Abstract
Background and Objectives
This study was designed to test whether a brief quantitative sensory testing (QST) assessment could be used to detect hyperalgesia in patients with suspected opioid-induced hyperalgesia.
Methods
Twenty patients on long-term opioid therapy with suspected opioid-induced hyperalgesia were recruited along with and 20 healthy controls. Pressure pain threshold, Pain50, a measure of intermediate suprathreshold pressure pain sensitivity, and tolerance levels, were evaluated. As a secondary outcome, changes in pressure pain sensitivity following intravenous administration of placebo (saline) and fentanyl (1.5 μg/kg) were assessed.
Results
There were no significant differences in pain measures between healthy controls and patients. However, there was an association between higher doses of opioids and having a lower pain tolerance (r= -0.46, P=0.041) and lower Pain50 (r=-0.46, P = 0.044), which was consistent with the hypothesis. Patients on >100 mg oral morphine equivalents (OME) displayed decreased pressure pain tolerance compared to patients taking <100 mg OME (P = 0.042). In addition, male patients showed a hyperalgesic response to fentanyl administration, which was significant for the Pain50 measure (P=0.002).
Conclusions
Whereas there were no differences between patients suspected of having opioid-induced hyperalgesia and the healthy controls, the finding that higher doses of opioids were associated with more sensitivity suggests that dose might be an important factor in the development of hyperalgesia. In addition, male patients demonstrated a hyperalgesic response after a bolus of fentanyl. Future studies are needed to develop better diagnostics for detecting hyperalgesia in the clinical setting.
Introduction
The estimated number of opioid analgesic prescriptions in the United States increased by 104% from 43.8 million in 2000 to 89.2 million in 2010.1 Opioid diversion, addiction and overdose has been the fastest growing drug problems in the United States prompting the Centers for Disease Control and Prevention to label pharmaceutical opioid overdose as a national epidemic.1,2 Admissions to substance-abuse treatment programs increased by 400% between 1998 and 2008, with prescription opioids being the second most prevalent type of abused drug after marijuana.3 Compounding the problem is that the intended result of improving chronic non-malignant pain has not been achieved.4 Our group previously demonstrated that patients on opioids with persistently high pain scores reported a phenotype consistent with having a more centralized pain state, which suggests the potential presence of opioid-induced hyperalgesia (OIH).5
OIH has been well demonstrated in the preclinical literature6 and is defined in animal studies as a decrease in pain threshold from baseline after single or repeated administration of opioids.7; 8 Clinically, OIH is characterized by (1) an increase in pain intensity over time, (2) the spreading of pain to other locations beyond the initial painful site, and (3) an increase in pain sensation to external stimuli.9 However, there is still no accepted clinical method to diagnose OIH or differentiate it from pharmacological tolerance, including no accepted standard for OIH-specific quantitative sensory testing (QST). In addition, there are limited data as to how patients with presumed OIH respond to an opioid challenge test using QST. Studies have examined pain sensitivity in patients with opioid addiction maintained on methadone using thermal-, electrical-, and pressure-based models of QST.10 These studies show a modality-specific increased sensitivity to cold pressor pain compared with matched or healthy controls. In contrast, hyperalgesia to electrical pain was weak or absent as was hyperalgesia in mechanically evoked pain models.10 Clinical data supporting OIH are still sparse, and the appropriate method of QST to potentially detect OIH remains unclear.9
Given the limited efficacy of long-term opioid management and concern for harm, many patients are being referred to pain clinics for opioid detoxification programs; however, data to drive these recommendations are limited. Malinoff and colleagues previously observed that the use of buprenorphine (a partial mu agonist and kappa antagonist11) in the detoxification of chronic pain patients from high-dose mu opioid agonists resulted in a significant decrease in pain reporting and improved functional capacity and mood in a number of patients.12 One of the proposed reasons for the decrease in pain scores may be due to the amelioration of OIH through the use of buprenorphine. One of the proposed mechanisms for OIH may occur via the action of dynorphin at the kappa receptor which is inhibited by buprenorphine.13 The primary objective of this study was to assess evoked pressure pain in chronic pain patients with presumed OIH that were referred for opioid cessation or transition to buprenorphine therapy compared to a healthy control cohort. This was done using a QST device designed for clinic settings.14 As a secondary outcome, we measured pressure pain sensitivity before and after an acute opioid challenge with intravenous fentanyl injection in both groups. We hypothesized that patients having features of OIH would exhibit increased pain sensitivity, as well as a decreased analgesic response to the IV fentanyl challenge, in comparison with healthy, opioid-naive controls.
Methods
The study was approved by the Institutional Review Board at the University of Michigan Medical School (Ann Arbor, MI). Written informed consent was obtained from all participants.
