Abstract
In this study, we examined the possible mechanisms of trehalose (Tre) in improving copper-stress (Cu-stress) tolerance in rice seedlings. Our findings indicated that pretreatment of rice seedlings with Tre enhanced the endogenous Tre level and significantly mitigated the toxic effects of excessive Cu on photosynthesis- and plant growth-related parameters. The improved tolerance induced by Tre could be attributed to its ability to reduce Cu uptake and decrease Cu-induced oxidative damage by lowering the accumulation of reactive oxygen species (ROS) and malondialdehyde in Cu-stressed plants. Tre counteracted the Cu-induced increase in proline and glutathione content, but significantly improved ascorbic acid content and redox status. The activities of major antioxidant enzymes were largely stimulated by Tre pretreatment in rice plants exposed to excessive Cu. Additionally, increased activities of glyoxalases I and II correlated with reduced levels of methylglyoxal in Tre-pretreated Cu-stressed rice plants. These results indicate that modifying the endogenous Tre content by Tre pretreatment improved Cu tolerance in rice plants by inhibiting Cu uptake and regulating the antioxidant and glyoxalase systems, and thereby demonstrated the important role of Tre in mitigating heavy metal toxicity. Our findings provide a solid foundation for developing metal toxicity-tolerant crops by genetic engineering of Tre biosynthesis.
Environmental pollution by heavy metals has been known for a long time; however, exposure to heavy metals still continues and is worsening, particularly in less developed countries1,2,3. In this context, copper (Cu) has emerged as a serious pollutant in the past few decades because of its excessive use in the manufacturing and agricultural industries4,5. In Bangladesh, cultivated lands are highly contaminated with Cu because of uncontrolled and repeated application of Cu-containing pesticides, pig and poultry slurries, and untreated wastewater from industrial establishments6. Rice (Oryza sativa) is an important cereal crop grown across the world and is also considered a staple food in many Asian countries, including Bangladesh, India, China, and Japan. Bangladesh is the world’s fourth biggest producer of rice, with 11.54 million ha under cultivation7. Cultivation of crops, such as rice, in Cu-polluted lands, results in yield loss, reduced seed quality, and Cu toxicity in humans. Therefore, developing rice varieties tolerant to Cu stress or understanding the overall mechanism of rice plant response to Cu toxicity is important for sustainable rice production.
Cu, as a co-factor of numerous proteins and enzymes, is involved in plant growth, development, and protective mechanisms8. However, a slightly higher than optimal level of Cu is toxic and can detrimentally affect biochemical and physiological processes, including photosynthesis, nitrogen metabolism, senescence, membrane integrity, and mineral uptake in plants9,10. Cu toxicity also causes abnormal root morphology, chlorosis, necrosis, and rolling in leaves, all of which hinder plant growth and development, and ultimately lead to reduced crop productivity10,11,12,13. The inherent redox active nature of Cu also potentiates Cu toxicity by generating reactive oxygen species (ROS), such as superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radical (OH•)12,14,15. To combat oxidative stress, plant cells are well equipped with intrinsic antioxidant capacity that comprises enzymatic components, such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), glutathione S-transferase (GST), ascorbate peroxidase (APX), dehydroascorbate reductase (DHAR), monodehydroascorbate reductase (MDHAR), and glutathione reductase (GR), as well as non-enzymatic components, such as ascorbic acid (AsA) and glutathione (GSH)16.
Methylglyoxal (MG), a highly reactive compound, is known to accumulate under abiotic stresses, including heavy metal toxicity17,18. Besides its direct cytotoxic effects on biological biomolecules, MG induces oxidative stress either directly through increasing ROS formation or indirectly by forming advanced glycation end products (AGEs)19,20. To detoxify MG, plants possess a GSH-dependent glyoxalase (Gly) system that converts MG into D-lactate by employing Gly I and Gly II21. Transgenic plants overexpressing Gly genes have shown higher tolerance to toxic levels of zinc, cadmium, and lead22, as well as higher ROS detoxification capacity17,23. Efficient induction of the antioxidant defense and Gly systems correlates with increased tolerance to abiotic stresses12,24,25.
Regulating Cu homeostasis is crucial in maintaining the intracellular Cu level to avoid toxicity. Plants have developed various mechanisms to restrict Cu toxicity, such as inhibition of Cu uptake by binding with root exudates like organic acids, intracellular sequestration by strong ligands like cysteine-rich compounds and phytochelatins, and exclusion of excessive Cu from the cells by sugar alcohols like trehalose (Tre)26,27,28. Tre, a non-reducing disaccharide of glucose, protects plant cells against long-term desiccation by stabilizing enzymes, proteins, and biological membranes under dehydration29. Tre is highly compatible with cellular metabolism because it is non-reactive even at higher concentrations29. Tre has the added advantage of being a signaling and antioxidant molecule and it can mitigate several types of abiotic stress, including heat, drought, and salinity30,31,32,33,34. However, the beneficial role of Tre and the associated mechanisms involved in protecting plants against heavy metal toxicity remain elusive.
Thus, in the current study, we examined the effects of excessive Cu on growth and development of the economically important rice crop, as well as various physiological and biochemical parameters in the plants. More important, to gain an insight into the physiological and biochemical mechanisms Tre uses to enhance tolerance in rice plants to Cu stress, we investigated the effects of exogenous Tre on (i) Cu uptake and accumulation, (ii) Cu-induced changes in growth parameters and the levels of oxidative parameters, (iii) Cu-induced modulation of non-enzymatic antioxidants, and (iv) the activities of the enzymes involved in the antioxidant defense and Gly systems in rice seedlings under Cu stress.
Results
Plant growth parameters
Several plant growth parameters, including plant height, fresh weight (FW), and dry weight (DW), were determined to estimate the negative effects of excessive Cu on plant growth and the potential mitigation effects of Tre on Cu-stressed rice seedlings. The height of the Cu-stressed seedlings decreased by 13 and 18% at days 4 and 7, respectively, compared with control (Table 1). However, under Cu stress, Tre pretreatment resulted in a significant increase in plant height, almost reaching that of control, compared with Cu-stressed only seedlings. FW of the Cu-stressed seedlings decreased by 33 and 65%, whereas DW decreased by 17 and 39% at days 4 and 7, respectively, compared with control. However, FW and DW remained significantly higher at both days 4 and 7 in the Tre-pretreated Cu-stressed seedlings relative to the Cu-stressed only seedlings. Tre pretreatment alone did not markedly affect plant height, FW, and DW compared with control over the experimental period. These results demonstrated that Tre pretreatment could enhance tolerance of rice seedlings under Cu stress.
Table 1. Effect of exogenous trehalose on plant growth, relative water content (RWC), total chlorophyll (Chl) content, and proline (Pro) content in rice seedlings with or without Cu stress.
| Duration | Treatment | Plant height (cm seedling−1) | FW (g seedling−1) | DW (g seedling−1) | Leaf RWC (%) | Total Chl (mg g−1 FW) | Pro (μmol g−1 FW) |
|---|---|---|---|---|---|---|---|
| Day 4 | Control | 13.57 ± 0.60b | 0.124 ± 0.003c | 0.029 ± 0.001b | 98.78 ± 0.19c | 1.50 ± 0.05b | 0.28 ± 0.07a |
| Tre | 13.07 ± 0.46b | 0.129 ± 0.004c | 0.031 ± 0.001b | 98.34 ± 0.90c | 1.50 ± 0.02b | 0.25 ± 0.12a | |
| Cu | 11.80 ± 0.62a | 0.083 ± 0.003a | 0.024 ± 0.001a | 81.41 ± 2.14a | 1.00 ± 0.08a | 1.65 ± 0.11c | |
| Tre+Cu | 13.07 ± 0.38b | 0.105 ± 0.006b | 0.029 ± 0.001b | 93.57 ± 2.12b | 1.46 ± 0.01a | 0.89 ± 0.08b | |
| Day 7 | Control | 14.40 ± 0.85b | 0.180 ± 0.006c | 0.036 ± 0.001c | 98.44 ± 1.40c | 1.53 ± 0.05c | 0.35 ± 0.04a |
| Tre | 14.23 ± 0.40b | 0.180 ± 0.007c | 0.036 ± 0.001c | 98.55 ± 0.83c | 1.49 ± 0.04c | 0.29 ± 0.06a | |
| Cu | 11.77 ± 0.76a | 0.067 ± 0.006a | 0.022 ± 0.001a | 67.05 ± 2.19a | 0.60 ± 0.06a | 2.33 ± 0.25c | |
| Tre+Cu | 14.00 ± 0.46b | 0.117 ± 0.006b | 0.027 ± 0.001b | 79.01 ± 2.03b | 0.86 ± 0.05b | 1.11 ± 0.13b |
Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively. FW, fresh weight; DW, dry weight. Values are means ± SD of three independent replications (n = 3). Different letters within the column indicate statistically significant differences between treatments, according to Duncan’s multiple range test (p < 0.05).
