Abstract
Background
In Central Europe, cold-induced injuries are much less common than burns. In a burn center in western Germany, the mean ratio of these two types of injury over the past 10 years was 1 to 35. Because cold-induced injuries are so rare, physicians often do not know how to deal with them.
Methods
This article is based on a review of publications (up to December 2014) retrieved by a selective search in PubMed using the terms “freezing,” “frostbite injury,” “non-freezing cold injury,” and “frostbite review,” as well as on the authors’ clinical experience.
Results
Freezing and cold-induced trauma are part of the treatment spectrum in burn centers. The treatment of cold-induced injuries is not standardized and is based largely on case reports and observations of use. A distinction is drawn between non-freezing injuries, in which there is a slow temperature drop in tissue without freezing, and freezing injuries in which ice crystals form in tissue. In all cases of cold-induced injury, the patient should be slowly warmed to 22°–27°C to prevent reperfusion injury. Freezing injuries are treated with warming of the body’s core temperature and with the bathing of the affected body parts in warm water with added antiseptic agents. Any large or open vesicles that are already apparent should be debrided. To inhibit prostaglandin-mediated thrombosis, ibuprofen is given (12 mg/kg body weight b.i.d.).
Conclusion
The treatment of cold-induced injuries is based on their type, severity, and timing. The recommendations above are grade C recommendations. The current approach to reperfusion has yielded promising initial results and should be further investigated in prospective studies.
Patients with frostbite or other cold-induced trauma are treated in burn centers. However, despite the similarity in clinical findings, the differences in etiology and pathophysiology between cold-induced injuries and burns affect the treatment and prognosis. Both types of injury lead to damage or even loss of the skin and the cutaneous and subcutaneous capillary system with planar wounds. Depending on the site and severity of cold-induced injury, the spectrum of treatment extends from conservative management to complex surgical reconstruction. The outcome may be anywhere between full recovery with no consequences and death from the associated hypothermia or infections. The resulting extensive wounds require elaborate sterile treatment. Centers for treatment of severe burns offer the necessary infrastructure and experience. The environment of the patient’s room can be adjusted as required, and there are similarities in the surgical treatment of burns and cold-induced injuries, from superficial removal of dead skin to large-scale amputation.
Cold-induced injuries are divided into frostbite and non-freezing cold injuries (NFCI) (Table 1).
Table 1. Comparison of non-freezing cold injury (NFCI) and frostbite.
| Non-freezing cold injury | Frostbite |
|---|---|
| Caused by slow temperature decrease in affected tissue with hypothermia, no direct frostbite | Formation of ice crystals in tissue |
| Alternating phases of vasoconstriction and vasodilation in protracted hypothermia | Tissue damage is immediate |
| Incomplete damage of tissue, nerve fibers react at early stage | Direct damage of entire tissue by formation of ice crystals |
| Four clinical phases (Table 4) | Course often very protracted, complete recovery rare |
| Blister formation rare | Blister formation in stages II and III |
| Slow rewarming | Rewarming in warm water (37 to 39 °C) for 15 to 60 min |
| Slight loading of the affected area possible, no bandaging necessary | Sterile und protective bandages, topical antiseptics as required, splinting of affected extremities |
Owing to the relatively low prevalence in Central Europe (1 out of every 35 patients in our collective), there are far fewer publications on frostbite and its treatment than on burns; the level of evidence is low. Only a small number of prospective randomized clinical trials have been carried out, with low numbers of cases. The literature consists largely of case reports and observational studies (Table 2).
Table 2. Overview of the relevant literature (January 2009 to December 2014).
