasma thromboplastin antecedent; Factor XI) gene C22771T polymorphism (rs2289252) | ||||
| C/C | 47 (7) | 35 (19) | ||
| C/T | 40 (6) | 46 (25) | ||
| T/T | 13 (2) | 19 (10) | ||
| Factor 12 (Hageman factor; Factor XII) gene C46T polymorphism (rs1801020) | ||||
| C/C | 53 (8) | 55 (30) | ||
| C/T | 47 (7) | 33 (18) | ||
| T/T | 0 (0) | 12 (7) | ||
| PAI-1 (plasminogen activator inhibitor-1) gene -675 4G/5G polymorphism (rs1799768) | ||||
| 4G/4G | 77 (10) | 44 (23) | ||
| 4G/5G | 23 (3) | 40 (21) | ||
| 5G/5G | 0 (0) | 16 (8) | ||
| GP3a (glycoprotein IIIa) gene T196C polymorphism (rs5918) | ||||
| T/T | 100 (15) | 100 (52) | ||
| T/C | 0 (0) | 0 (0) | ||
| C/C | 0 (0) | 0 (0) | ||
| MTHFR (methylenetetrahydrofolate reductase) gene C677T polymorphism (rs1801133) | ||||
| C/C | 47 (7) | 58 (32) | ||
| C/T | 47 (7) | 38 (21) | ||
| T/T | 6 (1) | 4 (2) | ||
aNumbers of individuals varied between different loci due to missing genotypes in some subjects.
*Fisher’s Exact p < .05 between the groups.
Relationships between haemostatic and cardiovascular variables
Platelet number
Significant Posture*PLT interactions were obtained for vagus tone (lnHF-HRV) and vagus reactivity (reHF-HRV) measures (Table 3). Figure 2A,B present this platelet number effect on the profile of between-posture HF-HRV changes with 242 as the cut-off point for this haemostatic factor (Pillai’s Traces = 0.25 and 0.21, Fs(2) = 6.46 and 5.04, ps = .004 and .011 for absolute and relative scores, respectively). Individuals with a lower platelet number showed a flatter profile of HF-HRV changes in response to posture changes compared to those with a higher platelet number. Post-hoc analysis found that parameter estimates of reHF-HRV during the lying1 (baseline) position were significantly lower, but during standing were higher in individuals with a lower platelet number compared to those with a higher platelet number (Bs[SE] = −793.3[307.1] and 777.5[246.2], t[p] = −2.58[.014] and 3.16[.003], η2 = .15 and .20, respectively). Bootstrap procedure confirmed the observed PLT effects with 95% confidence (data not shown).
Table 3. Effect sizes (Pillai’s Trace or η2) of simple and interaction effects of between-subject differences in platelet number (PLT), mean platelet volume (MPV) and within-subject posture changes (Posture) on interbeat intervals (IBI) and a power of a high frequency band of heart rate variability (HF-HRV) (Univariate and Multivariate Analyses).
| Independent Variables |
Dependent Variables |
|||
|---|---|---|---|---|
| df | IBI | lnHF-HRV | reHF-HRV | |
| Model 1 | ||||
| PLT | 1 | 0 | 0 | 0.03 |
| Posture | 2 | 0.03 | 0 | 0.05 |
| PLT*Posture | 2 | 0.09 | 0.20* | 0.14* |
| Model 2 | ||||
| MPV | 1 | 0.03 | 0.07 | 0 |
| Posture | 2 | 0.24** | 0.26** | 0.27** |
| MPV*Posture | 2 | 0.11 | 0.14* | 0.11 |
| Model 3 | ||||
| PLT | 1 | 0.01 | 0.14* | 0.01 |
| MPV | 1 | 0 | 0.1 | 0 |
| Posture | 2 | 0 | 0 | 0.1 |
| PLT*MPV | 1 | 0.01 | 0.15* | 0.01 |
| PLT*MPV*Posture | 2 | 0.03 | 0.02 | 0.20* |
*−p− < 0.05; **−p < 0.005.
All data are adjusted for age and sex.
re- prefix of reactivity transformation of raw data; ln- prefix of natural log transformation of raw data.
Figure 2.
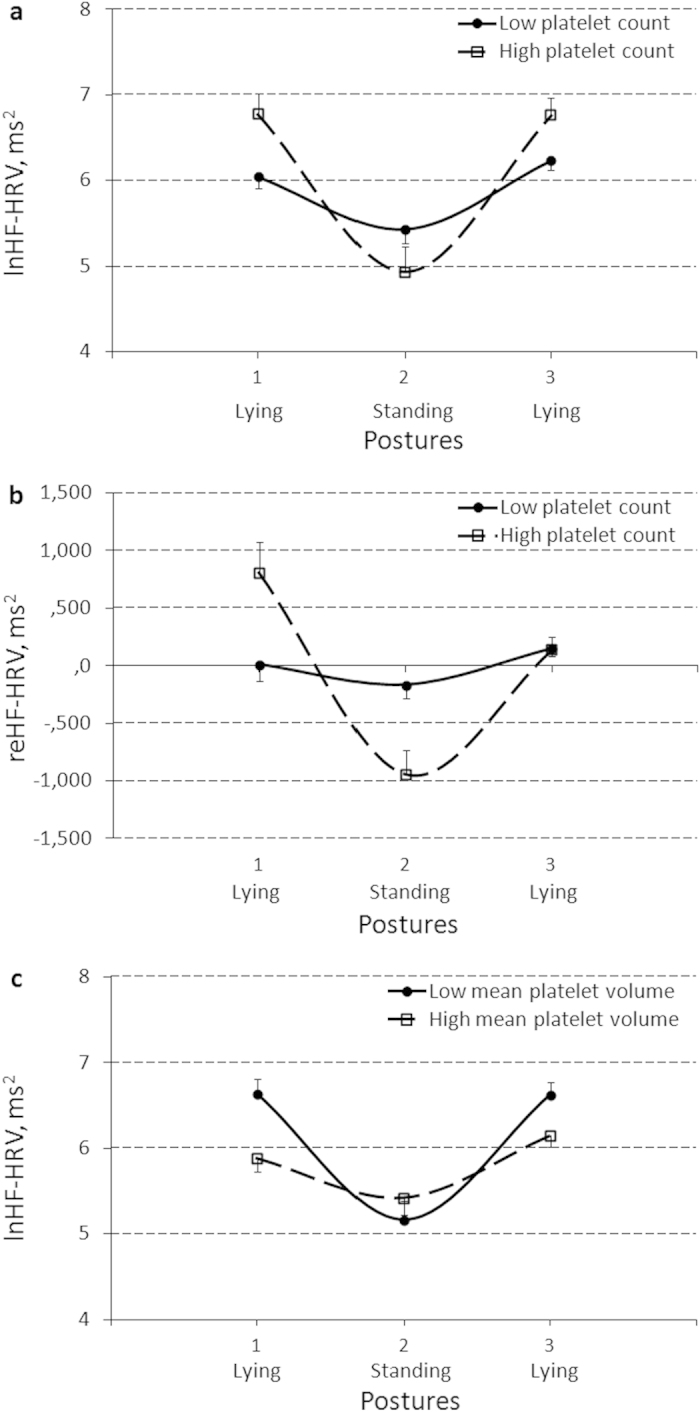
Effect of platelet count (with a score of 242 as the cut-off point) on the profile of between-posture changes of a natural log(ln)-transformed (lnHF-HRV) (a), and a reactivity-transformed (reHF-HRV) (b) power of a high frequency component of heart rate variability (HF-HRV), and effect of mean platelet volume (with a score of 8.6 as the cut-off point) on the profile of between-posture lnHF-HRV changes (с). Results are expressed as means and their standard errors.
Mean platelet volume
Significant Posture*MPV interaction effect on lnHF-HRV was obtained (Table 3). Figure 2C presents the mean platelet volume effect on a profile of between-posture HF-HRV changes with 8.6 as the cut-off point of this haemostatic factor (Pillai’s Trace = 0.24, F(2) = 5.84, p = .006). Individuals with a higher mean platelet volume showed a flatter profile of HF-HRV changes in response to posture changes compared to those with a lower mean platelet volume. Post-hoc analysis found that parameter estimates of lnHF-HRV during both the lying1 (baseline) and lying2 (recovery) positions were significantly lower in individuals with a higher mean platelet volume compared to those with a lower mean platelet volume (Bs[SE] = − 0.75[0.23] and −0.47[0.20], t[p] = −3.24[.002] and − 2.35[.024], η2 = .21 and .12, respectively). Bootstrap procedure confirmed the observed MPV effect with 95% confidence (data not shown).