Relative water content (RWC), and chlorophyll (Chl), and proline (Pro) content
Under Cu stress, RWC and total Chl content decreased significantly at days 4 and 7 compared with control (Table 1). The decreases were notably drastic (32 and 61% for RWC and Chl content, respectively) after 7 days of Cu treatment. In contrast, a lower decrease in RWC (5 and 20% at days 4 and 7, respectively) and total Chl content (3 and 44% at days 4 and 7, respectively) was observed in the Tre-pretreated Cu-stressed seedlings compared with control. However, RWC and total Chl content remained significantly higher in the Tre-pretreated Cu-stressed seedlings compared with the Cu-stressed only seedlings, suggesting that Tre could alleviate the negative effects of excessive Cu on the RWC and Chl content of rice plants. Under non-stressed conditions, no significant changes in RWC and total Chl content were observed in the Tre-pretreated seedlings relative to control. The level of Pro, which plays an osmoprotectant role against osmotic disturbance in plant cells caused by various abiotic stresses, including heavy metal stress35,36, increased by 489 and 566% at days 4 and 7, respectively, in the Cu-stressed seedlings compared with control (Table 1). On the other hand, compared with the Cu-stressed rice plants, the Tre-pretreated Cu-stressed seedlings showed a lower increase in Pro content (218 and 217%, respectively) over that of control. No significant change in Pro content was noted upon Tre pretreatment under non-stressed conditions over the experimental period.
Cu content in roots and leaves
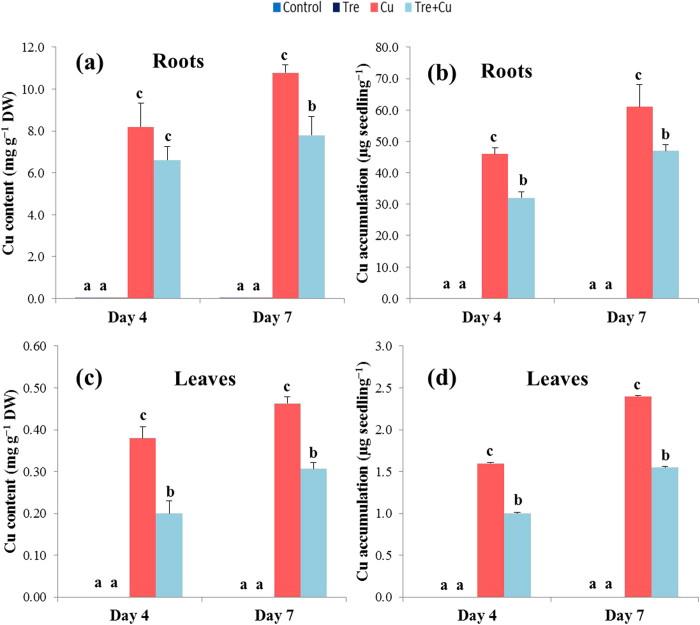
To examine whether the positive impact of Tre pretreatment on Cu-stressed seedlings was associated with its ability to reduce Cu uptake, the Cu content and accumulation were determined in the roots and leaves of the rice seedlings with or without treatment with Cu stress. Cu content in the roots of the Cu-stressed seedlings was 24 and 38% higher at days 4 and 7, respectively, compared with the Tre-pretreated Cu-stressed seedlings (Fig. 1a). The accumulation of Cu decreased by 30 and 23% at days 4 and 7, respectively, in the Tre-pretreated Cu-stressed seedlings compared with those in the seedlings treated with Cu stress alone (Fig. 1b). Similarly, Cu content in the leaves of Cu-stressed seedlings was 90 and 48% higher, but Cu accumulation decreased by 38 and 35% at days 4 and 7, respectively, compared with the Cu-stressed only seedlings (Fig. 1c,d)
Figure 1. Effect of exogenous trehalose on Cu content and accumulation in rice seedlings with or without Cu stress.
(a) Cu content in roots, (b) Cu accumulation in roots, (c) Cu content in leaves, and (d) Cu accumulation in leaves. Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively. Bars represent standard deviation (SD) of the mean (n = 3). Different letters indicate significant differences among treatments at p < 0.05, according to Duncan’s multiple range test.
Malondialdehyde (MDA) and H2O2 content and lipoxygenase (LOX) activity
H2O2 and MDA content were measured as the index of oxidative stress. Both these oxidative parameters significantly increased in the Cu-stressed seedlings over control at both days 4 and 7 (Table 2), but the increases were more drastic (151 and 119% for H2O2 and MDA, respectively) at day 7 of Cu treatment. On the other hand, the levels of H2O2 and MDA in the Cu-stressed seedlings pretreated with Tre were 5 and 17% lower at day 4, and 43 and 30% lower at day 7, respectively, compared with those in the Cu-stressed only seedlings, indicating that Tre could protect rice plants against excessive Cu-induced oxidative stress. Under non-stressed conditions, H2O2 content decreased by 11% at day 4 in the Tre-pretreated seedlings compared with the untreated control; however, no change in MDA content was observed at both days 4 and 7. LOX, as an oxidative enzyme, can also initiate lipid peroxidation, leading to the formation of MDA37. In the Cu-stressed seedlings, LOX activity increased by 63 and 61% at days 4 and 7, respectively, compared with control. However, LOX activity was 12 and 24% lower at days 4 and 7, respectively, in the Cu-stressed seedlings pretreated with Tre compared with the Cu-stressed only seedlings. Under non-stressed conditions, a significant change in LOX activity was recorded after only 4 days of Tre pretreatment of rice seedlings, with an increase of 26% in the Tre-pretreated seedlings relative to control.
Table 2. Effect of exogenous trehalose on levels of malondialdehyde (MDA) and hydrogen peroxide (H2O2), and lipoxygenase (LOX) activity in rice seedlings with or without Cu stress.
| Duration | Treatment | H2O2 (nmol g−1 FW) | MDA (nmol g−1 FW) | LOX (nmol min−1 mg−1 protein |
|---|---|---|---|---|
| Day 4 | Control | 14.43 ± 1.65a | 16.19 ± 1.45b | 10.24 ± 1.47a |
| Tre | 15.41 ± 1.25a | 14.38 ± 0.56a | 12.95 ± 1.64b | |
| Cu | 24.06 ± 1.44b | 35.79 ± 2.74d | 16.69 ± 1.34d | |
| Tre+Cu | 22.88 ± 2.39b | 29.69 ± 1.60c | 14.72 ± 1.53c | |
| Day 7 | Control | 16.72 ± 0.98a | 17.39 ± 1.90a | 14.67 ± 1.28a |
| Tre | 18.23 ± 1.83a | 16.61 ± 1.32a | 15.41 ± 1.68a | |
| Cu | 36.58 ± 2.18c | 43.72 ± 1.74c | 23.63 ± 1.70c | |
| Tre+Cu | 20.96 ± 1.53b | 30.69 ± 1.78b | 17.92 ± 1.90b |
Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively. FW, fresh weight. Values are means ± SD of three independent replications (n = 3). Different letters within the column indicate statistically significant differences between treatments, according to Duncan’s multiple range test (p < 0.05).
ROS (O2 •– and H2O2) accumulation
ROS level is also a good indicator of oxidative stress in plants. The accumulation of O2•− and H2O2 in the second leaf of the rice seedlings was recorded at day 7 of Cu treatment (Fig. 2). NBT staining indicated an increased amount of O2•– as scattered dark blue spots in the leaf plate of the Cu-stressed seedlings compared with the non-treated control (Fig. 2a). Similarly, DAB staining confirmed a marked increase in brown polymerization products, which indicated the over-accumulation of H2O2 in the leaves of the Cu-stressed seedlings relative to control (Fig. 2b). More important, the accumulation of O2•– and H2O2 in the leaves of the Tre-pretreated Cu-stressed seedlings diminished considerably compared with those of the Cu-stressed only seedlings. Pretreatment with exogenous Tre might therefore enhance tolerance in rice plants against oxidative stress induced by excessive Cu.
Figure 2. Effect of exogenous trehalose on ROS accumulation in leaves of rice seedlings with or without Cu stress.
(a) superoxide (O2∙−) and (b) hydrogen peroxide (H2O2) production in rice leaves were detected using nitroblue tetrazolium (NBT) solution and 3,3'-diaminobenzidine (DAB), respectively, at day 7 of Cu stress. Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively.
Ascorbic acid (AsA) content and AsA to dehydroascorbic acid (DHA) ratio (AsA/DHA)
Compared with the untreated control, total AsA content declined significantly at both days 4 and 7 in the Cu-stressed seedlings, with a more drastic decrease (54%) observed at day 7 (Table 3). On the other hand, total AsA content in the Tre-pretreated Cu-stressed seedlings was 39 and 93% higher at days 4 and 7, respectively, compared with the Cu-stressed only seedlings. Total AsA content in the Tre-pretreated Cu-stressed seedlings was 15% higher at day 7 than at day 4. Tre pretreatment significantly increased the levels of total AsA in the rice seedlings compared with control during the experiment, under normal conditions. The AsA/DHA ratio decreased significantly in the Cu-stressed seedlings at both days 4 and 7 relative to control, and this decrease was more severe (241%) at day 7. Although the AsA/DHA ratio in the Tre-pretreated Cu-stressed seedlings was lower than that of control, the values were still significantly higher than those of the Cu-stressed seedlings at both days 4 and 7.