| Author | Type of publication | Date of publication | Treatment/goal | Relevant conclusion |
|---|---|---|---|---|
| Saemi et al. (35) | Case report | November 2009 | Intra-arterial administration of tPA after previous papaverine infusion and warming | Complete recovery owing to the combined treatment |
| Imray et al. (2) | Review | September 2009 | Description of the treatment options (NSAR, vasodilators, hyperbaric oxygen treatment, inhibitors of coagulation) | Review of epidemiology, diagnosis, classification, and preclinical and clinical treatment options with differentiation of frostbite from non-freezing cold injury |
| Sheridan et al. (36) | Case report | December 2009 | Angiography of the affected area before and after intra-arterial administration of a vasodilator (nitroglycerin); in persisting underperfusion, repeated slow local administration of tPA via an intra-arterial catheter, systemic heparinization | Recommendation of the treatment strategy on the basis of the excellent clinical outcome |
| Hallam et al. (10) | Review | November 2010 | Presentation of an algorithm for the treatment of acute frostbite | Review of pathophysiology, risk factors, diagnosis; recommendation to administer vasodilattors and tPA |
| Wagner and Pannucci (37) | Case report/review | January 2011 | Intra-arterial administration of tPA after warming; detailed discussion of lysis treatment |
Good clinical result with demonstrated revascularization |
| Cauchy et al. (38) | Original article | January 2011 | Aspirin 250 mg/d + α -adrenoceptor antagonist vs. prostacyclin vs. prostacyclin and tPA |
Best result with administration of prostacyclin; treatment with α -adrenoceptor antagonist significantly inferior to other procedures |
| Johnson et al. (30) | Original article | December 2011 | Intravenous administration of tPA and heparin | Threatened amputation of 73 fingers/ toes reduced to 43: recommendation of lysis treatment; development of a treatment protocol |
| Woo et al. (39) | Original article | September 2013 | NSAR, prostaglandin E1 (PGE1) (alprostadil 5 µg/d) | All patients with second-degree frostbite recovered; two of five patients with third-degree frostbite recovered; others required surgery |
| Kemper et al. (23) | Case report/review | January 2014 | Hyperbaric oxygen treatment | Good clinical result; recommendation to use hyperbaric oxygen treatment as additive procedure |
| Handford et al. (24) | Review | April 2014 | Description of the relevant treatment options (NSAR, vasodilators, hyperbaric oxygen treatment, inhibitors of coagulation) | Administration of vasodilators or lysis recommended in the early phase of treatment (up to 24h) |
NSAR, non-steroidal antirheumatics
Methods
We conducted a selective survey of the literature in PubMed up to December 2014, using the search terms “frostbite injury”, “non-freezing cold injury”, and “frostbite review”. We also drew on our own clinical experience.
Pathophysiology
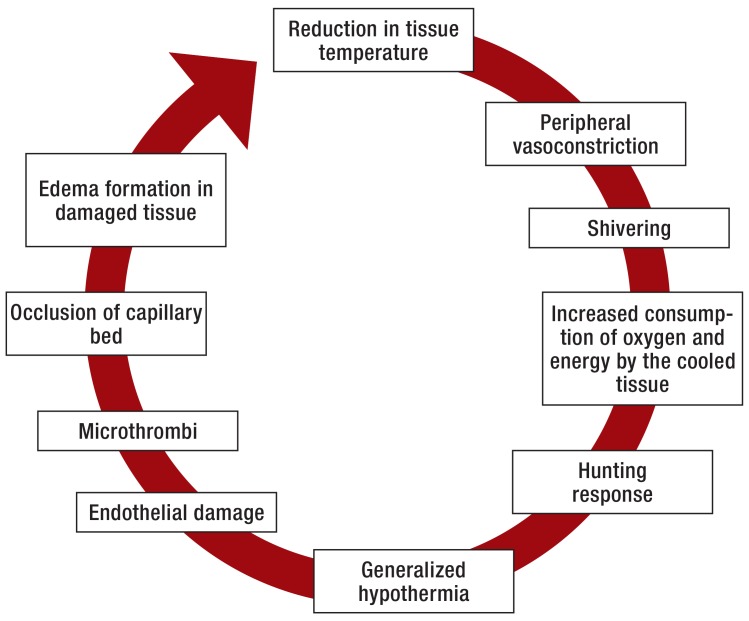
The tissue damage in frostbite is caused by formation of ice crystals (1). The severity of injury ranges from circumscribed superficial cutaneous lesions to necrosis of all layers of the skin (2) (Figures 1 and 2, Table 3). As well as the above-mentioned similarities between frostbite and burns, there are also distinct differences. For example, angiogenesis begins much sooner in frostbite injuries, and inflammation is less accentuated than in burns but persists much longer (3). In contrast to burn injuries, frostbite does not completely destroy the extracellular matrix. Extracellular ice crystals damage the cell membrane, dehydration of the cells ensues, and the result is hyperosmolar cell death (4). Migrating fibroblasts replace the dead cells (3).
Figure 1.
Stages of tissue damage and systemic reaction in protracted hypothermia
Figure 2.