Platelet number & Mean platelet volume
Moderation analysis showed that platelet count and mean platelet volume interacts in their effects on the between-subject difference in mean lnHF-HRV level (PLT*MPV interaction) and the profile of reHF-HRV changes (Posture*PLT*MPV interaction) (Table 3). Bootstrap procedure confirmed the observed interaction effects with 95% confidence (data not shown). The Johnson–Neyman technique with the use of bootstrap confidence intervals detected that mean platelet volume and mean lnHF-HRV level had a significant negative relationship only when the platelet count was higher than 223, and the platelet count and mean lnHF-HRV level had a significant negative relationship only when the mean platelet volume was higher than 9.1. Mean platelet volume and reHF-HRV change (increase) from standing to lying2 (i.e., recovery) had a significant negative relationship only when the platelet count was higher than 246, and the platelet count and reHF-HRV change had a significant positive relationship only when the mean platelet volume was lower than 8.1. No evidence of significant interaction effects of platelet number and mean platelet volume was found for the reHF-HRV change (decrease) from lying1 (baseline) to standing (i.e., for adaptation).
Effects of the Sport factor on haemostatic and cardiovascular variables
Significant Sport factor simple effects were obtained for IBI and MPV, and Posture*Sport interactions were obtained for IBI, lnHF-HRV, and reHF-HRV (Table 4). Figure 3 presents the latter Sport effect on a profile of between-posture CV changes. Non-athletes showed a flatter profile of CV changes in response to posture changes compared to athletes. Post-hoc analysis found that parameter estimates were significantly lower in non−athletes compared to athletes in general, and particularly during both the lying1 (baseline) and lying2 (recovery) positions for IBI (Bs[SE] = −0.08[0.03], −0.13[0.04], and −0.14[0.04], t[p] = −2.42 [.018], −3.25[.002], and −3.42[.001], η2 = .07, .12, and .13, respectively) and during both the lying1 (baseline) and lying2 (recovery) positions for lnHF−HRV (Bs[SE] = −0.90[0.28] and −0.54[0.25], t[p] = −3.26[.002] and −2.15[.035], η2 = .12 and .06, respectively). Parameter estimates of reHF-HRV during the lying1 (baseline) position were significantly lower, but during standing were higher in non-athletes compared to athletes (Bs[SE] = −466.0[208.9] and 536.1[199.8], t[p] = −2.23[.029] and 2.68[.009], η2 = .06 and .09, respectively). Mean platelet volume was higher in non-athletes compared to athletes (B[SE] = 0.90[0.35], t[p] = 2.56[.015], η2 = .14). Bootstrap procedure confirmed the observed Sport factor effects with 95% confidence (data not shown). Since the number of female athletes was only 2 out of 19, the GLM and post-hoc regression analyses with the bootstrapping procedure were redone in males alone and validated the findings (data not shown).
Table 4. Effect size (Pillai’s Trace or η2) of between-subject differences in the Sport factor (athletes vs. non-athletes) and Gene polymorphisma on platelet number (PLT), mean platelet volume (MPV) and in interaction with within-subject posture changes (Posture) on interbeat intervals (IBI) and a power of a high frequency band of heart rate variability (HF-HRV) (Univariate and Multivariate Analyses).
| Independent Variables |
Dependent variables |
|||||
|---|---|---|---|---|---|---|
| df | IBI | lnHF-HRV | reHF-HRV | PLT | MPV | |
| Model 1 | ||||||
| Sport | 1 | 0.07* | 0.05 | 0.01 | 0.01 | 0.14* |
| Posture | 2 | 0.09* | 0.06 | 0.14** | ||
| Sport*Posture | 2 | 0.27*** | 0.14** | 0.09* | ||
| Model 2 | ||||||
| Factor 12 | 1 | 0.11** | 0.15** | 0.01 | 0.01 | 0.02 |
| Posture | 2 | 0.08 | 0.07 | 0.15* | ||
| Factor 12*Posture | 2 | 0.09 | 0.19*** | 0.03 | ||
| Model 3 | ||||||
| MTHFR | 1 | 0 | 0.04 | 0.09* | 0.06 | 0.01 |
| Posture | 2 | 0.11* | 0.08 | 0.20*** | ||
| MTHFR*Posture | 2 | 0.06 | 0.01 | 0.28*** | ||
| Model 4 | ||||||
| PAI-1 | 1 | 0.02 | 0.04 | 0 | 0.21* | 0.01 |
| Posture | 2 | 0.07 | 0.05 | 0.14* | ||
| PAI-1*Posture | 2 | 0 | 0.02 | 0.06 | ||
| Model 5 | ||||||
| Sport | 1 | 0.03 | 0.03 | 0.38*** | 0.01 | 0.12* |
| MTHFR | 1 | 0 | 0.04 | 0.01 | 0.04 | 0 |
| Sport*MTHFR | 1 | 0 | 0.01 | 0.37*** | 0.01 | 0.03 |
| Posture | 2 | 0.13* | 0.08 | 0.30*** | ||
| Sport*Posture | 2 | 0.25*** | 0.24*** | 0.72** | ||
| MTHFR*Posture | 2 | 0.09* | 0.02 | 0.69*** | ||
| Sport*MTHFR*Posture | 2 | 0.09* | 0.11* | 0.68*** | ||
| Model 6 | ||||||
| Sport | 1 | 0.13** | 0.06 | 0.03 | 0.08 | 0.09 |
| PAI-1 | 1 | 0.02 | 0 | 0.01 | 0.17* | 0.02 |
| Sport*PAI-1 | 1 | 0.08* | 0.02 | 0.02 | 0 | 0 |
| Posture | 2 | 0.11* | 0.06 | 0.15* | ||
| Sport*Posture | 2 | 0.38*** | 0.31*** | 0.34*** | ||
| PAI-1*Posture | 2 | 0.12* | 0.15* | 0.32*** | ||
| Sport*PAI-1*Posture | 2 | 0.12* | 0.13* | 0.30*** | ||
| Model 7 | ||||||
| Factor 11 | 1 | 0.02 | 0.07* | 0.01 | 0.03 | 0.04 |
| Factor 12 | 1 | 0.07* | 0.04 | 0 | 0 | 0.02 |
| Factors 11*12 | 1 | 0.02 | 0.14** | 0.01 | 0 | 0.07 |
| Posture | 2 | 0.08 | 0.07 | 0.13* | ||
| Factor 11*Posture | 2 | 0.04 | 0 | 0.01 | ||
| Factor 12*Posture | 2 | 0.03 | 0.15* | 0.02 | ||
| Factors 11*12*Posture | 2 | 0.03 | 0 | 0 | ||
*−p < 0.05; **−p < 0.005; ***−p < 0.001.
All data are adjusted for age and sex.
re- prefix of reactivity transformation of raw data; ln- prefix of natural log transformation of raw data.
a-C- vs. non-C-allele carrier grouping of MTHFR C677T, F11 C22771T, and F12 C46T variants and 5G- vs. non-5G-allele carrier grouping of PAI-1 -675 4G/5G variants were used in these models.
Figure 3.