Table 3. Effect of exogenous trehalose on the levels of non-enzymatic antioxidants (ascorbic acid, AsA and glutathione, GSH) and their redox ratios (AsA/DHA, GSH/GSSG) in rice seedlings with or without Cu stress.
| Duration | Treatment | Total AsA (nmol g−1 FW) | AsA/DHA ratio | GSH (nmol g−1 FW) | GSH/GSSG ratio |
|---|---|---|---|---|---|
| Day 4 | Control | 3979.25 ± 68.80c | 3.68 ± 0.51b | 492.56 ± 27.33a | 31.04 ± 3.71c |
| Tre | 4133.52 ± 199.36c | 4.83 ± 0.26c | 462.74 ± 32.20a | 32.04 ± 3.07c | |
| Cu | 2412.68 ± 215.14a | 1.21 ± 0.24a | 815.54 ± 69.93c | 17.38 ± 1.26a | |
| Tre+Cu | 3344.98 ± 294.46b | 3.16 ± 0.47b | 767.92 ± 57.03b | 20.07 ± 0.56b | |
| Day 7 | Control | 4264.65 ± 213.20c | 4.86 ± 0.63c | 573.38 ± 41.18a | 33.22 ± 0.15c |
| Tre | 4401.74 ± 139.84c | 6.03 ± 0.64d | 580.56 ± 46.11a | 37.59 ± 4.43d | |
| Cu | 1981.15 ± 159.26a | 0.97 ± 0.18a | 992.46 ± 20.12c | 12.45 ± 2.31a | |
| Tre+Cu | 3829.16 ± 150.62b | 3.31 ± 0.31b | 632.80 ± 53.85b | 15.35 ± 1.07b |
Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively. AsA, ascorbic acid; DHA, dehydroascorbic acid (oxidized ascorbic acid); FW, fresh weight; GSH, reduced glutathione; GSSG, glutathione disulfide (oxidized glutathione).Values are means ± SD of three independent replications (n = 3). Different letters within the column indicate statistically significant differences between treatments, according to Duncan’s multiple range test (p < 0.05).
GSH content and GSH to oxidized GSH (GSSG) ratio (GSH/GSSG)
In the Cu-stressed seedlings, the GSH level increased by 66 and 73% at days 4 and 7, respectively, over control (Table 3). On the other hand, Tre pretreatment restricted the increase in the GSH level in the Tre-pretreated Cu-stressed seedlings to 6 and 36% at days 4 and 7, respectively, compared with the Cu-stressed only seedlings. However, the GSH levels were still significantly higher (56 and 10% at days 4 and 7, respectively) in the Tre-pretreated Cu-stressed seedlings relative to the non-treated control. No significant change in the level of GSH was noted upon Tre pretreatment only over non-treatment at both days 4 and 7. The GSH/GSSG ratio showed a significant decrease in Cu-stressed seedlings compared with control at both time points. However, GSH/GSSG ratios were significantly higher in the Tre-pretreated Cu-stressed seedlings than those of the Cu-stressed only seedlings at both days 4 and 7.
Antioxidant enzymes SOD, CAT, GPX, and GST
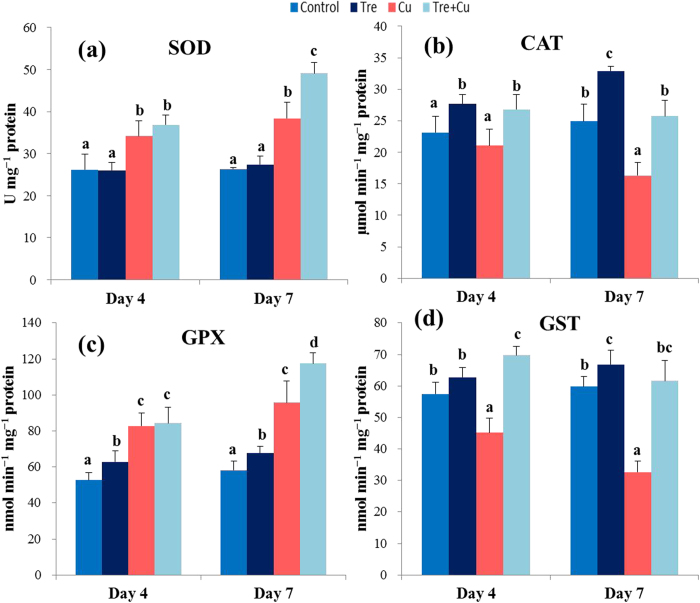
Tre pretreatment affected the activities of the enzymes in a time-dependent manner. SOD activity increased by 31 and 46% at days 4 and 7, respectively, in the Cu-stressed seedlings relative to control (Fig. 3a). SOD activity was observed to further increase in the Cu-stressed seedlings subjected to Tre pretreatment, and compared with the Cu-stressed seedlings alone, its activity increased by 8 and 28% at days 4 and 7, respectively. Tre pretreatment did not alter SOD activity under non-stressed conditions compared with control over the experimental period. As shown in Fig. 3b, CAT activity remained unchanged at day 4 but drastically decreased (34%) at day 7 in the Cu-stressed seedlings compared with control. However, CAT activity in the Tre-pretreated Cu-stressed seedlings remained at the level of control at both days 4 and 7. CAT activity increased by 19 and 32% at day 4 and day 7, respectively, in the Tre-pretreated only seedlings compared with control. GPX activity increased by 56 and 65% at days 4 and 7, respectively, in the Cu-stressed seedlings relative to control (Fig. 3c). On the other hand, an increase in GPX activity was also observed (2 and 23% at days 4 and 7, respectively) in the Tre-pretreated Cu-stressed seedlings compared with the Cu-stressed only seedlings. Under non-stressed conditions, GPX activity in the Tre-pretreated seedlings increased by 19 and 16% at days 4 and 7, respectively, compared with control. GST activity, upon Cu exposure, drastically decreased by 31 and 45% at days 4 and 7, respectively, compared with control (Fig. 3d). However, an increase in GST activity (54 and 89% at day 4 and day 7) was recorded in the Tre-pretreated seedlings compared with the Cu-stressed only seedlings. A significant increase in GST activity was also recorded in the Tre-pretreated seedlings, but only at day 7, relative to control.
Figure 3. Effect of exogenous trehalose on the activities of antioxidant enzymes in rice seedlings with or without Cu stress.
(a) superoxide dismutase (SOD), (b) catalase (CAT), (c) glutathione peroxidase (GPX), and (d) glutathione reductase (GST). Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively. Bars represent standard deviation (SD) of the mean (n = 3). Different letters indicate significant differences among treatments at p < 0.05, according to Duncan’s multiple range test.
Ascorbate-glutathione cycle enzymes APX, MDHAR, DHAR, and GR
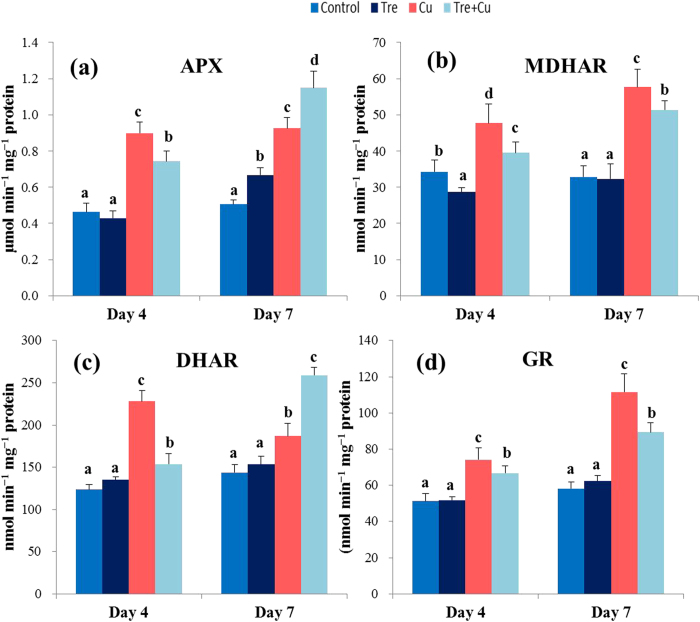
The results related to the activities of the ascorbate-glutathione cycle enzymes are depicted in Fig. 4. Under Cu stress, APX activity increased by 96 and 82% at days 4 and 7, respectively, compared with control (Fig. 4a). APX activity did not increase at day 4, but increased by 23% at day 7 in the Tre-pretreated Cu-stressed seedlings compared with the Cu-stressed only seedlings. APX activity increased significantly in the Tre-pretreated seedlings at day 7 only, relative to control. MDHAR activity increased by 39 and 76% at days 4 and 7, respectively, in the Cu-stressed seedlings compared with control (Fig. 4b). On the other hand, MDHAR activity did not increase in the Tre-pretreated Cu-stressed seedlings compared with the Cu-stressed only seedlings, but the activity was still higher than that of control, by 15 and 57% at days 4 and 7, respectively. DHAR activity increased by 85 and 30% at days 4 and 7, respectively, in the Cu-stressed seedlings compared with control (Fig. 4c). On the other hand, DHAR activity did not increase at day 4, but increased by 39% at day 7 in the Tre-pretreated Cu-stressed seedlings compared with the Cu-stressed only seedlings. No significant change in DHAR activity was observed in the rice seedlings under non-stressed conditions upon Tre pretreatment over the experimental period. GR activity increased by 45 and 92% at days 4 and 7, respectively, in the Cu-stressed seedlings relative to control (Fig. 4d). In contrast, GR activity did not increase in the Tre-pretreated Cu-stressed seedlings compared with the Cu-stressed only seedlings, but it was 30 and 54% higher at days 4 and 7, respectively, than that of control.