Study setting and participants
Twenty long-term opioid-using patients being treated at the Back and Pain Center (Department of Anesthesiology, Division of Pain Medicine, Ann Arbor, MI) and 20 healthy controls were recruited for this study. All patients had been maintained on opioids for at least 4 months, with the majority reporting opioid use for more than 1 year. The experimental group were part of a longitudinal study evaluating patients transitioning off full mu agonist opioids and on to buprenorphine in order to treat suspected OIH. Healthy participants completed the baseline visit only. Patients were evaluated by 1 of 2 physicians with expertise in managing addiction and OIH (HM or DB) as part of their standard clinic practice. If it was recommended that the patient stop full mu agonist opioids and transition to buprenorphine based on the clinical suspicion of OIH, the patient was informed that he/she may be eligible to participate in a study. Clinical suspicion included an increase in pain intensity over time despite the chronic use of opioids and the spreading of pain to other locations beyond the initial painful site. Interested patients were contacted by a study team member either in person at the clinic or via telephone and screened for study eligibility. The healthy controls were recruited through flyers posted on the University of Michigan (UM) campus and UM clinical studies'Website. Participants were paid $100.
Eligible participants were between the ages of 18-65 and had to be able to understand and be willing to cooperate with all study procedures. Exclusion criteria for all participants included BMI > 40, medical conditions capable of causing patients' symptoms and/or would make it unsafe for them to take part in the study (including, but not limited to autoimmune/inflammatory diseases, cardiopulmonary disorders, uncontrolled endocrine disorders, malignancy, pregnancy, or breast feeding), untreated active addiction to illicit substances, history of consistent alcohol consumption exceeding 7 drinks/week for females or 14 drinks/week for males, severe psychiatric illness, and a prior history of allergies or intolerance to buprenorphine or fentanyl. In addition to the above exclusion criteria, healthy controls were excluded if they endorsed concurrent use of benzodiazepine medications or sedative hypnotics, current smoking, and any current or prior history of chronic pain. Participants had a driver to take them home after the fentanyl challenge.
Study Procedures
All participants were evaluated at the research lab at the Back and Pain Center. Participants were instructed not to eat anything 6 hours prior to the baseline visit and not to drink any liquids 3 hours prior to the visit. Female participants were given a pregnancy test to verify that they were not pregnant. Participants were weighed in order to calculate fentanyl dose (1.5 μg per kg). In the first part of the study, participants completed study questionnaires (see Pain Phenotyping below). Once these were completed, a board certified anesthesiologist took a medical history to assess for potential contraindications to an intravenous fentanyl challenge. Next, the physician placed an intravenous line into the participant's non-dominant arm.
Pain Phenotyping
Demographic variables were collected and current medications were recorded. The average daily dose of opioids was obtained and converted to oral morphine equivalents (OME)15; 16 (SDC Table 1). In addition, patients were phenotyped using a battery of validated self-report measures, including pain severity and pain interference (The Brief Pain Inventory [BPI]),17 fibromyalgia survey score,18 neuropathic pain descriptors (PainDETECT [PDQ]),19 depressive and anxiety symptoms (Hospital Anxiety and Depression Scale [HADS])20 pain catastrophizing (Coping Strategies Questionnaire),21 sleep disturbance (PROMIS Sleep Disturbance Short Form Questionnaire).22; 23 fatigue (PROMIS Fatigue Questionnaire 7),22; 23 and physical function (PROMIS Physical Function Questionnaire).22; 23
Table 1. Baseline descriptive data.
| Healthy Controls | Patients | P-value | |
|---|---|---|---|
| N = 20 | N = 20 | ||
| Demographics | |||
|
| |||
| Age | 29.2 (13.2) | 44.5 (9.2) | < 0.001 |
| Sex (% Male) | 50% | 35% | 0.337 |
| Race (% Caucasian) | 61% | 95% | 0.022 |
|
| |||
| Pain Phenotype | |||
|
| |||
| Pain Severity (BPI) | 0.1 (0.2) | 6.2 (1.3) | < 0.001 |
| Neuropathic pain (PainDETECT) | 0.3 (1.0) | 19.7 (7.4) | < 0.001 |
| Fibromyalgia Survey Score | 1.1 (1.2) | 14.2 (7.6) | < 0.001 |
| Catastrophizing | 1.5 (2.7) | 17.8 (8.4) | < 0.001 |
| Depressive symptoms (HADS) | 0.9 (1.1) | 9.2 (3.8) | < 0.001 |
| Anxiety symptoms (HADS) | 1.5 (1.7) | 9.4 (3.7) | < 0.001 |
| Fatigue (PROMIS) | 10.1 (1.0) | 24.3 (5.2) | < 0.001 |
| Sleep (PROMIS) | 12.3 (3.0) | 33.3 (5.7) | < 0.001 |
The patient group was significantly older and had a higher percentage of Caucasians. As expected, the patient group also reported a significantly worse pain phenotype.
Note: Means and standard deviations are presented for continuous variables, and percentages are presented for dichotomous variables. Mean differences assessed using t-test, and percent differences were assessed using the chi-square test.
BPI = Brief Pain Inventory; HADS = Hospital Anxiety and Depression Scale; PROMIS = Patient Reported Outcomes Measurement Information System.