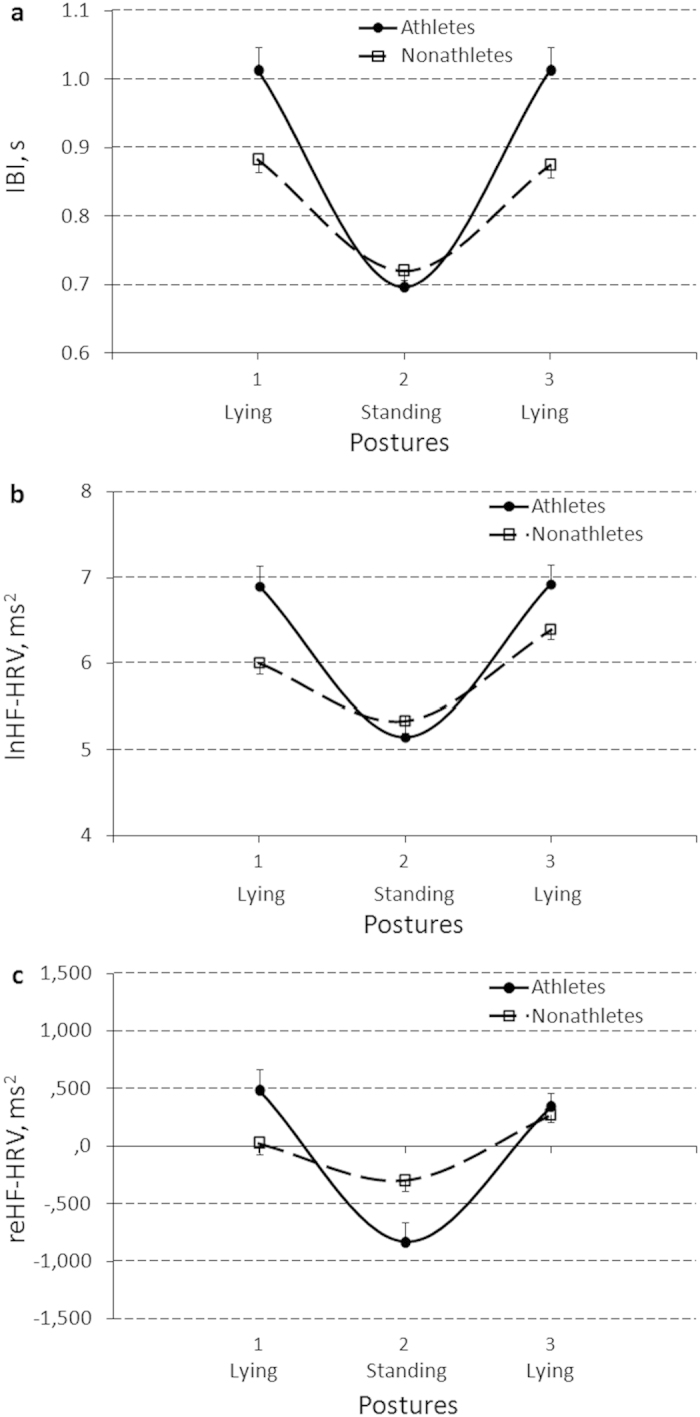
Sport (athletes vs non-athletes) effect on the profile of between-posture changes of interbeat intervals (IBI) (a), a natural log(ln)-transformed (lnHF-HRV) (b), and a reactivity-transformed (reHF-HRV) (c) power of a high frequency component of heart rate variability (HF-HRV). Results are expressed as means and their standard errors.
Bootstrapping procedure used here as a robust alternative to inference based on parametric assumptions, confirmed the findings obtained by parametric analyses and additionally detected that the Sport factor changed individual mean and reactivity HF-HRV scores through a mediation mechanism associated with the mean platelet volume. Sport activity decreases mean platelet volume, which in turn increases mean lnHF-HRV during lying (baseline and recovery) positions (Bs[SE] = −0.26[0.18] and −0.20[0.17], 95% CIs = −0.82 - −0.03 and −0.73 - −0.01, respectively), and decreases reHF-HRV during standing (B[SE] = 162.5[130.8], 95% CI = 6.40 - 570.50). Though mean platelet volume and platelet number were significantly negatively correlated (r = −.51, p = .001), the same analyses did not show evidence for platelet number as an outcome of the Sport factor effect nor as a single mediator of the Sport factor effect on HF-HRV measures when compared to mean platelet volume. Since these measures shared a common variance a two-mediator serial model with mean platelet volume and platelet number as mediating variables associated with a common latent (haemostasis) mechanism was tested and confirmed. The serial chain from mean platelet volume to platelet number transmitted the Sport factor effect onto absolute lnHF-HRV value during standing (Sport factor - > MPV- > PLT -> standing lnHF-HRV; B[SE] = 0.22[0.14], 95% CI = 0.01 - 0.60). Sport activity decreases mean platelet volume, which in turn increases platelet number with a decreased mean lnHF-HRV during standing position as outcome.
Effects of Gene polymorphism on haemostatic and cardiovascular variables
In the present sample, the Factor 2 and GP3a genes were not polymorphic (Table 2), and no evidence was found for effects related to a Factor 5 gene polymorphism.
Factor 12
Significant simple effects of Factor 12 gene polymorphism were obtained for IBI and lnHF-HRV, and a Posture*Factor 12 interaction effect was obtained for lnHF-HRV (Table 4). Post-hoc analysis found similar effects for CC and CT genotype carriers and, therefore, parameter estimates and Fig. 4A represent simple and interaction effects with the Factor 12 gene after regrouping to C- vs. non-C-allele carriers. Non-C-allele carriers had significantly lower IBI and lnHF-HRV in general (Bs[SE] = −0.13[0.04] and −0.95[0.28], t[p] = −2.91 [.005] and −3.42[.001], η2 = .11 and .15, respectively), and particularly during both the lying1 (baseline) and lying2 (recovery) positions for IBI (Bs[SE] = −0.16[0.05] and −0.16[0.05], t[p] = −3.15[.002] and −2.99[.004], η2 = 13 and .12, respectively) and during the lying (baseline) position for lnHF-HRV (B[SE] = −1.67[0.36], t[p] = −4.68[.000], η2 = .25). Bootstrap procedure confirmed the observed Factor 12 effects with 95% confidence (data not shown).
Figure 4.
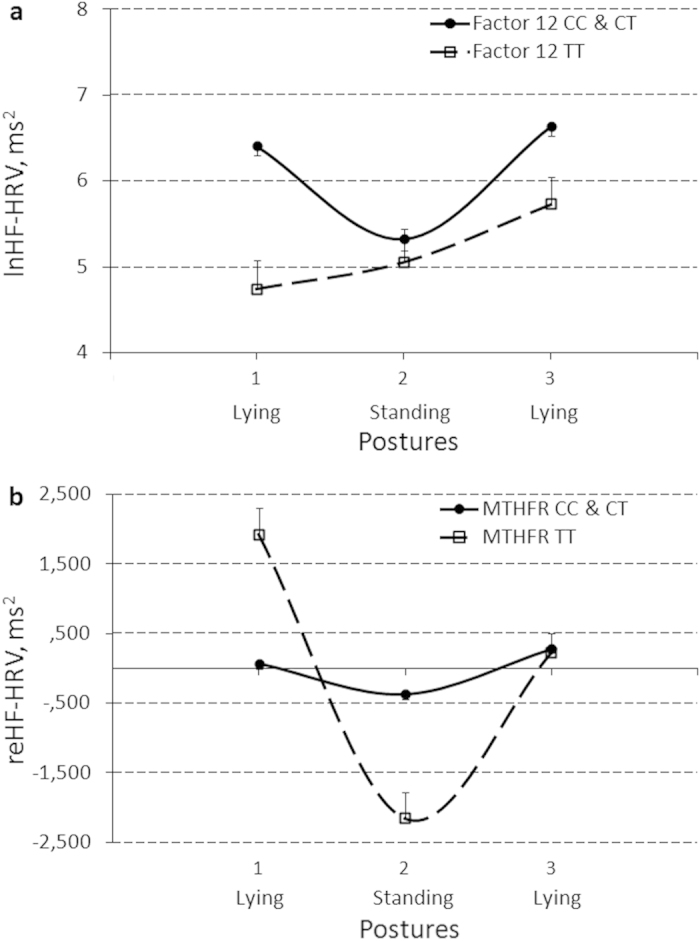
Effects of polymorphism of the Factor12 gene (CC and CT genotype carriers vs. TT genotype carriers) on between-posture changes of a natural log(ln)-transformed power of a high frequency component of heart rate variability (lnHF-HRV) (a) and polymorphism of the MTHFR gene (CC and CT genotype carriers vs. TT genotype carriers) on between-posture changes of a reactivity-transformed power of a high frequency component of heart rate variability (reHF-HRV) (b). Results are expressed as means and their standard errors.