Figure 4. Effect of exogenous trehalose on activity of ascorbate-glutathione cycle enzymes in rice seedlings with or without Cu stress.
(a) ascorbate peroxidase (APX), (b) monodehydroascorbate reductase (MDHAR), (c) dehydroascorbate reductase (DHAR), and (d) glutathione reductase (GR). Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively. Bars represent standard deviation (SD) of the mean (n = 3). Different letters indicate significant differences among treatments at p < 0.05, according to Duncan’s multiple range test.
MG content and activity of Gly enzymes
Under Cu stress, the MG level increased by 106 and 156% at days 4 and 7, respectively, compared with control (Fig. 5a). In comparison with the seedlings treated with Cu stress alone, the MG level decreased by 27 and 35% at days 4 and 7, respectively, in the seedlings treated with both Tre and Cu. Tre did not affect the level of MG in the Tre-pretreated seedlings under non-stressed conditions. On the other hand, the MG detoxifying enzymes Gly I and Gly II showed varying responses to Tre pretreatment in the presence or absence of Cu stress. Gly I activity increased by 22% at day 4 and decreased by 25% at day 7 in the Cu-stressed plants compared with control (Fig. 5b). Under Tre pretreatment, Gly I activity further increased in the Tre-pretreated Cu-stressed seedlings, making it higher than that of the Cu-stressed only seedlings by 14 and 45% at days 4 and 7, respectively. Under non-stressed conditions, Gly I activity increased by 20% in the Tre-pretreated seedlings at day 7 only relative to control. Gly II activity (Fig. 5c) increased 47% in the Cu-stressed seedlings over control at day 4, but the activity returned to the level in control at day 7. Conversely, Gly II activity did not increase significantly at day 4, but increased significantly at day 7 in the Tre-pretreated Cu-stressed seedlings compared with the Cu-stressed only seedlings. No significant change in Gly II activity was found under non-stressed conditions upon Tre pretreatment compared with control over the experimental period.
Figure 5. Effect of exogenous trehalose on activity of glyoxalase cycle enzymes in rice seedlings with or without Cu stress.
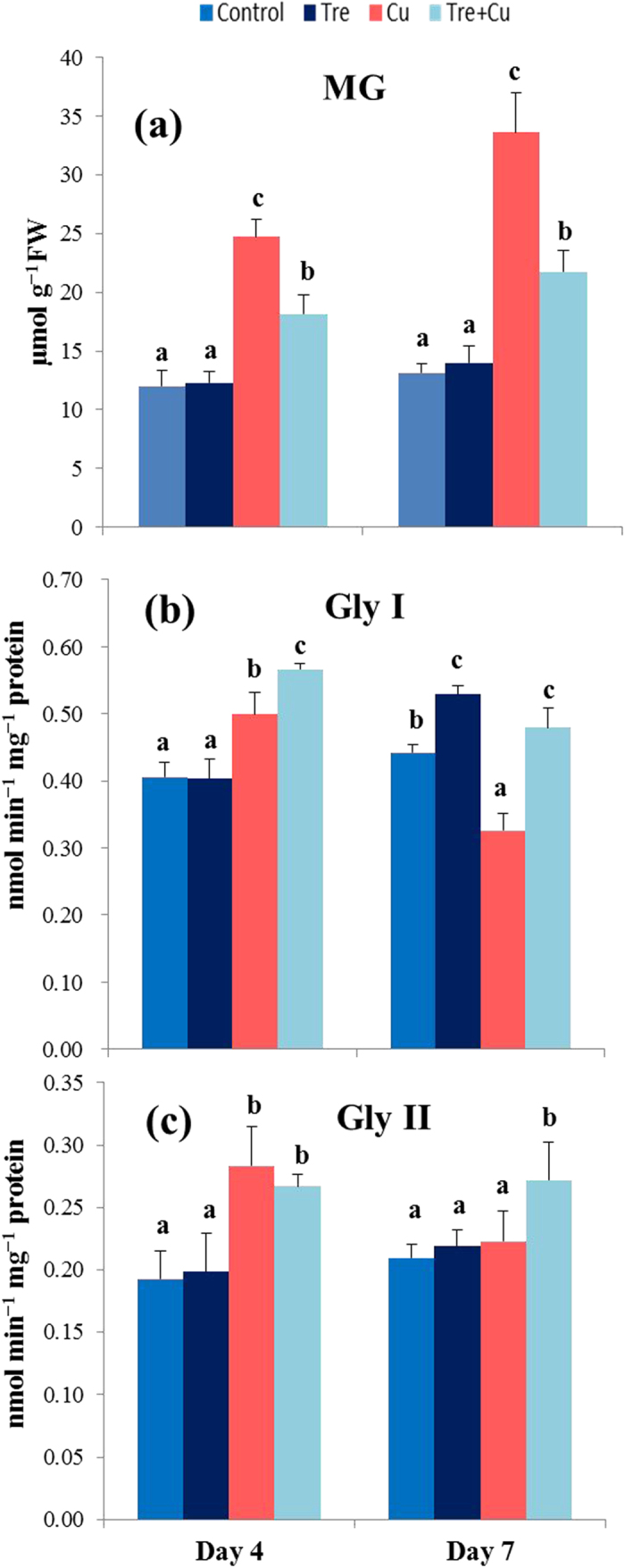
(a) methylglyoxal (MG) content, (b) glyoxalase I (Gly I) activity, and (c) glyoxalase II (Gly II) activity. Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively. Bars represent standard deviation (SD) of the mean (n = 3). Different letters indicate significant differences among treatments at p < 0.05, according to Duncan’s multiple range test.
Tre content
Tre level was estimated to observe the influence of exogenous Tre on the endogenous content of Tre and its contribution to the enhanced tolerance of the rice plants to Cu stress. Under Cu stress, the endogenous level of Tre increased in the stressed seedlings by 45 and 50% at days 4 and 7, respectively, relative to the unstressed control (Fig. 6). A further increase in the level of Tre was noted in the Cu-stressed seedlings due to Tre pretreatment, making the endogenous Tre level in the Tre-treated Cu-stressed seedlings significantly higher (41 and 39% at days 4 and 7, respectively) than that in the Cu-stressed only seedlings. Tre content also markedly increased (64 and 60% at days 4 and 7, respectively) in the Tre-pretreated seedlings compared with control.
Figure 6. Effect of exogenous trehalose on endogenous trehalose content in rice seedlings with or without Cu stress.
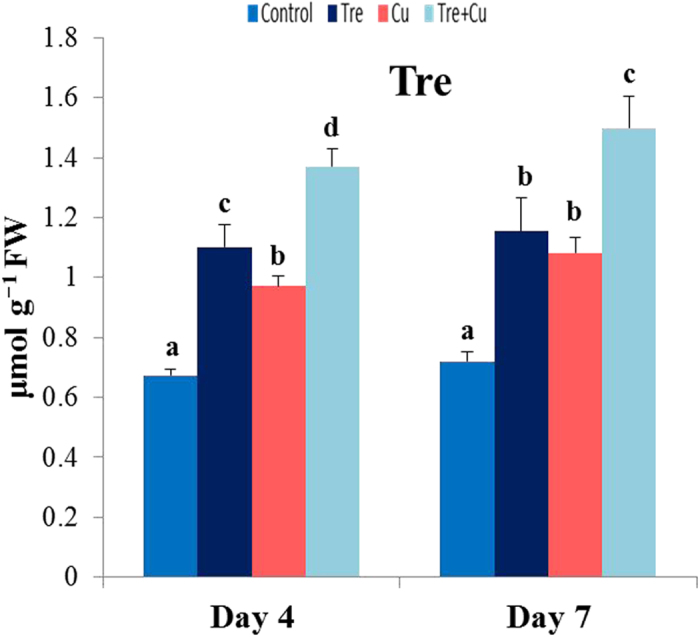
Control, Tre, Cu, and Tre+Cu correspond to control, 10 mM trehalose, 100 μM CuSO4, and 10 mM trehalose + 100 μM CuSO4, respectively. Bars represent standard deviation of the mean (n = 3). Different letters indicate significant differences among treatments at p < 0.05, according to Duncan’s multiple range test.