Quantitative Sensory Testing (QST)
The Multi-modal Automated Sensory Testing (MAST) System was used to assess pressure pain sensitivity. The MAST device is a small, portable device designed for research, as well as potential point-of-care applications.14; 24; 25 It applies discrete pressure stimuli to the thumbnail bed. Our group has extensive experience using thumbnail pressure as an evoked pain stimulus and its validity in the measurement of pain sensitivity has been discussed extensively.26,27; 28
The MAST System features a control computer that coordinates testing protocols and program execution. A second computer displays pain rating scales and captures participant feedback at predetermined times on a touch screen monitor. A wireless thumbnail stimulator serves as an actuator device to evoke pressure pain. Both the operator and the participant have a stop button and are able to stop the testing at any point. The thumbnail stimulator applies blunt force, delivered by a 1 cm2 rubber probe, to the thumbnail bed. The probe is attached to a cylindrical transducer housed inside a plastic joystick designed to be held comfortably in either hand. The transducer is driven by a miniature servo-motor and a digital load-cell measures the exact pressure applied to the thumb to ensure accurate and repeatable testing. After the intravenous line was placed, participants received training on how to use the MAST device including a series a practice pressures before starting data collection. QST was conducted three times in the following order: before IV infusions (baseline), after saline placebo administration, and after fentanyl administration (see below). The MAST system delivered an ascending series of discrete 5-s duration stimuli to the patient's dominant thumbnail beginning at 0.50 kg/cm2 and increasing 0.50 kg/cm2 steps up to the patient's pain tolerance or to a maximum of 10 kg/cm2. This test was designed to be brief for implementation in clinical settings and is typically completed within 5-6 minutes. The pain intensity evoked by each pressure was rated on a 0-100 numerical rating scale (0 = no sensation, 20 = just noticeable pain, and 100 = worst pain imaginable) displayed on the touch screen monitor. Participants used a stylist or their finger to tap the touch screen and select their pain rating. They then hit a “confirm” button to proceed to the next pressure. Subsequent pressures were delivered 20 seconds after each rating confirmation. Stimulus-response curves were constructed from each test and three measures were derived: pain (1) Threshold - first pressure sensation rated as painful (i.e., first rating ≥ 20/100); (2) Pain50-pressure that evoked an intermediate suprathreshold pain intensity rating halfway between pain threshold and tolerance and (3) Tolerance - the maximum pressure tolerated, or rated ≥ 80/100, or a maximum of 10 kg/cm2. The primary outcome of the study was differences in pressure pain sensitivity between suspected OIH patients and healthy controls.
Fentanyl Challenge
As a secondary outcome of this study, changes in pressure pain sensitivity following a placebo injection and a fentanyl challenge were assessed. Following the baseline QST assessment, there was a 3-minute rest period before beginning the fentanyl challenge. The participants first received an intravenous bolus of 3 mL of saline (placebo) over 1 minute to which the patient, but not the investigator, was blinded. Five minutes post-injection, post-placebo MAST testing began. Thus participants had a minimum of 9 minutes of rest between MAST tests. Once post-placebo MAST testing was completed, there was another 3-minute rest period.
The fentanyl challenge consisted of the administration of a 1.5 μg/kg dose of fentanyl (50 μg/mL) intravenously over one minute. This dosage was selected based on Stoelting's recommendation of 1-2 μg/kg intravenous as the dose associated with analgesia.29 MAST testing began 5 minutes following the injection of fentanyl. All subjects were required to meet the University of Michigan's Back and Pain Center's criteria for being discharged before they left with a driver.
Statistical Analysis
The primary outcome analysis was the baseline QST comparisons between the suspected OIH (patient) group and the healthy control group. Demographic and pain phenotype variables were compared between groups using t-tests or chi squared tests as appropriate. Univariate differences in QST by participant type were analyzed using independent samples t-tests. Multivariate linear regressions were also conducted to test for differences in baseline QST by participant type, controlling for age and sex. To explore the influence of patients' opioid use on QST results, correlations between OME values and QST outcomes were conducted. Given existing data suggesting that patients on doses of >100 mg OME are clinically different and have greater morbidity and mortality,30 differences in QST outcomes between patients taking < 100 mg OME, patients taking >100 mg OME, and healthy controls were assessed with ANOVA followed by Tukey post hoc comparisons.
A secondary analysis of QST response to a fentanyl challenge was conducted by creating difference scores for pressure pain threshold, Pain50, and tolerance, by subtracting the placebo score from the fentanyl score. The difference scores can be interpreted such that a difference score greater than 0 means the person's score increased from placebo to fentanyl (analgesia). Difference scores less than 0 indicate the person's score went down from placebo to fentanyl (hyperalgesia). Mean differences in difference scores by participant type, gender, and their interaction were assessed using ANOVA. Significant interactions were further analyzed with paired contrasts using Bonferroni adjusted P values of .025 to correct for the 2 contrasts conducted after each significant interaction. Data were analyzed using Stata version 13.1 (StataCorp LP, College Station, TX) and SPSS 22 (IBM, Armonk, NY).