MTHFR
Significant simple effect of MTHFR gene polymorphism and Posture*MTHFR interaction effect were obtained for reHF-HRV (Table 4). Post-hoc analysis found similar effects for CC and CT allele carriers, therefore, parameter estimates and Fig. 4B represent effects with the MTHFR gene after regrouping to C- vs. non-C-allele carriers. Non-C-allele carriers had significantly higher reHF-HRV during lying1 (baseline) and lower reHF-HRV during standing positions (Bs[SE] = 1847.4[397.2] and −1794.2[382.8], t[p] = 4.65[.000] and −4.69[.000], η2 = .25 and .25, respectively). Bootstrap procedure confirmed the observed MTHFR effects with 80% confidence (data not shown).
PAI-1
A significant effect of PAI-1 gene polymorphism was obtained for PLT (Table 4) with an increase in platelet number from non-5G to 5G allele carriers (Fig. 5A). Bootstrap procedure confirmed the observed PAI-1 effect with 95% confidence (data not shown). Mediation analysis by bootstrapping procedure detected that a 5G allele polymorphism of the PAI-1 gene indirectly increased lnHF-HRV power fluctuation through the mechanism of increasing the platelet number, which was mainly apparent in the increased mean lnHF-HRV during both lying (baseline and recovery) positions (Bs[SE] = 0.27[0.17] and 0.19[0.13], 95% CIs = 0.04-0.79 and 0.04-0.73, respectively).
Figure 5.
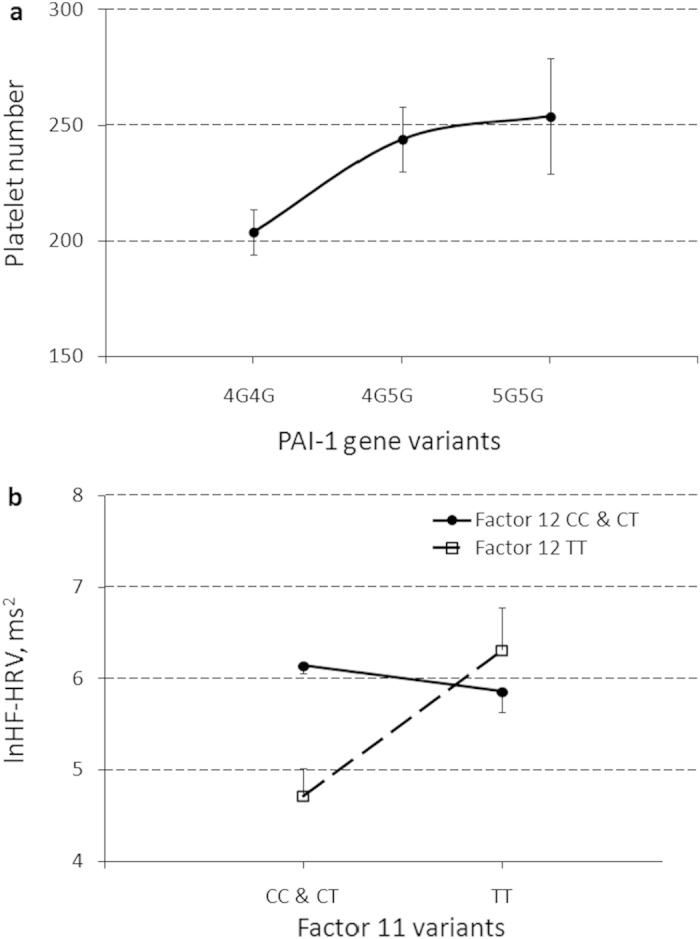
Simple effect of polymorphism of the PAI-1 gene (4G4G, 4G5G, and 5G5G variants) on platelet count (a) and the interaction effect of Factor 12 * Factor 11 gene (CC and CT genotype carriers vs. TT genotype carriers) on natural log(ln)-transformed values of the mean power of a high frequency component of heart rate variability (lnHF-HRV) (b). Results are expressed as means and their standard errors.
Gene-gene and gene-environment interactions
Several significant effects of gene-gene interaction (Factors 11*12) for mean lnHF-HRV level, gene-environment interaction (Sport*MTHFR and Sport*PAI-1) for mean reHF-HRV and IBI, respectively, and gene-environment interactions (Sport*MTHFR*Posture and Sport*PAI-1*Posture) for between-posture IBI, lnHF-HRV, and reHF-HRV changes were obtained (Table 4). Although a bootstrap procedure confirmed the observed interaction effects with 95% confidence (data not shown), these interactions should be evaluated with caution as preliminary due to the small sample size. The TT genotype of Factor 12 compared to CC and CT genotypes of Factor 12 significantly decreased mean lnHF-HRV level in only the presence of the CC and CT genotypes (N = 5 with Factor 12 TT vs. N = 52 with Factor 12 CC and CT) (B[SE] = −1.41[0.31], t[p] = −4.62[.000], η2 = .29), but not in the presence of the TT genotype of Factor 11 (N = 2 with Factor 12 TT vs. N = 9 with Factor 12 CC and CT) (B[SE] = 0.22[0.49], t[p] = 0.46[.659], η2 = .03) (Fig. 5B). The most pronounced gene-environment effects with increased effects of size were detected for reHF-HRV. The Sport factor had an increased effect on reHF-HRV fluctuating abilities (higher lying1 and lower standing scores) in non-C allele carriers of the MTHFR gene and 5G-allele carriers of the PAI-1 gene (Fig. 6). The GLM analyses of these gene-environment interaction effects with the bootstrapping procedure were also redone in males alone and validated the findings (data not shown).
Figure 6.
Gene-environment interaction effects on between-posture changes of a reactivity-transformed power of a high frequency component of heart rate variability (reHF-HRV) present in athletes (a) and non-athletes (b) with CC and CT genotype carriers vs. TT genotype carriers of the MTHFR gene, and in athletes (c) and non-athletes (d) with the 4G4G vs. 4G5G and 5G5G genotype carriers of the PAI-1 gene. Results are expressed as means and their standard errors.
Discussion
The study confirmed the hypothesis of an association between the haemostatic factors assessed by the standard complete blood count procedure and cardiovascular fluctuation processes associated with parasympathetic nervous activity (vagus tone and reactivity). Individuals with a lower platelet number or a higher mean platelet volume, which predisposes them to increased bleeding, show a flatter profile of changes of the high frequency component of heart rate variability, either due to low vagus tone or flatter vagus reactivity and recovery during posture changes. These haemostatic factors interact in their associations with the mean and recovery of vagus tone, which suggests a compensatory feedback between these two haemostatic mechanisms in the regulation of parasympathetic nervous activity. Mechanisms associated with mean platelet volume regulate vagal tone in parallel with vagal recovery (both improving with a decrease in volume) when the platelet count is higher than a specific cut-off point (>246). The mechanism regulating platelet count also regulates vagal tone (improving with a decrease in platelet count) separately from vagal recovery (improving with an increase in platelet count) when the mean platelet volume is higher (>9.1) or lower (<8.1) than a specific cut-off point, respectively. These two haemostatic mechanisms seem to compensate for each other as a protection against extensive inhibition of vagal tone (i.e., vagal tone being extensively decreased) if one of these mechanisms becomes too active (i.e., associated with either a high mean platelet volume or a high platelet count). Vagal recovery seems be regulated differently and the systems cooperate in its regulation to prevent this recovery process from becoming too extensive (i.e., extensive vagal rebound or overshoot after suppression).
Environmental factors such as sport activity indirectly increase individual vagus tone during lying (rest) and its reactivity (inhibition) in response to standing through regulation of a haemostatic mechanism that decreases the mean platelet volume. Despite the athletes sample size was small, the assumption of the effects and the path was validated through a robust bootstrap procedure. The study also detected evidence that some genetic factors associated with haemostatic mechanisms determine predisposition to low or high vagus tone during lying (rest) and its reactivity (inhibition) in response to standing either directly (Factor 12 and MTHFR gene polymorphisms; the robustness of the latter gene effect was increased in interaction with the Sport activity factor, see below) or indirectly through a platelet count regulation mechanism (PAI-1 gene polymorphism). Although a group of athletes showed a significant difference in Factor 5 Leiden polymorphism compared to non-athletes, it was not associated with between-group differences in vagus activity and measures of the platelet index.