Discussion
In this study, we have provided an insight into how Tre regulates Cu homeostasis to provide protection to rice seedlings against excessive Cu. Prolonged Cu stress resulted in a decrease in the overall growth of rice seedlings (Table 1), perhaps by disturbing the functions of Cu-containing proteins and the cell metabolism of plants, which was attributed to Cu-induced phytotoxicity. However, Tre pretreatment enhanced the growth and biomass of Cu-stressed rice seedlings, demonstrating the ameliorating action of Tre in reducing Cu toxicity (Table 1), as also observed in maize under drought and salinity conditions31,38, which suggests the protecting role of Tre against a wide range of environmental stresses. Disturbance of water uptake and destruction of Chl, and thereby photosynthesis, have been regarded as characteristic symptoms of Cu stress11,12,14, as observed in this study (Table 1). More important, Tre-induced restoration of the levels of RWC and Chl in the Cu-stressed seedlings indicated an osmoprotective and membrane-protecting role of Tre for plants exposed to Cu stress. In addition, plants can respond rapidly to water imbalance by accumulating various osmolytes like Pro39. Many plants have been shown to accumulate Pro in large quantities when exposed to heavy metal stress35,36. However, despite its beneficial effects, Pro may be toxic if over-accumulated or applied in excessive concentrations39. In this work, we found that Cu stress induced a high increase in the Pro level, whereas Tre pretreatment reduced the accumulated Pro level in the Cu-stressed seedlings, which was still higher than that of control (Table 1). This result might indicate that Cu stress induced such a high increase in the Pro level that it caused metabolic perturbation, and that Tre pretreatment could provide osmotic protection to cells by adjusting the accumulated Pro to an optimal level.
In the current study, the degree of Cu toxicity was clearly seen with the levels of Cu uptake and accumulation in the roots and leaves upon extending the period of exposure to Cu, and thereby contributing to the reductions in plant growth and biomass. Our results indicated that Cu uptake and accumulation were much higher in the roots than in the leaves of the rice seedlings (Fig. 1), demonstrating that the roots are the primary site of Cu accumulation. Our results are in well agreement with the findings of Bouazizi et al.4 and Brackhage et al.40 who observed higher accumulation of Cu and arsenic in the roots of bean and wheat, respectively. Tre pretreatment significantly compromised Cu uptake and accumulation (Fig. 1), and was therefore useful in reducing Cu phytotoxicity. The restriction of Cu uptake and accumulation might have occurred because of the formation of a complex between Cu ions and Tre molecules, as observed between Tre and Cd when these were co-applied in a solution41. This pattern of Cu homeostasis might constitute an avoidance or exclusion strategy in reducing Cu accumulation and translocation in leaves, and thus allows higher Cu tolerance in rice seedlings.
In accordance with the higher accumulation of Cu, the present study demonstrated that increased levels of O2•– and H2O2 (Table 2; Fig. 2) was associated with Cu-mediated oxidative burst in the rice seedlings. Cu-induced ROS levels also correlated with the substantial increase in MDA level (Table 2), which indicated severe membrane damage in the rice seedlings caused by Cu stress. In addition, increased LOX activity (Table 2) might have also contributed to oxidative damage by peroxiding membrane lipids. Tre treatment prior to Cu stress resulted in lower ROS production, as well as reduced LOX activity and MDA content, which might be achieved through Tre-mediated reduced Cu uptake (Fig. 1), direct ROS scavenging (Fig. 2), membrane stabilizing (Table 2), and modulation of the antioxidative mechanism involved in eliminating ROS (Figs 3,4). When the ROS level increases in plants exposed to stress, enhanced production of non-enzymatic antioxidants in plant cells, such as AsA and GSH, is crucial for minimizing ROS-induced oxidative stress16. The synchronized actions of these antioxidants drive the ascorbate-glutathione cycle to protect cells from ROS toxicity. Moreover, the reduced oxidized ratios of these antioxidants (AsA/DHA and GSH/GSSG) govern the redox balance of the cell16. The current study revealed that excessive Cu resulted in a decrease in the AsA level and AsA/DHA and GSH/GSSG ratios, but an increase in the GSH level (Table 3), which thereby impaired the ROS detoxification mechanism and consequently increased oxidative stress. Our results are supported by the work of Thounaojam et al.14 and Drazkiewicz et al.15, who investigated the effect of Cu stress on rice and Arabidopsis thaliana, respectively. On the other hand, Tre-induced amendment of the AsA level and redox status augmented the detoxification of ROS, which prevented oxidative damage in the Cu-exposed seedlings. Additionally, induced GSH synthesis was observed in this study in the rice plants treated with excessive Cu (Table 3), as also reported in other plant species under heavy metal stress36,42. However, Tre pretreatment resulted in a decreased GSH level in Cu-stressed seedlings (Table 3), which might be attributed to the Tre-induced reduction in Cu uptake and accumulation (Fig. 1). Furthermore, the increased activities of GSH-metabolizing enzymes (GPX, GST, DHAR, and Gly I) observed in the Cu-stressed plants upon Tre pretreatment might also curtail the cellular GSH level (Table 3; Figs 3c,d,4c,5b).
SOD, CAT, peroxidases, and ascorbate-glutathione cycle enzymes constitute the major enzymatic network that detoxifies ROS15. SOD, a frontline protective enzyme, converts O2•− into H2O2, which is then subsequently removed by CAT and peroxidases. SOD and CAT activities in seedling leaves considered as biomarkers for growth and development under heavy metal stresses43. In this study, a Cu-induced increase in SOD activity did not correlate with the level of O2•− (Figs 2a,3a), indicating that this level of SOD activity might not be sufficient to neutralize O2•− toxicity. In addition, a profound decrease in CAT activity in the Cu-stressed seedlings trapped H2O2 and accelerated the accumulation of H2O2 (Table 2; Figs 2b,3b). As a consequence, excessive Cu might have amplified the Haber-Weiss reaction to produce deadly OH•. However, Tre pretreatment resulted in a further increase in SOD activity and countered the inhibition of CAT activity, particularly at day 7 of Cu exposure, which implied that Tre provided a safeguard against O2•− and H2O2, as well as OH•. A similar phenomenon was found in wheat under heat stress30, which supports our finding. Another important mechanism in regulating heavy metal-induced toxicity is associated with the GSH-dependent conjugation of lipid hydroperoxides and endobiotic substrates by GPX and GST26. Studies on hyperaccumulating and transgenic plants suggested that enhanced activities of GPX and GST conferred higher tolerance to abiotic stresses, including heavy metals44. In the present study, the enhanced activities of GPX and GST (Fig. 3c,d) due to Tre pretreatment suggested that there might be GSH-dependent peroxide scavenging that led to reduced oxidative damage. It might also be that the higher GST activity enhanced the membrane-protecting role of Tre by conjugating GSH with various Cu-induced electrophiles, which thereby prevented them from further damaging the membrane.
The ascorbate-glutathione cycle involves a series of reactions with four enzymes (APX, MDHAR, DHAR, and GR) that act in concert in H2O2 metabolism16,26. This cycle also appears to play a key role in maintaining cellular redox balance, especially under metal stress conditions25,45. However, in this study, despite the stimulation of the activities of all four enzymes upon Cu stress, the H2O2 level remained significantly higher (Table 2; Fig. 4a–d), which indicated that the amount of stimulation was not up to the requisite level in lowering excess H2O2. In another study, Thounaojam et al.14 observed an accumulation of H2O2 even after increasing the activity of H2O2-scavenging enzymes, suggesting that production of H2O2 exceeded ROS scavenging capacity. In contrast, Tre, by intensifying APX and DHAR activities as well as keeping MDHAR and GR activities above the control level, coordinates the activities of these four enzymes with enhanced CAT and GPX activities, and thereby contributes well to regulating the H2O2 level (Table 2; Figs 2, 3, 4).
Under abiotic stress conditions, MG is overproduced, which in turn contributes to cellular toxicity as well as oxidative damage in plant tissues18,19,21. Plant cells detoxify MG by using the Gly system21. In the present study, a time-dependent increase in the MG level correlated with the decreased activities of Gly I and Gly II (Fig. 5), and thereby intensified Cu toxicity in the rice seedlings. In contrast, Tre pretreatment detoxified MG by up-regulating the Gly I and Gly II activities, which indicated a positive effect of Tre in amplifying MG detoxification under Cu stress. Several reports have suggested that the antioxidant and Gly systems are closely linked and they act simultaneously in conferring tolerance against multiple stresses, including heavy metals17,23,24,25. It has also been reported that the enhanced activities of Gly enzymes and antioxidant enzymes (APX, GST, and GPX) provided higher tolerance to Zn toxicity in Brassica oleracea46. Our study suggested that Tre might enhance rice tolerance to Cu stress by coordinating the actions of the antioxidant and Gly systems to mitigate Cu-induced ROS and MG toxicity.