Results
Demographics and Pain Phenotype
A total of 40 subjects were recruited with 20 in the healthy group and 20 in the patient group. The characteristics of each group are outlined in Table 1. There was a significant difference in age between the groups with the healthy group averaging 29.2 years versus 44.5 for the patient group (P< 0.001). There was also a significant difference in race between the groups with the healthy group being 61% Caucasian versus patients being 94.7% Caucasian (P< 0.05). As expected, there were significant differences between the groups with the patient group reporting higher pain severity, more neuropathic pain, and higher fibromyalgia survey criteria scores. Patients also reported a higher level of depression and anxiety, higher catastrophizing scores, more fatigue, and worse sleep when compared with the healthy control group (P< 0.001 for each comparison).
Baseline Quantitative Sensory Testing
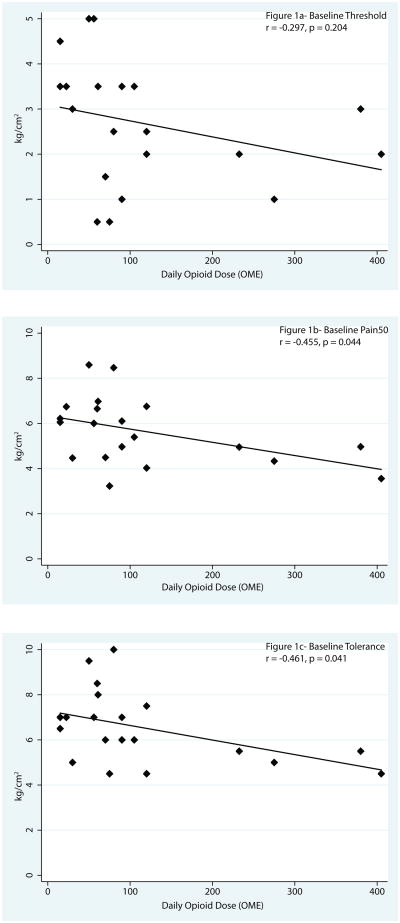
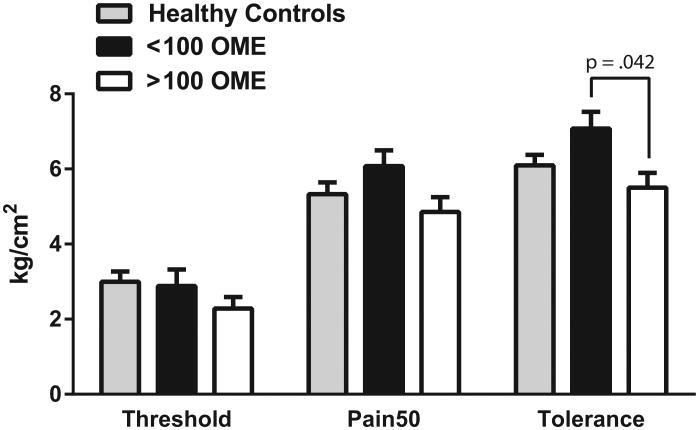
There were no differences in mean pressure pain threshold, Pain 50, or tolerance between patients and healthy controls (Table 2). Moreover, there were still no differences between groups when controlling for age and sex (Table 3). In a secondary analysis of the patient group, an association was detected for baseline QST and daily opioid dose (OME). Higher doses of opioids were associated with lower Pain50 (r (18) = -0.455, P = 0.044) and lower pain tolerance (r(18) =-0.461, P = 0.041), indicating increased suprathreshold pressure pain sensitivity with higher opioid dosing (Figure 1). When controlling for age and gender, there was still a trend towards patients on higher opioid doses being more pain sensitive (not shown). ANOVA comparing patients taking <100 mg OME, patients taking >100 mg OME, and healthy controls revealed that patients taking >100 mg OME had significantly lower pain tolerance than patients taking <100 mg OME (P = 0.042) (Fig. 2). There were no differences between either group of patients and healthy controls; however, there was a non-significant pattern of patients on <100 mg OME exhibiting decreased pressure pain sensitivity compared to controls, whereas those on the >100 mg OME were more sensitive than controls (Fig. 2).
Table 2. Quantitative Sensory Testing Results.
There were no differences detected in the mean (standard deviation) quantitative sensory testing (QST) results between suspected opioid-induced hyperalgesia (OIH) patients and healthy controls.
| Healthy | Patients | P-value | |
|---|---|---|---|
| N = 20* | N = 20 | ||
| Baseline QST | |||
|
| |||
| Threshold | 3.0 (1.2) | 2.7 (1.4) | 0.432 |
| Pain50 | 5.3 (1.4) | 5.7 (1.5) | 0.489 |
| Tolerance | 6.1 (1.2) | 6.5 (1.6) | 0.353 |
|
| |||
| Post-placebo QST | |||
|
| |||
| Threshold | 2.6 (1.1) | 2.9 (1.5) | 0.499 |
| Pain50 | 4.9 (1.7) | 5.7 (1.7) | 0.130 |
| Tolerance | 5.8 (1.7) | 6.6 (1.8) | 0.173 |
|
| |||
| Post-fentanyl QST | |||
|
| |||
| Threshold | 3.0 (1.3) | 3.6 (1.9) | 0.328 |
| Pain50 | 5.6 (2.0) | 6.0 (2.0) | 0.589 |
| Tolerance | 6.4 (1.9) | 6.9 (2.0) | 0.422 |
All quantitative sensory testing (QST) values listed are kg/cm2.