Polymorphism of the Factor 12 gene was found to determine predisposition to a higher vagus tone in C-allele carriers (showing higher plasma Factor XII level and activity38), but MTHFR gene polymorphism determined predisposition to a higher vagal reactivity (vagus activation at rest and inhibition in response to orthostasis) in non-C-allele carriers (predisposing to higher coagulation reactivity39). PAI-1 gene polymorphism determined predisposition to a higher vagus tone in 5G allele carriers through the increase of the number of platelets. The PAI-1 gene (-675 4G/5G) polymorphism influences the plasma levels of PAI-1, which is considered a regulator of fibrinolysis; 5G/5G carriers demonstrated a lower level of PAI-1, greater postoperative bleeding and blood loss compared to other variants40,41. Thus, increased vagus tone, along with platelet count in 5G allele carriers, may be considered a compensation or resilience mechanism, i.e., increased vagus pro-coagulation activity in these subjects compensates for their predisposition to extensive bleeding.
The gene-environment interaction analysis conducted in this study should be considered preliminary due to the small sample size. However bootstrap confidence intervals were sufficiently robust to validate their presentation and discussion. Sport activity and polymorphism of the Factor 12 gene were found to have independent effects on vagus tone that suggest the probability of an indirect modification of this gene effect by regular physical activity. However, sport activity directly modifies other genetic effects on vagus reactivity by increasing it in non-C allele carriers of the MTHFR gene and in 5G-allele carriers of the PAI-1 gene, most likely through epigenetic mechanisms, which may heighten the pro-coagulation bias determined by hyperhomocysteinemia in the former group of subjects39, but compensate for the coagulation deficit in the latter group of subjects40,41. An additional gene-gene interaction effect was also detected. The Factor 12 TT genotype, which is associated with a Factor XII deficit38, demonstrated its inhibitory effect on vagus tone only in the presence of Factor 11 CC and CT genotypes. The Factor 11 TT genotype seems have a protective effect on the pro-coagulation role of vagus tone by encoding high levels of Factor XI (another component of the intrinsic coagulation pathway42) in the presence of the impairing effect associated with Factor XII (activating Factor XI).
A challenge for researchers in the post-genomic era is not only to identify the genetic polymorphisms increasing the risk of diseases, but to also determine epigenetic factors and physiological conditioning mechanisms that can counteract the risk genotype by developing a resilient phenotype1,43. The complexity of the genotype–phenotype relationship of most physiological conditions requires a reliable biomarker of specific physiological resilience with clear mechanisms or paths underlying its phenotypic construction to develop respective therapeutic interventions. This study confirmed the assumption that the high frequency band of heart rate variability was a probable biomarker of such resilience with respect to haemostatic processes. Vagus activity, as a mechanism to determine the power of this heart rate fluctuation, is considered a factor that couples cardiovascular regulation with acquired (mean platelet volume) and innate (coagulation factors XI, XII, PAI-1 [via platelet count regulation], and MTHFR) haemostatic mechanisms, suggesting its prophylactic regulation for promoting advanced protection of an individual against haemorrhage in a variety of combat, occupational, and accidental traumas and injuries, especially in individuals with coagulation deficits. Findings of the present study (e.g., with respect to modifying the generalized effects of PAI-1 and MTHFR gene polymorphisms) may also be important for determining resilience mechanisms against mental health problems like mood disorders and schizophrenia, which severity and treatment outcome were found to be associated with mutations in some coagulation factor genes44,45,46,47.
The main limitation of this pilot study is concerned with the small sample size selected for this research. However, several points have been considered for reducing the likelihood of Type I (“false-positive”) and the Type II (“false-negative”) errors in our inferences. In this study more conservative two-tail tests with an alpha of 0.05 were taken while correct directions of most effects are predicted. For example, regular physical exercises (sport factor) have an extremely large effect on vagus tone and reactivity, assessed by HF-HRV. This is reliably defined in previous studies6,7,11,15. A sample of athletes was selected in the present study to increase the effect size of the between-subject difference in vagal tone and reactivity. A reasonable sample size of athletes was calculated to detect significance of the sport effect on vagal tone. An obtained direction and size of the effect in the present study are comparable with those from previous studies48.
The sample size to detect an association between genetic polymorphisms and vagus activity was chosen to be comparable with the magnitude of sport effect on vagus activity, thus having similar practical importance. In our present study larger group sample sizes would be relevant in the case of gene polymorphisms whose effects do not show evidence of practical importance or statistical significance to avoid type II (“false-negative”) errors in the future. Predicted directions of the relationships between platelet index (platelet count and mean platelet volume) and vagus activity measures28,29,37 and between polymorphisms of coagulation genes and vagus activity measures are also confirmed in present study. Higher platelet number, lower mean platelet volume and genetic polymorphisms (e.g., in C-allele carriers of the Factor 12 gene38 and non-C-allele carriers of the MTHFR gene39) that are associated with higher pro-coagulation effects are also associated with higher vagus activity. In the case of PAI-1 polymorphism (anti-fibrinolytic factor), the correspondence between two indicators of high pro-coagulation activity (high platelet number and high power of HF-HRV) in 5G/5G carriers of the PAI-1 gene (having decreased suppression of fibrinolysis40,41) permits considering the pattern as a part of a physiological mechanism that protect those with this genotype against extensive bleeding.
These results confirm that the present sample size has enough power to demonstrate the predicted vagus-related effects with respect to genetic, haemostatic and physical factors, thereby detecting the mediation relationships between them, in order to support the main hypothesis of their common mechanism. However, gene-gene and gene-environment interaction analyses should be evaluated with caution and repeated in a larger sample size to confirm null results with respect to other genes. Therefore further validation of these findings in a larger population involving more haemostatic factors is required.
Methods
Ethical information and participants
This research was performed in accordance with relevant guidelines and regulations, adhered to ethical research standards set by the latest revision of the Declaration of Helsinki, was approved by the Institutional Review Board of the Kazan State University, and informed consent was obtained from all subjects. Data were collected initially during annual health examinations (conducted over 3 different months in the outpatient clinic #4, Kazan) from 19 professional athletes (players and coaches; 2 females; all Caucasians) of the University basketball teams who were recruited by letters to their coaches (mean [range], age 22.5 [17–61]; body mass index 21.6 [15.6–32.5]). Sixty one non-athlete participants (students and faculty staff; 38 females; all Caucasians) were recruited by flyers on University bulletin boards during the same period of time as the annual health examinations (mean [range], age 28.6 [17–66]; body mass index 21.8 [14.9–34.8]).
The effective sample size for testing the main objective of this pilot study was calculated by the power analysis of within- and between-subject effects of physical activity and platelet indices on HF-HRV by using effect size data (η2 = .17 and Cohen’s d = 0.53 for exercise, R2 = 0.64 for MPV) of previous studies28,48 and respective total sample sizes = 12 and 10 were found effective for alpha = 0.05 and power = 0.95. However, we increased the total sample size to 80 (40 men and 40 women) to be confident in obtaining sufficient samples with genetic mutations for testing the additional hypothesis of innate mechanisms of these relationships with respect to SNP polymorphisms in genes of some coagulation factors. A sample size with sufficient statistical power is critical to the success of genetic association studies to detect causal effects of genes on physiological and biochemical measures and the effective sample size was computed using Quanto (a program for calculating required sample size for genetic studies) for genetic model with independent subject design and continuous outcomes49. Total sample sizes = 40 and 65 were found effective for alpha = 0.05 and power = 0.80 and 0.95 respectively with effect size η2 = 0.18 obtained from previous studies for gene-treatment effect on HF-HRV changes31. Allele frequencies for rare alleles of the selected factors were found to be from 1.2%–5.1% of 20210A F2 and 1691A F5 alleles to 10.5–49.5% of 46T F12, 22771T F11, 5G PAI-1, 196C GP3a, and 677T MTHFR alleles in European populations38,50,51,52,53,54,55.
The participants were qualified for the study after a careful medical record review to determine if inclusion and exclusion criteria were met. Subjects were excluded if they had a history of chronic cardiovascular, pulmonary, renal, liver, or haematological disease, intracranial haemorrhage and haemorrhagic stroke, gastrointestinal or urogenital bleeding, recent major surgery or trauma, a gastric or duodenal ulcer in the past 6 months, elevated levels of aspartate or alanine transaminase (twice above upper-normal range) in the past month, the use of a long-acting non-steroidal anti-inflammatory drug, pregnancy or lactation, or a malignancy.