Although Tre accumulates in negligible amounts in crop plants, it has been considered a quantitatively important compatible solute and stress protectant29. Non-stressed and stressed rice seedlings pretreated with Tre increased the endogenous level of Tre (Fig. 6), which indicated that Tre was readily absorbed by the roots and easily transported to the aerial parts. Our results corroborate previous findings28,30,31,32,33,34 and suggest that external application of Tre could be an alternative approach to modify the level of endogenous Tre, and thus potentially strengthen plant capacity to withstand the deleterious effects of environmental threats. From this point of view, we assume that additional protection against Cu toxicity in the rice seedlings was attributed to the increase in the endogenous level of Tre.
In conclusion, prolonged exposure to excessive Cu resulted in serious toxic effects on the rice seedlings by affecting their physiological and biochemical attributes. In contrast, Tre pretreatment has been shown to be beneficial in alleviating Cu toxicity, which was mainly attributed to the ability of Tre (i) to restrict Cu uptake and accumulation to maintain Cu homeostasis, and (ii) to induce production of antioxidant and Gly enzymes to alleviate excessive Cu-triggered oxidative stress. Current data suggests that enhancing the level of endogenous Tre by supplementation or genetic engineering could be used as an important strategy in mitigating Cu toxicity, and perhaps other heavy metal toxicity, in rice or other crop plants.
Methods
Plant materials, growth conditions, and treatments
Rice (Oryza sativa L. cv. BRRI dhan29) seeds were surface sterilized with 1% (v/v) sodium hypochlorite solution for 20 min, washed with distilled water, and imbibed for 24 h. The seeds were sown on plastic nets floating on distilled water in 250 mL plastic beakers and kept in the dark at 28 ± 2 °C for germination. After 48 h, uniformly germinated seeds were transferred to a growth chamber and grown in a commercial hydroponic solution (Hyponex, Japan) diluted according to the manufacturer’s instructions. The nutrient solution consisted of 8% N, 6.43% P, 20.94% K, 11.8% Ca, 8% S, 3.08% Mg, 0.07% B, 0.24% Fe, 0.03% Mn, 0.0014% Mo, 0.008% Zn, and 0.003% Cu. The seedlings were grown under controlled conditions (photon density: 100 μmol m−2 s−1, temperature: 26 ± 2 °C, RH: 65–70%). Each plastic beaker contained about 60 rice seedlings. The nutrient solution (pH 5.5) was renewed every four days. At day 10 after sowing, seedlings were pretreated with 10 mM Tre in the hyponex solution for 48 h. Tre-pretreated and non-pretreated rice seedlings were then subjected to 100 μM CuSO4 in the hyponex solution. This CuSO4 concentration was selected based on our previous experiments12,35,47. Based on literature33,34,48 and our preliminary experiments with a range of Tre concentrations (5, 10, 15, and 20 mM), we observed that 10 mM Tre was optimally effective in alleviating Cu-induced toxic symptoms. Therefore, our experiment consisted of 4 treatments as follows: (i) control, (ii) 10 mM trehalose (Tre), (iii) 100 μM CuSO4 (Cu), and (iv) 10 mM trehalose + 100 μM CuSO4 (Tre+Cu). The second leaf of rice seedlings was harvested at day 4 and 7 after Cu-stress treatment to determine various physiological and biochemical parameters. Each treatment was replicated three times under the same experimental conditions.
Determination of plant height, FW, DW, total Chl, RWC, and Pro content
Just after harvest, plant height was determined by measuring the length from the bottom of the main stem to the end of the emerging third leaf. To determine FW, seedlings were separated from the culture medium and the roots were washed thoroughly with distilled water followed by blotting with tissue paper. After separating adherent seeds, 10 seedlings from each treatment were weighed to determine FW (g seedling−1). To quantify DW, 10 seedlings from each treatment were oven dried at 80 °C for 48 h and expressed as g seedling−1. To estimate total Chl content, leaves (0.5 g) were extracted in 80% chilled acetone and Chl was estimated according to the formula of Arnon49.
Total Chl content (mg g−1 FW) = (20.2 × D645 + 8.02 × D663) × V/(1000 × W); where V = volume of 80% (v/v) acetone (mL), W = weight of sample (g).
RWC was determined as described by Mostofa et al.12. Pro content was determined according to the method of Bates et al.50 with minor modifications. Fresh leaf samples (0.5 g) were homogenized with 5 mL of 3% aqueous sulfosalicylic acid and the homogenate was centrifuged at 11,500 × g for 15 min. Supernatant (2 mL) was mixed with 2 mL of glacial acetic acid and 2 mL of acid ninhydrin solution. The resultant mixture was boiled at 100 °C for 1 h and then transferred to ice to stop the reaction. The developed red color was extracted with 4 mL toluene and absorption of the chromophore was measured at 520 nm. Pro concentration was calculated using a calibration curve developed with Pro standard.
Measurement of Cu content and its accumulation in roots and leaves
To determine Cu content, the root and leaf samples were harvested separately and the roots were washed thoroughly with distilled water to remove Cu ions adhering to the root surface. The root and leaf samples were oven dried at 80 °C for 48 h. Dried samples (0.1 g) were ground and digested with a HNO3:HClO4 (5:1 v/v) mixture at 80 °C until the yellow color disappeared. The Cu content in the roots and leaves was measured by using flame atomic absorption spectrophotometry (Z-5000; Hitachi, Japan). The concentration and accumulation of Cu in the roots and leaves were determined by the following formula:
Cu concentration (mg g−1 DW) = reading of AAS × dilution factor/dry wt. of roots or leaves
Cu accumulation (mg seedling−1) = conc. of Cu × dry wt. of roots or leaves of each seedling.
Determination of lipid peroxidation and H2O2 content
Lipid peroxidation of the second leaves was measured by estimating MDA according to the method of Heath and Packer51 using an extinction co-efficient of 155 mM−1 cm−1. H2O2 was extracted and determined after reaction with 0.1% TiCl4 in 20% H2SO4 following the method of Yu et al.52.
Histochemical detections of ROS (O2.– and H2O2)
O2.– and H2O2 were detected in rice leaves according to the method of Mostofa and Fujita12. In brief, the second leaves were stained in 0.1% nitroblue tetrazolium (NBT) solution or 1% 3,3’-diaminobenzidine (DAB) solution to detect O2.– and H2O2, respectively. After 24 h of incubation, leaves were decolorized by immersing them in boiling ethanol to detect the blue insoluble formazan (for O2.–) or deep brown polymerization product (for H2O2). After cooling, photographs were taken by placing the leaves between two glass plates.
Estimation of non-enzymatic antioxidants
Fresh leaves (0.5 g) were homogenized in 3 mL of ice-cold 5% meta-phosphoric acid containing 1 mM EDTA and centrifuged at 11,500 × g for 15 min. Reduced and total AsA content were determined following the method of Dutilleul et al.53 with minor modifications. To estimate the total AsA level, the oxidized fraction was reduced by 0.1 M dithiothreitol. Reduced and total AsA content were assayed at 265 nm in 100 mM K-phosphate buffer (pH 7.0) with 1.0 U of ascorbate oxidase (AO). DHA content was calculated by deducting the reduced AsA amount from total AsA content. Based on enzymatic recycling, reduced GSH, GSSG, and total glutathione (GSH + GSSG) content were determined according to the method of Griffiths54. GSSG content was determined after removing GSH by 2-vinylpyridine derivatization. GSH concentration was measured after subtracting the value of GSSG from the total GSH level.
Extraction and assay of enzymes
To extract enzymes, fresh leaf samples (0.5 g) were homogenized separately with a reaction mixture containing 50 mM K-phosphate buffer (pH 7.0), 100 mM KCl, 1 mM AsA, 5 mM β-mercaptoethanol, and 10% (w/v) glycerol in pre-chilled mortars. The homogenate was centrifuged at 11,500 × g for 15 min and the resultant supernatants were collected to analyze enzyme activities and protein content. All procedures were performed at 0–4 °C.