Note that 2 healthy participants do not have data included for placebo & fentanyl QST.
Table 3. Baseline Tolerance, Threshold, and Pain50 controlling for age and gender.
In this multivariate linear regression model controlling for age and sex, there were no differences between healthy controls and patients for measures of evoked pain.
| Threshold | |||
|---|---|---|---|
|
| |||
| Coefficient | SE | P-value | |
|
| |||
| Participant type | 0.37 | 0.47 | 0.439 |
| Age | -0.05 | 0.02 | 0.012 |
| Sex | -0.11 | 0.39 | 0.786 |
| Intercept | 4.41 | 0.61 | <0.001 |
|
| |||
| Pain50 | |||
|
| |||
| Coefficient | SE | P-value | |
|
| |||
| Participant type | 0.76 | 0.55 | 0.180 |
| Age | -0.02 | 0.02 | 0.240 |
| Sex | 0.43 | 0.46 | 0.361 |
| Intercept | 5.83 | 0.72 | <0.001 |
|
| |||
| Tolerance | |||
|
| |||
| Coefficient | SE | P-value | |
|
| |||
| Participant type | 0.77 | 0.55 | 0.176 |
| Age | -0.02 | 0.02 | 0.387 |
| Sex | 0.44 | 0.46 | 0.349 |
| Intercept | 6.41 | 0.72 | <0.001 |
Figure 1. Relationship between baseline quantitative sensory testing (QST) outcomes and opioid dose.
The relationship between the three baseline QST outcomes and the total opioid dose (oral morphine equivalents [OME]) in patients with suspected opioid induced hyperalgesia.
Figure 2. Baseline quantitative sensory testing results in healthy controls and patients.
Patients were divided by dose to those with average daily dosing above and below 100mg oral morphine equivalents (OME). There were no differences between healthy controls and either patient group; however, the patients in the >100 mg OME were significantly more sensitive for tolerance and showed similar trends for threshold and Pain50. When observing all 3 groups, a pattern was observed to show patients <100 mg OME being more pain tolerant and those >100 mg OME being more pain sensitive when compared to the healthy controls.
Fentanyl Challenge
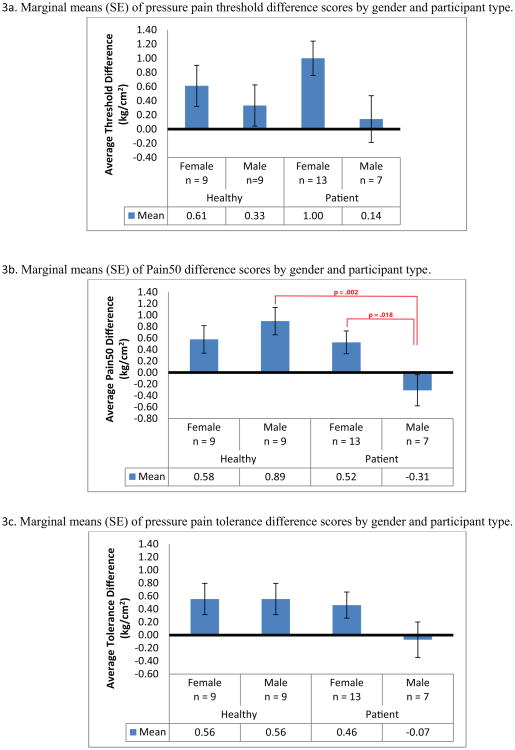
Grouped QST data following placebo and fentanyl administration are presented in Table 2. There were no differences in pressure pain sensitivity between groups following either injection. Fentanyl-induced changes in pressure pain sensitivity were also assessed across gender. As shown in Figure 3, male patients alone exhibited increased pain sensitivity to fentanyl administration (post-fentanyl minus post-placebo) on Pain50 and tolerance. There was a significant interaction of participant type and sex on Pain50 only (P = 0.021). Paired contrasts revealed significant differences between healthy males and male patients on Pain50 difference scores (p = 0.002) such that while healthy males' Pain50 scores increased from placebo to fentanyl, male patients' Pain50 scores decreased, which is consistent with a hyperalgesic response. Paired contrasts revealed a similar difference between male patients and female patients on Pain50 difference scores (p = 0.018), with male patients' Pain50 scores decreasing (hyperalgesia) from placebo to fentanyl and female patients' scores increasing (analgesia). No group differences were observed on pressure pain thresholds following fentanyl administration. Note that 2 healthy participants were removed from this analysis because they did not follow the same fentanyl challenge protocol as the remaining 38 participants.