Protocol
The study was conducted in the morning after an overnight (at least 8-h) fast during the same week as the annual medical examinations. Subjects were instructed not to consume any alcohol or high-fat meals on the day before the study. On arrival at the lab, participants signed a consent form indicating that the study would consist of (i) measuring height and body weight, causal systolic and diastolic blood pressure and heart rate; (ii) attachment of a device to record respiration parameters; (iii) attachment of a device to record an electrocardiogram (ECG) in response to an orthostatic challenge; (iv) psychological testing (not presented here); as well as (v) obtaining venous blood samples for a complete blood count including platelet number (PLT) and average platelet size (mean platelet volume; MPV), and for identifying SNPs in 7 haemostatic genes, which were of major interest in the study. The procedures were conducted by qualified staff nurses of the clinic in different isolated rooms specifically equipped for each respective procedure. Blood samples were always obtained at the end of the experiment.
Physiological Measures
Two hundred cycles of a standard limb 3-lead electrocardiogram (ECG) were recorded with a 500 Hz sampling rate in subjects in each of the following positions: lying (first), standing, and lying (second). Measurements were recorded with a “Valenta+” (ECG+respiration) monitoring system (Neo Company, Russia) consisting of a PBS-05 3-lead ECG machine, an MN-02-05.1.1 acquisition system, and a Valenta 1.4 program. The processes that were conducted by the same program included processing ECGs for uneven RR interval series (ms), treating for artefacts, calculating the heart rate (HR; estimated as RR or interbeat interval [IBI] Mode, Mo, s), 10 Hz resampling to the IBI series, detrending, tapering, Fourier transformation and spectral-power value integration in three bands of HR variability (HRV; ms2) of which only high frequency (HF-HRV, 0.15–0.40 Hz) was considered as a measure of parasympathetic (PNS) influences on the heart in this study. Because the distribution of the HRV measures was skewed (exponentially distributed), reactivity- and natural log(ln)-transformed HF-HRV indices, as respective relative changes about individual means representing vagus reactivity (reHF-HRV) and absolute values representing vagus tone (lnHF-HRV), were used in statistical analyses56. The Valenta 1.4 program also provided a calculation of respiration parameters (respiration rate, vital capacity, forced vital capacity, forced expiratory volume at the 1st s, Tiffeneau Pinelli Index, forced expiratory flow at 25–75%, maximal voluntary ventilation and tidal volume in BTPS expressions) by acquiring the signal from the spirometer. Between-subject variations in the respiration parameters were used to evaluate their possible confounding effects with respect to HRV.
Blood samples, complete blood count, genotyping procedures and analysis
Blood samples (5 ml) for complete blood count (CBC) and genotyping procedures were obtained from the median cubital vein using vacutainer tubes with K3EDTA. For the CBC, all blood samples were analysed within 4 hours of collection. Platelet number (PLT) and mean platelet volume (MPV) values were obtained using the Coulter AcT 5-part differential (5 diff) autoloader (AL) haematology analyser from Beckman Coulter (Fullerton, CA, USA).
Total genomic DNA was isolated from peripheral blood samples using a ‘DNA-Express-Blood’ Kit (Lytech, Russia) according to the manufacturer’s protocol. SNP genotyping of F2 G20210A (rs1799963), F5 G1691A (rs6025), F11 C22771T (rs2289252), F12 C46T (rs1801020), PAI-1 4G/5G (rs1799768), GP3a T196C (rs5918), and MTHFR C677T (rs1801133)39,54,57,58,59,60,61 was performed using a TaqMan probe-based assay (SibDNA, Russia) and a CFX96 Thermal Cycler (Bio-Rad Laboratories, USA). Each sample was processed in duplicate for each genotype analysis. The end-point readings were analysed according to the manufacturer’s instructions.
Of the original sample of 80 subjects, 15 athletes and 55 non-athletes provided DNA and were included in the model analysing genetic effects. Other subjects did not provide DNA due to a variety of reasons (for example, ethical issues concerning a genetic study or genotyping failure).
Statistical Analysis
Descriptive and inferential analyses were performed with SPSS (SPSS Science, Chicago, IL) software using General Linear Models by the Type III method (GLM), SPSS built-in bootstrapping option for computing confidence intervals for regression estimates in GLM, and the SPSS macro command set to evaluate the significance of moderation and mediation effects62. Values of p < .05 were regarded as statistically significant. All parameter estimates are expressed as non-standardized (B) regression coefficients and their standard errors (SE) in the text, and as means and their standard errors in the figures. Where necessary, a partial η2 was reported as a measure of strength of associations (effect size). Sex, as categorical, and age (its natural log(ln)-transformed value), as quantitative, variables were included in all models to adjust for factors. Body mass index (BMI) was not included in the analyses because it did not show any significant effect.
To confirm the main hypothesis that vagus activity was a generalized protective factor (the general resilience hypothesis), the first group of GLM analyses was conducted to test interaction effects between a within-subject factor (Posture: lying1, standing, lying2) of repeated IBI and HRV measures and between-subject measures of haemorrhages (separately PLT or MPV) treated as continuous independent variables on IBI and HF-HRV measures treated as continuous dependent variables (Fig. 1).
The GLM Repeated Measures procedure provided multivariate analyses of interaction effects of this within-subject factor and haemostatic measures on absolute and relative CV (IBI, lnHF-HRV, reHF-HRV) changes as profile analyses (PLT*Posture and MPV*Posture). These profile analyses tested the ‘parallelism’ null hypothesis63, which asked whether CV changes between postures showed the same pattern (i.e., similar profile) with respect to individual differences in the haemostatic variables. The multivariate approach to repeated measures does not require the compound symmetry and sphericity assumptions. All multivariate F values were obtained by the Pillai’s Trace statistic, which is equivalent to the partial η2 measure of effect size.
A second group of GLM analyses was conducted to evaluate three other hypotheses; i.e., that the vagus-related and haemostatic variances could be explained by including: (i) an acquired mechanism (an acquired resilience hypothesis) determined by a conditioning process that is different in athletes and non-athletes and treated as a categorical ‘Sport’ factor (e.g., Sport*Posture) (Fig. 1b); (ii) innate mechanisms (an innate resilience hypothesis) determined by polymorphisms of some genes related to haemostatic traits (coagulation factors II, V, XI, XII, PAI-1, GPIIIa, and MTHFR; e.g., MTHFR*Posture) treated as categorical independent variables (e.g., with respect to the MTHFR C677T genotype as CC, CT, or TT variant carriers) (Fig. 1c); and (iii) gene-gene and gene-environment interactions (e.g., Factors 11*12 and Sport*MTHFR*Posture) (Fig. 1d). Analyses of the gene-gene and gene-environment interaction effects were conducted and presented as preliminary to provide the effect size for conducting power analysis in future studies of the topic. In addition, significant relationships were evaluated for best-fit cut-off points of respective PLTs and MPVs for presenting these relationships in figures and their possible practical implication.
A third group of mediation and moderation analyses was conducted to evaluate the path and interactions of different resilience mechanisms. All mediation effects were evaluated for significance by the bootstrapping procedure included in the SPSS macro command set. The Johnson–Neyman technique included in the same set was used to detect regions of significant relationships in the cases of significant moderation effects. Degrees of freedom could vary between some analyses due to missing genotypes in some subjects. Compliance with the Hardy–Weinberg equilibrium and the distribution of tested genotypes between groups (athletes and non-athletes) were assessed using the Chi-square and the Freeman-Halton extension of Fisher’s Exact test. Multiple inferences (comparisons) were controlled against Type I errors by cross-validation of observations utilizing the application of different analytic techniques (i.e., parametric and non-parametric profile, bootstrapping mediation, and Johnson–Neyman moderation analyses).
In this study, a bootstrapping procedure (i.e., a procedure with a number of resamples with replacement) was additionally used to validate the findings of regression analyses with uncertain stability (e.g., due to small sample size) by generating 95% confidence intervals for regression estimates in GLM and mediation analyses. Bootstrapping is often used as a robust nonparametric alternative to statistical inference based on parametric assumptions (such as normally distributed errors) and allows assigning measures of accuracy defined in confidence intervals when those assumptions and the stability of the results are in doubt. It can provide more accurate inferences when the data are not well behaved or when the sample size is small64. Even when the sample size is as small as 15, the empirical level of the bootstrap test is consistently close to the nominal level65. Moreover, the robustness of some conclusions was further increased by applying the bias-corrected and accelerated bootstrap method to the analysis of more complex, indirect or mediating, relationships between grouping factors and dependent measures62.