LOX (EC 1.13.11.12) activity was estimated according to the method of Doderer et al.55 by monitoring the increase in absorbance at 234 nm using linoleic acid as a substrate. SOD (EC 1.15.1.1) activity was estimated according to the method of El-Shabrawi et al.24, which is based on a xanthine-xanthine oxidase system. The reaction mixture contained K-phosphate buffer (50 mM), NBT (2.24 mM), CAT (0.1 units), xanthine oxidase (0.1 units), xanthine (2.36 mM), and enzyme extract. SOD activity was expressed as units (that is, the amount of enzyme required to inhibit NBT reduction by 50%) min−1 mg−1 protein. CAT (EC 1.11.1.6) activity was measured according to the method of Mostofa et al.56. APX (EC 1.11.1.11) activity was determined by monitoring the decrease in absorbance at 290 nm as AsA was oxidized, according to the method of Nakano and Asada57. MDHAR (EC 1.6.5.4) activity was measured by using 1 U of AO and the oxidation rate of NADPH was determined at 340 nm58. DHAR (EC 1.8.5.1) activity was measured by monitoring the formation of AsA from DHA at 265 nm using GSH57. GR (EC 1.6.4.2) activity was quantified by monitoring the decrease in the absorbance of NADPH at 340 nm for GSSG-dependent oxidation of NADPH, as described by Foyer and Halliwell59. GST (EC 2.5.1.18) activity was determined using the method of Mostofa et al.11. GPX (EC: 1.11.1.9) activity was measured as described by Mostofa et al.56 using H2O2 as a substrate. Gly I (EC 4.4.1.5) assay was carried out according to the method of Hossain et al.18 using an extinction co-efficient of 3.37 mM−1 cm−1. Gly II (EC 3.1.2.6) activity was determined according to the method of Mostofa et al.56 using an extinction co-efficient of 13.6 mM−1 cm−1. Total protein content in the extracts was estimated with the dye-binding method of Bradford60 using bovine serum albumin (BSA) as standard.
Determination of Tre content
Tre content in the second leaves was determined following the method described by Li et al.61 with minor modifications. The leaves (1.0 g) were homogenized in 5 mL of 80% (v/v) hot ethanol and centrifuged at 11,500 × g for 20 min. The supernatants were dried at 80 °C followed by re-suspension in 5 mL distilled water. The solution (100 μL) was mixed with 150 μL 0.2 N H2SO4 and boiled at 100 °C for 10 min to hydrolyze any sucrose or glucose-1-phosphate, then chilled on ice. NaOH (0.6 N, 150 μL) was added to the above mixture and boiled for 10 min to destroy reducing sugars, then chilled again. Anthrone reagent (2.0 mL; 0.2 g anthrone per 100 mL of 95% H2SO4) was added to the above mixture and boiled for 10 min to develop a color and then chilled again. The absorbance was recorded at 630 nm and Tre concentration was calculated as μmol g−1 FW using a standard curve developed with commercial Tre.
Determination of MG content
MG content was measured following the method described by Wild et al.62 with modifications. Leaves (0.25 g) were homogenized in 2.5 mL 0.5 M perchloric acid and incubated for 15 min on ice. The extract was centrifuged for 10 min at 11,200 × g at 4 °C and 1 mL supernatant was transferred to a fresh microcentrifuge tube. Charcoal (10 mg mL−1) was added and kept at room temperature for 15 min to decolorize the supernatant. The mixture was centrifuged for 10 min at 11,000 × g and the supernatant was neutralized by saturated K2CO3. The extract was kept at room temperature for 15 min and centrifuged at 11,200 × g for 10 min. In a total volume of 1 mL, 650 μL of neutralized supernatant, 330 μL of 100 mM sodium dihydrogen phosphate buffer (pH 7.0), and 20 μL of freshly prepared 0.5 M N-acetyl-L-cysteine were added and incubated for 15 min. Formation of N-α-acetyl-S-(1-hydroxy-2-oxo-prop-1-yl) cysteine was recorded at a wavelength of 288 nm and MG content was calculated by using a standard curve of known concentration of MG.
Statistical analysis
The data were subjected to one-way analysis of variance (ANOVA) and different letters indicate significant differences between treatments at p < 0.05, according to Duncan’s multiple range test (DMRT) using IRRISTAT version 3 (International Rice Research Institute, Biometrics Unit, Manila, Philippines). Data represented in the Tables and Figures are means ± standard deviations (SD) of three replicates for each treatment.
Additional Information
How to cite this article: Mostofa, M. G. et al. Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci. Rep. 5, 11433; doi: 10.1038/srep11433 (2015).
Acknowledgments
M.G.M. thankfully acknowledges the financial support from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Footnotes
Author Contributions M.G.M. conceived, designed, and conducted the experiments. M.G.M. and L.S.T. analyzed the data and results. M.G.M., M.A.H. and L.S.T. wrote the manuscript. M.F. monitored the experimental work and critically commented on the manuscript. All authors read and approved the final manuscript.
References
- Nagajyoti P. C., Lee K. D. & Sreekanth T. V. M. Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 8, 199–216 (2010). [Google Scholar]
- Shahid M. et al. Heavy metal -induced reactive oxygen species: phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 232, 1–44 (2014). [DOI] [PubMed] [Google Scholar]
- Hughes D. J. et al. Ecological impacts of large-scale disposal of mining waste in the deep sea. Sci. Rep. 5, 9985 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouazizi H., Jouili H., Geitmann A. & El Ferjani E. Cell wall accumulation of Cu ions and modulation of lignifying enzymes in primary leaves of bean seedlings exposed to excess copper. Biol. Trace Elem. Res. 139, 97–107 (2011). [DOI] [PubMed] [Google Scholar]
- Cambrollé J. et al. Effects of copper sulfate on growth and physiological responses of Limoniastrum monopetalum. Environ. Sci. Pollut. Res. 20, 8839–8847 (2013). [DOI] [PubMed] [Google Scholar]
- Rahman S. H. et al. Assessment of heavy metal contamination of agricultural soil around Dhaka Export Processing Zone (DEPZ), Bangladesh: implication of seasonal variation and indices. Appl. Sci. 2, 584–601 (2012). [Google Scholar]
- Karim M. R., Ishikawa M., Ikeda M. & Islam M. T. Climate change model predicts 33% rice yield decreases in 2100 in Bangladesh. Agron. Sustain. Dev. 32, 821–830 (2012). [Google Scholar]
- Yruela I. Copper in plants: acquisition, transport and interactions. Func. Plant Biol. 36, 409–430 (2009). [DOI] [PubMed] [Google Scholar]
- Yruela I. Copper in plants. Braz. J. Plant Physiol. 17, 145–156 (2005). [Google Scholar]
- Burkhead J. L., Reynolds K. A. G., Abdel-Ghany S. E., Cohu C. M. & Pilon M. Copper homeostasis. New Phytol. 182, 799–816 (2009). [DOI] [PubMed] [Google Scholar]
- Bazihizina N., Colzi I., Giorni E., Mancuso S. & Gonnelli C. Photosynthesizing on metal excess: Copper differently induced changes in various photosynthetic parameters in copper tolerant and sensitive Silene paradoxa L. populations. Plant Sci. 232, 67–76 (2015). [DOI] [PubMed] [Google Scholar]
- Mostofa M. G. & Fujita M. Salicylic acid alleviates copper toxicity in rice (Oryza sativa L.) seedlings by up-regulating antioxidative and glyoxalase systems. Ecotoxicology 22, 959–973 (2013). [DOI] [PubMed] [Google Scholar]
- Xu J. et al. Toxicity of copper on rice growth and accumulation of copper in rice grain in copper contaminated soil. Chemosphere 62, 602–607 (2006). [DOI] [PubMed] [Google Scholar]
- Thounaojam T. C. et al. Excess copper induced oxidative stress and response of antioxidants in rice. Plant Physiol. Biochem. 53, 33–39 (2012). [DOI] [PubMed] [Google Scholar]
- Drazkiewicz M., Skorzynska-Polit E. & Krupa Z. Responses of the ascorbate glutathione cycle to excess copper in Arabidopsis thaliana (L.). Plant Sci. 164, 195–202 (2003). [Google Scholar]
- Gill S. S. & Tuteja N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930 (2010). [DOI] [PubMed] [Google Scholar]
- Yadav S. K., Singla-Pareek S. L., Ray M., Reddy M. K. & Sopory S. K. Transgenic tobacco plants overexpressing glyoxalase enzymes resist an increase in methylglyoxal and maintain higher reduced glutathione levels under salinity stress. FEBS Lett. 579, 6265–6271 (2005). [DOI] [PubMed] [Google Scholar]
- Hossain M. A., Hossain M. Z. & Fujita M. Stress induced changes of methylglyoxal level and glyoxalase I activity in pumpkin seedlings and cDNA cloning of glyoxalase I gene. Aust. J. Crop Sci. 3, 53–64 (2009). [Google Scholar]
- Saito R., Yamamoto H., Makino A., Sugimoto T. & Miyake C. Methylglyoxal functions as Hill oxidant and stimulates the photoreduction of O2 at photosystem I: a symptom of plant diabetes. Plant Cell Environ. 34, 1454–1464 (2011). [DOI] [PubMed] [Google Scholar]
- Thornalley P. J. Glyoxalase I-structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 31, 1343–1348 (2003). [DOI] [PubMed] [Google Scholar]