Figure 3. Change in quantitative sensory testing (QST) after the fentanyl challenge.
The figure shows the changes in the 3 QST outcomes when subtracting the post-fentanyl challenge results from the post-placebo challenge. Male patients demonstrated a hyperalgesic response to the fentanyl challenge (change score < 0) when compared with healthy males and female patients.
Discussion
The primary objective of this study was to assess experimentally evoked pain sensitivity in chronic pain patients on opioids who have been referred for opioid cessation or transition to buprenorphine therapy for presumed OIH. Contrary to our hypothesis, we found no differences in pressure pain sensitivity between patients and healthy controls (Table 2, Fig. 2). Although this group difference was not significant, we did see an intriguing pattern in the evoked pain responses when dividing patients into high (>100 mg OME) and low (<100 mg OME) dose opioid subgroups. Specifically patients on lower doses were less sensitive when compared with healthy individuals, while those on higher doses were more sensitive when compared with the healthy subjects (Fig. 2). Consistent with our hypothesis, patients on higher doses were significantly more sensitive in Pain50 and pain tolerance (Figure 1b and 1c), while there was a non-significant trend in the same direction for pain threshold (Fig. 1a). Whereas the total number of participants is small and the age differences between groups make comparisons challenging, these data suggest that a correlation exists between opioid dose and the development of OIH. Studies supporting the development of OIH in patients with chronic pain are generally lacking. Our results, however, are consistent with one study in chronic pain patients which suggests an association between opioid dosage and the development of OIH.31 In this study, chronic pain patients were not on opioids at the start of the study and were given opioids over a 4-week period. They found a positive correlation with opioid dose and OIH for heat pain intensity when compared with controls. In our study, patients had both chronic pain and were on opioids for > 4 months prior to participation and the OME covered a much larger dosage spectrum. Clinical experience suggests that some patients on higher doses describe symptoms of hyperalgesia and improve after opioid cessation.12 These data suggest that dose may be an important factor to consider when assessing a patient for OIH.
Although OIH has been shown to exist in animals, the existence for this in humans has been a source of debate. For example, a recent systematic review showed that a number of studies using a variety of different QST modalities and measures including cold pain, heat pain, pressure pain, electrical pain, ischemic pain, and injection pain were not capable of detecting hyperalgesia in chronic pain patients on long-term opioids.9 However, only a few of these clinical studies included individuals on the very high doses of opioids being taken by many of the participants in this study, and those are amongst the few cross-sectional studies that demonstrated hyperalgesia. Moreover, only a few of these clinical studies performed QST prior to and following opioid administration (fentanyl challenge) similar to the preclinical studies which defined OIH in animals as a decrease in pain threshold from baseline after administration of opioids.32
We were able to find a significant association between higher doses of opioids and having a lower Pain50 and pain tolerance. Most QST devices/procedures are designed for laboratory research and are not practical or too burdensome for use in routine clinical care. The rationale for testing pressure pain sensitivity with the MAST system in this study was its compact and user-friendly design and its ability to conduct a fully computer-automated test procedure in 10 minutes or less, which could potentially allow for use in a clinical setting. The ability to utilize an office-based protocol as demonstrated above may allow clinicians to identify patients with opioid induced hyperalgesia and alter the treatment plan accordingly.
Male Patients Demonstrated Hyperalgesia Following a Fentanyl Bolus
As a secondary outcome, we measured pressure pain sensitivity before and after an opioid challenge with intravenous fentanyl administration in both healthy controls and patients. To our knowledge, this is the first time that such a test has been conducted on chronic pain patients with long-term opioid use. We hypothesized that patients having features of OIH would show a decreased analgesic response to the IV fentanyl challenge in comparison with healthy, opioid naive controls. In the analysis of the results of the fentanyl challenge, we showed that pressure pain sensitivity increased (hyperalgesic response) going from placebo to fentanyl in male patients only (Figure 3). In contrast, pressure pain sensitivity decreased (analgesic response) following fentanyl administration in females and healthy males.
Our results suggest that there may be a gender-specific response to hyperalgesia in males taking chronic opioids. This has not been described in humans before to our knowledge. This is in contrast to the finding in animal studies that have shown that female rats have been found to have a decreased tail-flick latency and hyperalgesia to mechanical stimuli after acute administration of subcutaneous morphine compared to male rats. 33; 34 However, one needs to be cautious about applying the animal literature to humans. In rodents, opioids with mu agonist activity were more effective in males than females, whereas in humans, opioids with mu agonist activity were more effective in females.35 No explanation of this apparent species difference has been proposed. Yet, the fact that the analgesic effects of morphine are more pronounced in male rodents, whereas the hyperalgesic effects appear more pronounced in female rodents seems to indicate a different mechanism involved in the anti-nociceptive and pro-nociceptive processes. In mice it has been shown that ovariectomy abolished the sex differences and resulted in females exhibiting the male-typical pattern whereas ovariectomy with estrogen replacement resulted in a similar opioid induced hyperalgesic response to intact female mice.36 This finding suggests that morphine hyperalgesia in male and female mice is mediated by different neurochemical substrates and that females possess the male typical hyperalgesic mechanism, but are protected by circulating ovarian sex steroids. Further studies have suggested that the sex-specific mediation of morphine-induced hyperalgesia is through NMDA and melanocortin-1 receptors in male and female mice respectively.37 Whether similar receptors are involved in humans, but with a species specific response, or different sex linked receptors are involved to account for the hyperalgesic response in males is unclear.