Additional Information
How to cite this article: Davydov, D. M. et al. Resilience to orthostasis and haemorrhage: A pilot study of common genetic and conditioning mechanisms. Sci. Rep. 5, 10703; doi: 10.1038/srep10703 (2015).
Acknowledgments
The authors are grateful to Dr. Svetlana Zhdanova, MD (Kazan Medical University, Russia) and the physicians of the outpatient clinic #4, Kazan for health control of the subjects. We thank Professor Dr. Fazly Ataullakhanov (Lomonosov Moscow State University), Professor Dr. Farida Alimova and Anastasia Doinikova, MSc (Kazan Federal University, Russia) for their help and support. We thank Dr. Malik Nurbekov (Sholokhov Moscow State University for the Humanities, Russia) for his valuable suggestions. The work was performed in frame of 2014–2016 State program to support scientific activity at the Kazan Federal University. The study was supported by the Russian Ministry of Science and Education and Kazan Federal University grants № 12–26 (2012–2013) and project № 14–80 (2014–2016), the Russian Humanitarian Scientific Foundation (№14–06–00304а), and “HemaCore” Ltd, Moscow, Russia (supplied devices and consumables). This work was also supported by personal awards from the Russian Institute for Advanced Study, Sholokhov Moscow State University for the Humanities granted to DMD and RIZ and of the A. von Humboldt Foundation (Bonn, Germany) granted to RIZ.
Footnotes
Author Contributions Authors R.I.Z. and V.G.D. designed the study as a whole, conducted the parts of the study related to recruiting subjects, cardiovascular, and blood sampling and count procedures; authors O.A.K., E.N.V. and M.L.F. designed and conducted the genotyping procedures and analysis; author D.M.D. conceived the hypotheses, designed the analyses, undertook the literature searches and analyses, and wrote the first and final drafts of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Davydov D. M., Stewart R., Ritchie K. & Chaudieu I. Resilience and mental health. Clin. Psychol. Rev. 30, 479–495 (2010). [DOI] [PubMed] [Google Scholar]
- Davydov D. M., Stewart R., Ritchie K. & Chaudieu I. Depressed mood and blood pressure: The moderating effect of situation-specific arousal levels. Int. J. Psychophysiol. 85, 212–223 (2012). [DOI] [PubMed] [Google Scholar]
- Davydov D. M. Resilience and adaptation: Two competing mechanisms of responding to challenges. in Applied Neuroscience and Social Well-being (eds. Varlamov A. A. & Tkachenko O. I. ) 8–9 (Scholokhov University, Moscow, 2013). [Google Scholar]
- Grant C. C., Clark J. R., Janse van Rensburg D. C. & Viljoen M. Relationship between exercise capacity and heart rate variability: supine and in response to an orthostatic stressor. Auton. Neurosci. 151, 186–188 (2009). [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., LaManca J. J. & Natelson B. H. A measure of heart rate variability is sensitive to orthostatic challenge in women with chronic fatigue syndrome. Exp. Biol. Med. (Maywood). 228, 167–174 (2003). [DOI] [PubMed] [Google Scholar]
- Gilder M. & Ramsbottom, R. Change in heart rate variability following orthostasis relates to volume of exercise in healthy women. Auton. Neurosci. 143, 73–76 (2008). [DOI] [PubMed] [Google Scholar]
- Kiviniemi A. M., Hautala A. J., Kinnunen H. & Tulppo M. P. Endurance training guided individually by daily heart rate variability measurements. Eur. J. Appl. Physiol. 101, 743–751 (2007). [DOI] [PubMed] [Google Scholar]
- Mourot L. et al. Decrease in heart rate variability with overtraining: assessment by the Poincaré plot analysis. Clin. Physiol. Funct. Imaging 24, 10–18 (2004). [DOI] [PubMed] [Google Scholar]
- Huovinen J. et al. Relationship between heart rate variability and the serum testosterone-to-cortisol ratio during military service. Eur. J. Sport Sci. 9, 277–284 (2009). [Google Scholar]
- Hynynen E., Konttinen N., Kinnunen U., Kyröläinen H. & Rusko H. The incidence of stress symptoms and heart rate variability during sleep and orthostatic test. Eur. J. Appl. Physiol. 111, 733–741 (2011). [DOI] [PubMed] [Google Scholar]
- Grant C. C., Viljoen M., Janse van Rensburg D. C. & Wood P. S. Heart rate variability assessment of the effect of physical training on autonomic cardiac control. Ann. Noninvasive Electrocardiol. 17, 219–229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uusitalo A. L., Uusitalo A. J. & Rusko H. K. Endurance training, overtraining and baroreflex sensitivity in female athletes. Clin. Physiol. 18, 510–520 (1998). [DOI] [PubMed] [Google Scholar]
- Uusitalo A. L., Uusitalo A. J. & Rusko H. K. Heart rate and blood pressure variability during heavy training and overtraining in the female athlete. Int. J. Sports Med. 21, 45–53 (2000). [DOI] [PubMed] [Google Scholar]
- Chen J.-L. et al. Parasympathetic nervous activity mirrors recovery status in weightlifting performance after training. J. Strength Cond. Res. 25, 1546–1552 (2011). [DOI] [PubMed] [Google Scholar]
- Botek M., McKune A. J., Krejci J., Stejskal P. & Gaba A. Change in performance in response to training load adjustment based on autonomic activity. Int. J. Sports Med. 35, 482–488 (2014). [DOI] [PubMed] [Google Scholar]
- Mejia-Rodriguez A. R., Gaitan-Gonzalez M. J., Carrasco-Sosa S. & Guillen-Mandujano A. Time varying heart rate variability analysis of active orthostatic and cold face tests applied both independently and simultaneously. Comput. Cardiol. 36, 361–364 (2009). [Google Scholar]
- Davydov D. M. Asymmetry in activity of cardiovascular system: Its origin and role in the maintenance of human resilience and adaptation to stressors. Pathogenesis 11, 26–31 (2013). [Google Scholar]
- Convertino V. A. Neurohumoral mechanisms associated with orthostasis: reaffirmation of the significant contribution of the heart rate response. Front. Physiol. 5, 236 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J. Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vimercati C. et al. Acute vagal stimulation attenuates cardiac metabolic response to β-adrenergic stress. J. Physiol. 590, 6065–6074 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czura C. J. et al. Vagus nerve stimulation regulates hemostasis in swine. Shock 33, 608–613 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezende-Neto J. B. et al. Vagus nerve stimulation improves coagulopathy in hemorrhagic shock: a thromboelastometric animal model study. J. Trauma Manag. Outcomes 8, 15 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manta S., Dong J., Debonnel G. & Blier P. Enhancement of the function of rat serotonin and norepinephrine neurons by sustained vagus nerve stimulation. J. Psychiatry Neurosci. 34, 272–280 (2009). [PMC free article] [PubMed] [Google Scholar]
- Lopez-Vilchez I., Diaz-Ricart M., White J. G., Escolar G. & Galan A. M. Serotonin enhances platelet procoagulant properties and their activation induced during platelet tissue factor uptake. Cardiovasc. Res. 84, 309–316 (2009). [DOI] [PubMed] [Google Scholar]
- Steptoe A. & Marmot M. Psychosocial, hemostatic, and inflammatory correlates of delayed poststress blood pressure recovery. Psychosom. Med. 68, 531–537 (2006). [DOI] [PubMed] [Google Scholar]
- Hamaad A., Sosin M. D., Blann A. D., Lip G. Y. H. & MacFadyen R. J. Markers of thrombosis and hemostasis in acute coronary syndromes: relationship to increased heart rate and reduced heart-rate variability. Clin. Cardiol. 32, 204–209 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Känel R. & Orth-Gomér K. Autonomic function and prothrombotic activity in women after an acute coronary event. J. Women’s Heal. 17, 1331–1337 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozdemir O. et al. Association between mean platelet volume and autonomic nervous system functions: Increased mean platelet volume reflects sympathetic overactivity. Exp. Clin. Cardiol. 9, 243–247 (2004). [PMC free article] [PubMed] [Google Scholar]