- Kaur C., Ghosh A., Pareek A., Sopory S. K. & Singla-Pareek S. L. Glyoxalases and stress tolerance in plants. Biochem. Soc. Trans. 42, 485–490 (2014). [DOI] [PubMed] [Google Scholar]
- Singla-Pareek S. L., Yadav S. K., Pareek A., Reddy M. K. & Sopory S. K. Transgenic tobacco overexpressing glyoxalase pathway enzymes grow and set viable seeds in zinc-spiked soils. Plant Physiol. 140, 613–623 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez Viveros M. F., Inostroza-Blancheteau C., Timmermann T., González M. & Arce-Johnson P. Overexpression of Gly I and Gly II genes in transgenic tomato (Solanum lycopersicum Mill.) plants confers salt tolerance by decreasing oxidative stress. Mol. Biol. Rep. 40, 3281–3290 (2013). [DOI] [PubMed] [Google Scholar]
- El-Shabrawi H. et al. Redox homeostasis, antioxidant defense, and methylglyoxal detoxification as markers for salt tolerance in Pokkali rice. Protoplasma 245, 85–96 (2010). [DOI] [PubMed] [Google Scholar]
- Upadhyaya C. P. et al. Transgenic potato overproducing L-ascorbic acid resisted an increase in methylglyoxal under salinity stress via maintaining higher reduced glutathione level and glyoxalase enzyme activity. Biotechnol Lett. 33, 2297–2307 (2011). [DOI] [PubMed] [Google Scholar]
- Hossain M. A., Piyatida P., Teixeira da Silva J. A. & Fujita M. Molecular mechanism of heavy metal toxicity and tolerance in plants: central role of glutathione in detoxification of reactive oxygen species and methylglyoxal and in heavy metal chelation. J. Bot. 2012, 1–37 (2012). [Google Scholar]
- Küpper H., Götz B., Mijovilovich A., Küpper F. C. & Meyer-Klaucke W. Complexation and toxicity of copper in higher plants. I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol. 15, 702–714 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins L. L. et al. Response to oxidative stress induced by cadmium and copper in tobacco plants (Nicotiana tabacum) engineered with the trehalose-6-phosphate synthase gene (AtTPS1). Acta Physiol. Plant. 36, 755–765 (2014). [Google Scholar]
- Fernandez O., Béthencourt L., Quero A., Sangwan R. S. & Clément C. Trehalose and plant stress responses: friend or foe? Trends Plant Sci. 15, 409–417 (2010). [DOI] [PubMed] [Google Scholar]
- Luo Y., Li F., Wang G. P., Yang X. H. & Wang W. Exogenously-supplied trehalose protects thylakoid membranes of winter wheat from heat-induced damage. Biol. Plant. 54, 495–501 (2010). [Google Scholar]
- Ali Q. & Ashraf M. Induction of drought tolerance in maize (Zea mays L.) due to exogenous application of trehalose: growth, photosynthesis, water relations and oxidative defense mechanism. J. Agron. Crop Sci. 197, 258–271 (2011). [Google Scholar]
- Ma C., Wang Z., Kong B. & Lin T. Exogenous trehalose differentially modulate antioxidant defense system in wheat callus during water deficit and subsequent recovery. Plant Growth Regul. 70, 275–285 (2013). [Google Scholar]
- Nounjan N., Nghia P. T. & Theerakulpisut P. Exogenous proline and trehalose promote recovery of rice seedlings from salt-stress and differentially modulate antioxidant enzymes and expression of related genes. J. Plant Physiol. 169, 596–604 (2012). [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Hossain M. A. & Fujita M. Trehalose pretreatment induces salt tolerance in rice (Oryza sativa L.) seedlings: oxidative damage and co-induction of antioxidant defense and glyoxalase systems. Protoplasma 252, 461–475 (2014). [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Seraj Z. I. & Fujita M. Interactive effects of nitric oxide and glutathione in mitigating copper toxicity of rice (Oryza sativa L.) seedlings. Plant Signal. Behav. 10, e9915701–4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary S. P., Oral H. V., Bhardwaj R., Yu J. Q. & Tran L. S. Interaction of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. J. Exp. Bot. 63, 5659–5675 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A. & Choudhuri M. A. Effects of salicylic acid on heavy metal-induced membrane deterioration mediated by lipoxygenase in rice. Biol. plant. 42, 409–415 (1999). [Google Scholar]
- Zeid I. M. Trehalose as osmoprotectant for maize under salinity-induced stress research. J. Agr. Biol. Sci. 5, 613–622 (2009). [Google Scholar]
- Hayat S. et al. Role of proline under changing environments. Plant Signal. Behav. 7, 1456–1466 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackhage C., Huang J.-H., Schaller J., Elzinga E. J. & Dudel E. G. Readily available phosphorous and nitrogen counteract for arsenic uptake and distribution in wheat (Triticum aestivum L.). Sci. Rep. 4, 4944 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman F., Aksoy A., Aydin Z. & Temizgul R. Effects of exogenous glycinebetaine and trehalose on cadmium accumulation and biological responses of an aquatic plant (Lemna gibba L). Water Air Soil Pollut. 217, 545–556 (2010). [Google Scholar]
- Sobrino-Plata J. et al. Differential alterations of antioxidant defenses as bioindicators of mercury and cadmium toxicity in alfalfa. Chemosphere 77, 946–954 (2009). [DOI] [PubMed] [Google Scholar]
- Song W.-Y., Yang H.-C., Shao H.-B., Zheng A.-Z. & Brestic M. The alleviative effects of salicylic acid on the activities of catalase and superoxide dismutase in malting barley (Hordeum uhulgare L.) seedling leaves stressed by heavy metals. CLEAN – Soil, Air, Water 42, 88–97 (2014). [Google Scholar]
- Roxas V. P., Lodhi S. A., Garrett D. K., Mahan J. R. & Allen R. D. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol. 41, 1229–1234 (2000). [DOI] [PubMed] [Google Scholar]
- Shao H. B., Chu L. Y., Shao M. A., Jaleel C. A., & Mi H. M. Higher plant antioxidants and redox signaling under environmental stresses. C. R. Biol. 331, 433–441 (2008). [DOI] [PubMed] [Google Scholar]
- Barrameda-Medina Y., Montesinos-Pereira D., Romero L., Blasco B. & Ruiz J. M. Role of GSH homeostasis under Zn toxicity in plants with different Zn tolerance. Plant Sci. 227, 110–121 (2014). [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Seraj Z. I. & Fujita M. Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251, 1373–1386 (2014). [DOI] [PubMed] [Google Scholar]
- Garcia A. B. et al. Effects of osmoprotectants upon NaCl stress in rice. Plant Physiol. 115, 159–169 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. T. Copper enzymes in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol. 24, 1–15 (1949). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates L. S., Waldren R. P. & Teare I. D. Rapid determination of free proline for water-stress studies. Plant Soil 39, 205–207 (1973). [Google Scholar]
- Heath R. L. & Packer L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stochiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198 (1968). [DOI] [PubMed] [Google Scholar]
- Yu C. W., Murphy T. M. & Lin C. H. Hydrogen peroxide-induces chilling tolerance in mung beans mediated through ABA independent glutathione accumulation. Funct. Plant. Biol. 30, 955–963 (2003). [DOI] [PubMed] [Google Scholar]
- Dutilleul C. et al. Functional mitochondrial complex I is required by tobacco leaves for optimal photosynthetic performance in photorespiratory conditions and during transients. Plant Physiol. 131, 264–275 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths O. W. Determination of glutathione and glutathione disulphide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 106, 207–212 (1980). [DOI] [PubMed] [Google Scholar]
- Doderer A. et al. Purification and characterization of two lipoxygenase isoenzymes from germinating barley. Biochim. Biophys. Acta 112, 97–104 (1992). [DOI] [PubMed] [Google Scholar]
- Mostofa M. G., Fujita M. & Tran L.–S. P. Nitric oxide mediates hydrogen peroxide- and salicylic acid-induced salt tolerance in rice (Oryza sativa L.) seedlings. Plant Growth Regul. DOI 10.1007/s10725-015-0061-y (2015). [DOI] [Google Scholar]
- Nakano Y. & Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 22, 867–880 (1981). [Google Scholar]
- Hossain M. A., Nakano Y. & Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in the regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 25, 385–395 (1984). [Google Scholar]
- Foyer C. H. & Halliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta 133, 21–25 (1976). [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976). [DOI] [PubMed] [Google Scholar]
- Li Z. G., Luo L. J. & Zhu L. P. Involvement of trehalose in hydrogen sulfide donor sodium hydrosulfide-induced the acquisition of heat tolerance in maize (Zea mays L.) seedlings. Bot. Stud. 55, 20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild R., Ooi L., Srikanth V. & Münch G. A quick, convenient and economical method for the reliable determination of methylglyoxal in millimolar concentrations: the N-acetyl-L-cysteine assay. Anal. Bioanal. Chem. 403, 2577–2581 (2012). [DOI] [PubMed] [Google Scholar]