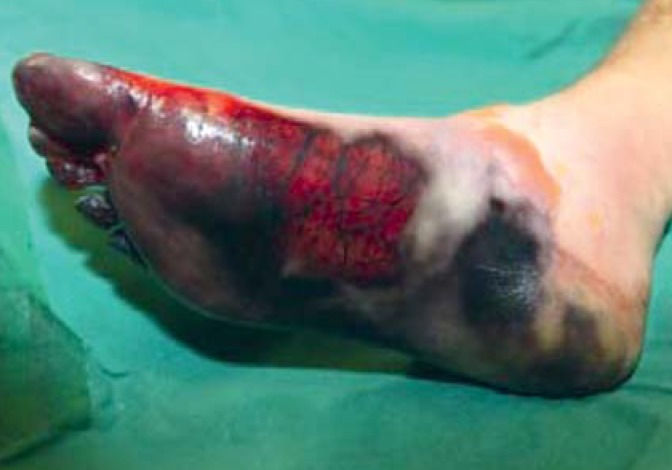
Fourth-degree frostbite of the foot: livid pattern of discoloration, thrombosed subcutaneous veins, and lack of sensation in the skin with slight edema
Table 3. Clinical appearance, morphology and treatment of frostbite according to severity (modified from Imray [2]).
| Degree of severity | Clinical appearance | Morphology | Action | |
|---|---|---|---|---|
| Superficial | I | Reddening, swelling, hyperemia, numbness | Incomplete intradermal frostbite | Warming according to protocol |
| II | Reddening, blisters (clear), superficial erosions, hyperalgesia to the point of loss of sensation |
Complete dermal frostbite | Application of dry protective bandage | |
| Deep | III | Livid discoloration, blisters (hemorrhagic), skin necroses, asensitive |
Complete frostbite of skin and subcutaneous tissue | Transfer to burn center |
| IV | Blotchy dark red/livid to black/necrotic discoloration, slight edema, asensitive | Necrosis of all layers: skin, subcutaneous tissue, muscle, bone | ||
Exposed parts of the body with a thin covering of skin and soft tissues, such as the fingers, toes, ears, and nose, are particularly at risk of frostbite.
In contrast to classic frostbite, NFCI are characterized by a slow decrease in tissue temperature without direct freezing (5, 6). The organism seeks to maintain local perfusion and the systemic temperature by means of a cascade of processes: First come shivering and peripheral vasoconstriction; this increases the susceptibility of the tissue to the low temperature. The greatest vasoconstriction is found at a tissue temperature of 15 °C (5). If the temperature falls further to 10 °C or exposure to cold continues, the vasoconstriction is repeatedly interrupted by phases of vasodilation (5). The extent of this vasodilation, known as “hunting response,” depends on the body temperature and is reduced in hypothermia (7– 9). This represents an attempt by the organism to prevent complete loss of perfusion in the affected area. The result is a decrease in core temperature (2). If the cold exposure persists, endothelial damage ensues with formation of microthrombi and ultimately occlusion of the capillary bed resulting in ischemia, degranulation of mast cells with excretion of histamine and subsequent edema (Figure 1) (2, 10– 16). Warming is followed by ischemia–reperfusion damage (2). Prostaglandin F 2 α (PGF2 α) and thromboxane A2 (TXA2) lead to thrombocyte aggregation and thus extension of the area of ischemia (2).
The principal factor in the development of NFCI is thought to be the persisting vasoconstriction (5). If the nerves are cooled to under 10 °C, they suffer relevant damage (17). Large myelinated fibers are more susceptible than small and unmyelinated fibers (17, e1– e5). The precise pathophysiological foundations of the neural damage have not yet been uncovered (18, 19, e1, e6). Hypothermia of the nerves is held to be responsible for the ensuing damage (Table 4).
Table 4. The four clinical phases that characterize the course of non-freezing cold injury*.