- Ihara A. et al. Relationship between hemostatic factors and the platelet index in patients with ischemic heart disease. Pathophysiol. Haemost. Thromb. 35, 388–391 (2006). [DOI] [PubMed] [Google Scholar]
- Wiggins R. C., Glatfelter A., Campbell A. M., Kunkel R. G. & Ulevitch R. J. Acute hypotension due to platelet serotonin-induced chemoreflexes after intravenous injection of dextran sulfate in the rabbit. Circ. Res. 57, 262–277 (1985). [DOI] [PubMed] [Google Scholar]
- Agorastos A. et al. The 5-HTTLPR genotype modulates heart rate variability and its adjustment by pharmacological panic challenge in healthy men. J. Psychiatr. Res. 50, 51–58 (2014). [DOI] [PubMed] [Google Scholar]
- Mercado C. P. et al. Impact of elevated plasma serotonin on global gene expression of murine megakaryocytes. PLoS One 8, e72580 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall G., Lane D., Carroll D. & Lip G. Y. H. A systematic review of the prothrombotic effects of an acute change in posture: a possible mechanism underlying the morning excess in cardiovascular events? Chest 132, 1337–1347 (2007). [DOI] [PubMed] [Google Scholar]
- Ikarugi H. et al. Norepinephrine, but not epinephrine, enhances platelet reactivity and coagulation after exercise in humans. J. Appl. Physiol. 86, 133–138 (1999). [DOI] [PubMed] [Google Scholar]
- Lee K. W., Blann A. D., Ingram J., Jolly K. & Lip G. Y. H. Incremental shuttle walking is associated with activation of haemostatic and haemorheological markers in patients with coronary artery disease: the Birmingham rehabilitation uptake maximization study (BRUM). Heart 91, 1413–1417 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrall G., Lane D., Carroll D. & Lip G. Y. H. A systematic review of the effects of acute psychological stress and physical activity on haemorheology, coagulation, fibrinolysis and platelet reactivity: Implications for the pathogenesis of acute coronary syndromes. Thromb. Res. 120, 819–847 (2007). [DOI] [PubMed] [Google Scholar]
- Ihara A. et al. Relationship between hemostatic markers and platelet indices in patients with aortic aneurysm. Pathophysiol. Haemost. Thromb. 35, 451–456 (2006). [DOI] [PubMed] [Google Scholar]
- Bach J. et al. Coagulation factor XII (FXII) activity, activated FXII, distribution of FXII C46T gene polymorphism and coronary risk. J. Thromb. Haemost. 6, 291–296 (2008). [DOI] [PubMed] [Google Scholar]
- Madonna P. et al. Hyperhomocysteinemia and other inherited prothrombotic conditions in young adults with a history of ischemic stroke. Stroke 33, 51–56 (2002). [DOI] [PubMed] [Google Scholar]
- Iribarren J. L. et al. Postoperative bleeding in cardiac surgery: the role of tranexamic acid in patients homozygous for the 5G polymorphism of the plasminogen activator inhibitor-1 gene. Anesthesiology 108, 596–602 (2008). [DOI] [PubMed] [Google Scholar]
- Jimenez Rivera J. J. et al. Factors associated with excessive bleeding in cardiopulmonary bypass patients: a nested case-control study. J. Cardiothorac. Surg. 2, 17 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi B., Cini M., Legnani C., Bernardi F. & Marchetti G. The F11 rs2289252 polymorphism is associated with FXI activity levels and APTT ratio in women with thrombosis. Thromb. Res. 130, 563–564 (2012). [DOI] [PubMed] [Google Scholar]
- Swart K. M. A. et al. Homocysteine and the methylenetetrahydrofolate reductase 677C-->T polymorphism in relation to muscle mass and strength, physical performance and postural sway. Eur. J. Clin. Nutr. 67, 743––748. (2013). [DOI] [PubMed] [Google Scholar]
- López-León S. et al. Meta-analyses of genetic studies on major depressive disorder. Mol. Psychiatry 13, 772–785 (2008). [DOI] [PubMed] [Google Scholar]
- Lewis S. J., Zammit S., Gunnell D. & Smith G. D. A meta-analysis of the MTHFR C677T polymorphism and schizophrenia risk. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 135B, 2–4 (2005). [DOI] [PubMed] [Google Scholar]
- Tsai S.-J., Hong C.-J., Liou Y.-J., Yu Y. W.-Y. & Chen T.-J. Plasminogen activator inhibitor-1 gene is associated with major depression and antidepressant treatment response. Pharmacogenet. Genomics 18, 869–875 (2008). [DOI] [PubMed] [Google Scholar]
- Hyun Y. S. et al. Association of PAI-1 polymorphism with schizophrenia in Korean population. Mol. Cell. Toxicol. 2, 212–215 (2009). [Google Scholar]
- Albinet C. T., Boucard G., Bouquet C. A. & Audiffren M. Increased heart rate variability and executive performance after aerobic training in the elderly. Eur. J. Appl. Physiol. 109, 617–624 (2010). [DOI] [PubMed] [Google Scholar]
- Gauderman W. J. Sample size requirements for association studies of gene-gene interaction. Am. J. Epidemiol. 155, 478–484 (2002). [DOI] [PubMed] [Google Scholar]
- Clark J. S. C., Adler G., Salkic N. N. & Ciechanowicz A. Allele frequency distribution of 1691G >A F5 (which confers Factor V Leiden) across Europe, including Slavic populations. J. Appl. Genet. 54,441––446. (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margaglione M. et al. The PAI-1 gene locus 4G/5G polymorphism is associated with a family history of coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 18, 152–156 (1998). [DOI] [PubMed] [Google Scholar]
- Yang B. et al. Associations of MTHFR gene polymorphisms with hypertension and hypertension in pregnancy: a meta-analysis from 114 studies with 15411 cases and 21970 controls. PLoS One 9, e87497 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. C., Bunce M., Fuggle S. V, Young N. T. & Marshall S. E. Human platel et al. loantigens (HPAs): PCR-SSP genotyping of a UK population for 15 HPA alleles. Eur. J. Immunogenet. 30, 415–419 (2003). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Genetic variants associated with deep vein thrombosis: the F11 locus. J. Thromb. Haemost. 7, 1802–1808 (2009). [DOI] [PubMed] [Google Scholar]
- De Stefano V. et al. Prothrombin G20210A mutant genotype is a risk factor for cerebrovascular ischemic disease in young patients. Blood 91, 3562–3565 (1998). [PubMed] [Google Scholar]
- Ellis R. J., Sollers Iii J. J., Edelstein E. A. & Thayer J. F. Data transforms for spectral analyses of heart rate variability. Biomed. Sci. Instrum. 44, 392–397 (2008). [PubMed] [Google Scholar]
- Cooper P. C. & Rezende S. M. An overview of methods for detection of factor V Leiden and the prothrombin G20210A mutations. Int. J. Lab. Hematol. 29, 153–162 (2007). [DOI] [PubMed] [Google Scholar]
- Blondon M., Hwang M. & Smith N. L. Genotyping in Prothrombotic States: Implications for the Clinician. Curr. Cardiovasc. Risk Rep. 5, 525–532 (2011). [Google Scholar]
- Morange P. E. & Tregouet D. A. Lessons from genome-wide association studies in venous thrombosis. J. Thromb. Haemost. 9 Suppl 1, 258–264 (2011). [DOI] [PubMed] [Google Scholar]
- Calafell F. et al. Sequence variation and genetic evolution at the human F12 locus: mapping quantitative trait nucleotides that influence FXII plasma levels. Hum. Mol. Genet. 19, 517–525 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharska-Newton A. M. et al. Association of the platelet GPIIb/IIIa polymorphism with atherosclerotic plaque morphology: the Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 216, 151–156 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. F. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. (Guilford Press, New York, 2013). [Google Scholar]
- Bray J. H. & Maxwell S. E. Multivariate Analysis of Variance. (SAGE Publications, Inc., Newbury Park, CA, 1985). [Google Scholar]
- Fox J. Bootstrapping regression models. in Applied Regression Analysis and Generalized Linear Models (ed. Fox J. ) 587–606 (SAGE Publications, Thousand Oaks, CA, 2008). [Google Scholar]
- Hall P. & Hart J. D. Bootstrap test for difference between means in nonparametric regression. J. Am. Stat. Assoc. 85, 1039–1049 (1990). [Google Scholar]