Limitations
There are limitations of our study. The most significant is that the sample size is small with only 20 participants per group. This is a challenging patient population to recruit and although the sample size is small, trends in the data suggest that a larger study could reveal important results. Another limitation of the study was that the patient population and the sample population were significantly different in terms of both age and race. It has been shown that algometric mechanical pain thresholds increase with age,38 although in our statistical analyses we controlled for age. As was previously discussed, the lack of a gold standard for clinical OIH diagnosis also limits the interpretation of these findings.
Conclusions
In the present study, we were unable to detect a difference in measures of evoked pain between healthy controls and patients with suspected OIH. However, we did observe a relationship between opioid dose and pain sensitivity, such that patients on higher doses of opioids were significantly more sensitive. These data suggests that negative findings of this and previous clinical studies of OIH may be in part due to dose effects. Future studies of larger cohorts are still needed to better elucidate the importance of dose and the potential ways to differentiate patients using phenotype and possibly experimental pain testing.
Supplementary Material
Acknowledgments
The authors would like to thank Kevin K. Tremper, PhD, MD, Professor and Chairman of the Department of Anesthesiology at the University of Michigan, and Daniel Clauw, MD, Professor of Anesthesiology, Medicine (Rheumatology) and Psychiatry and Director of the Chronic Pain and Fatigue Research Center at the University of Michigan, for guidance and support, as well as the staff at the Back and Pain Center.
Financial Support: The study was supported by a Michigan Institute for Clinical and Health Research Grant, 2UL1TR000433.
Footnotes
Prior Presentation: None
Disclosures: Dr. Brummett receives research funding from Neuros Medical, Inc. (Willoughby Hills, OH) and is a consultant for Tonix Pharmaceuticals (New York, NY). Dr. Hassett has received research funding from and has been a consultant for Bristol-Myers Squibb (New York, NY) and Pfizer (New York, NY). Dr. Harte has received research funding from Cerephex (Palo Alto, CA), Forest Laboratories (New York, NY), and Merck (White House Station, NJ); and serves as a consultant for Pfizer (New York, NY), Analgesic Solutions (Natick, MA), Regeneron (Tarrytown, NY), and deCode Genetics (Reykjavik, Iceland). Dr. Harte is co-inventor of the MAST device used in this study. For the remaining authors, no potential conflicts were declared.
References
- 1.Sites BD, Beach ML, Davis MA. Increases in the use of prescription opioid analgesics and the lack of improvement in disability metrics among users. Regional anesthesia and pain medicine. 2014;39:6–12. doi: 10.1097/AAP.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow NDMT. Curtailing diversion and abuse of opioid analgesics without jeopardizing pain treatment. JAMA. 2011;305:1346–1347. doi: 10.1001/jama.2011.369. [DOI] [PubMed] [Google Scholar]
- 3.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 4.Manchikanti L, Abdi S, Atluri S, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: Part I--evidence assessment. Pain Physician. 2012;15:S1–65. [PubMed] [Google Scholar]
- 5.Wasserman RA, Brummett CM, Goesling J, Tsodikov A, Hassett AL. Characteristics of chronic pain patients who take opioids and persistently report high pain intensity. Reg Anesth Pain Med. 2014;39:13–17. doi: 10.1097/AAP.0000000000000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Angst MS, Clark JD. Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiology. 2006;104:570–87. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 7.Celerier E, Rivat C, Jun Y, et al. Long-lasting hyperalgesia induced by fentanyl in rats: Preventive effect of ketamine. Anesthesiology. 2000;92:465–72. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 8.Van Elstraete AC, Sitbon P, Trabold F, Mazoit JX, Benhamou D. A single dose of intrathecal morphine in rats induces long-lasting hyperalgesia: The protective effect of prior administration of ketamine. Anesth Analg. 2005;101:1750–1756. doi: 10.1213/01.ANE.0000184136.08194.9B. [DOI] [PubMed] [Google Scholar]
- 9.Katz NP, Paillard FC, Edwards RR. Review of the performance of quantitative sensory testing methods to detect hyperalgesia in chronic pain patients on long-term opioids. Anesthesiology. 2015;122:677–685. doi: 10.1097/ALN.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 10.Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14:145–161. [PubMed] [Google Scholar]
- 11.Walsh SL, Eissenberg T. The clinical pharmacology of buprenorphine: Extrapolating from the laboratory to the clinic. Drug Alcohol Depend. 2003;70:S13–27. doi: 10.1016/s0376-8716(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 12.Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12:379–384. doi: 10.1097/01.mjt.0000160935.62883.ff. [DOI] [PubMed] [Google Scholar]
- 13.Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol. 2010;6:191–197. doi: 10.1038/nrrheum.2010.24. [DOI] [PubMed] [Google Scholar]
- 14.Harte SE, Mitra M, Ichesco EA, et al. Development and validation of a pressure-type automated quantitative sensory testing system for point-of-care pain assessment. Med Biol Eng Comput. 2013;51:633–644. doi: 10.1007/s11517-013-1033-x. [DOI] [PubMed] [Google Scholar]
- 15.Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose ratios for opioids. A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672–687. doi: 10.1016/s0885-3924(01)00294-9. [DOI] [PubMed] [Google Scholar]
- 16.McCaffery M, Pasero C. Pain: Clinical Manual. Second. St. Louis, MO: Mosby Inc.; 1999. Opioid Analgesics. [Google Scholar]
- 17.Cleeland CS, Ryan KM. Pain assessment: Global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 18.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: A modification of the ACR preliminary diagnostic criteria for fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 19.Freynhagen R, Baron R, Gockel U, Tolle TR. Paindetect: A new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. 2006;22:1911–20. doi: 10.1185/030079906X132488. [DOI] [PubMed] [Google Scholar]
- 20.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 21.Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: Relationship to patient characteristics and current adjustment. Pain. 1983;17:33–44. doi: 10.1016/0304-3959(83)90125-2. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Riley W, Stone A, et al. The patient-reported outcomes measurement information system (promis) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63:1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cella D, Yount S, Rothrock N, et al. The patient-reported outcomes measurement information system (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med Care. 2007;45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmidt-Wilcke T, Ichesco E, Hampson JP, et al. Resting state connectivity correlates with drug and placebo response in fibromyalgia patients. Neuroimage Clin. 2014;6:252–261. doi: 10.1016/j.nicl.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrepf A, Bradley CS, O'Donnell M, et al. Toll-like receptor 4 and comorbid pain in interstitial cystitis/bladder pain syndrome: A multidisciplinary approach to the study of chronic pelvic pain research network study. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.03.003. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geisser ME, Glass JM, Rajcevska LD, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9:417–422. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Giesecke T, Williams DA, Harris RE, et al. Subgrouping of fibromyalgia patients on the basis of pressure-pain thresholds and psychological factors. Arthritis Rheum. 2003;48:2916–2922. doi: 10.1002/art.11272. [DOI] [PubMed] [Google Scholar]
- 28.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: Effects of stimulus type and mode of presentation. Pain. 2003;105:403–413. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 29.Stoelting RK. Pharmacology and Physiology in Anesthetic Practice. 3rd. Philadelphia, PA: Lippincott-Raven; 1999. Opioid agonists and antagonists. [Google Scholar]
- 30.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305:1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 31.Suzan E, Eisenberg E, Treister R, Haddad M, Pud D. A negative correlation between hyperalgesia and analgesia in patients with chronic radicular pain: Is hydromorphone therapy a double-edged sword? Pain Physician. 2013;16:65–76. [PubMed] [Google Scholar]
- 32.Tompkins DA, Campbell CM. Opioid-induced hyperalgesia: Clinically relevant or extraneous research phenomenon? Curr Pain Headache Rep. 2011;15:129–136. doi: 10.1007/s11916-010-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holtman JR, Jr, Wala EP. Characterization of morphine-induced hyperalgesia in male and female rats. Pain. 2005;114:62–70. doi: 10.1016/j.pain.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Bodnar RJ, Kest B. Sex differences in opioid analgesia, hyperalgesia, tolerance and withdrawal: Central mechanisms of action and roles of gonadal hormones. Horm Behav. 2010;58:72–81. doi: 10.1016/j.yhbeh.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 35.Craft RM. Sex differences in opioid analgesia: “From mouse to man”. Clin J Pain. 2003;19:175–186. doi: 10.1097/00002508-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Juni A, Klein G, Kowalczyk B, Ragnauth A, Kest B. Sex differences in hyperalgesia during morphine infusion: effect of gonadectomy and estrogen treatment. Neuropharmacology. 2008;54:1264–1270. doi: 10.1016/j.neuropharm.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Juni A, Cai M, Stankova M, et al. Sex-specific mediation of opioid-induced hyperalgesia by the melanocortin-1 receptor. Anesthesiology. 2010;112:181–188. doi: 10.1097/ALN.0b013e3181c53849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magerl W, Krumova EK, Baron R, Tolle T, Treede RD, Maier C. Reference data for quantitative sensory testing (QST): Refined stratification for age and a novel method for statistical comparison of group data. Pain. 2010;151:598–605. doi: 10.1016/j.pain.2010.07.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.