| Phase | Clinical signs |
|---|---|
| Prehyperemic phase | Pale, white skin, disturbances of sensation, loss of deep sensation, occasionally blisters |
| Cyanotic phase | Early phase of rewarming with appearance of pain |
| Hyperemic phase | In the hours after rewarming: pain, swelling, reddening, capillary supply still impaired, petechiae |
| Posthyperemic phase | A phase lasting weeks or months featuring cold sensitivity, pain, hyperhidrosis, and reduced sensation |
* Modified from (2)
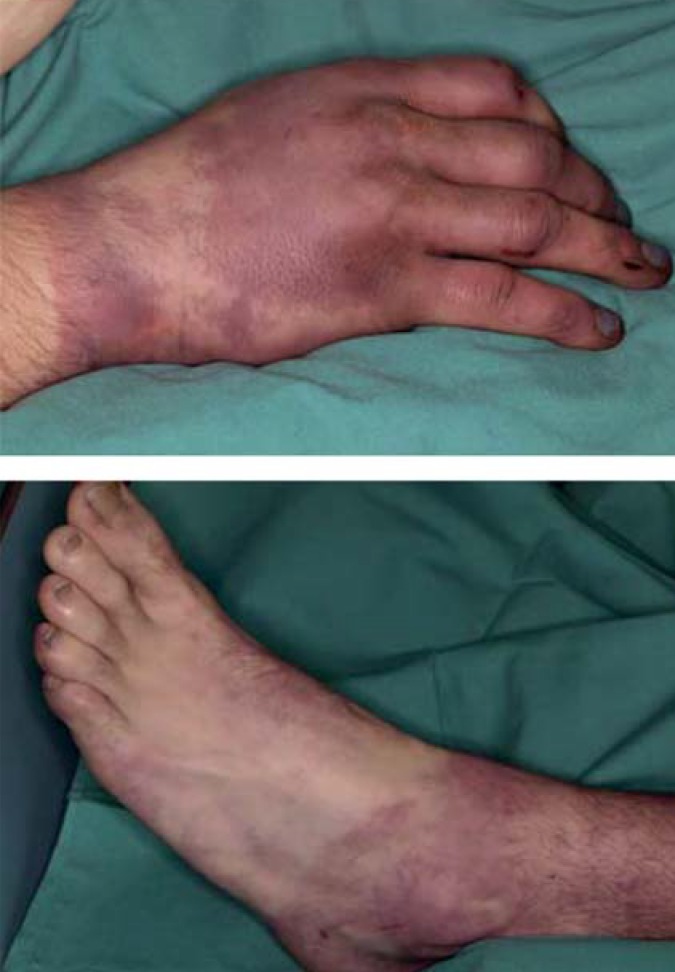
A rough division of cold-induced injuries into superficial and deep injuries, on the basis of the clinical appearance, has become established in clinical routine and in the preclinical setting (Table 2) (20). If the injury is accompanied by formation of blood blisters or full-thickness necrosis, it is classified as deep (Figures 2 and 3).
Figure 3.
Third-degree frostbite of the extremities after 24 h of cold exposure following a road traffic accident with delayed rescue
In the German-speaking countries, however, cold-induced injuries are classically divided into four categories, in analogy to burns (Table 3).
Treatment
The appropriate treatment for frostbite depends on the severity of injury and the time point (Table 3, Figure 3, Box).á
Box. Protocol for initial treatment of frostbite*.
Admit the patient to a burn center
Increase the body core temperature, exclude severe accompanying injuries
Warm the affected part(s) of the body in water at 37 to 39 °C with added antiseptics (polyhexanide, iodine) for 15 to 60 min or until the skin color changes to red/violet
Debride open or large blisters; small, intact blisters can be left untreated
Splint and elevate the affected extremity
Tetanus prophylaxis
Adequate analgesia (use opiates if necessary) and hydration
Ibuprofen 12 mg/kg body weight b.i.d.
Daily bathing in warm water containing antiseptics with active and passive mobilization
Sterile bandages (including between the fingers/toes)
Strictly no smoking
Regular monitoring for reperfusion; if no reperfusion occurs, experimental treatments such as sympathectomy, thrombolytic therapy or vasodilatation are further options to be considered.
*Modified from (2)
In the prehospital phase, the first priority is to rule out severe accompanying injuries, particularly after falls or road traffic accidents. Next, the patient should be transferred to a protected environment in order to minimize the likelihood of further exposure to cold (21). Wet clothing must be removed immediately. The administration of aspirin (75 mg) to inhibit thrombocyte aggregation is controversial because of the non-selective inhibition of prostaglandin synthesis. Ibuprofen (12 mg/kg body weight [BW] b.i.d.) should be given because of its anti-prostaglandin and analgetic effects (evidence level 3) (2), with the principal aim of inhibiting the thrombogenic action of PGF2 α and TXA2. Direct application of heat and warming of the affected part of the body by rubbing are not indicated. The affected area should not be warmed until renewed exposure to cold can be excluded, because alternating freezing and thawing processes can lead to severe thrombosis and ischemia.
In the hospital phase of treatment, the priority is increasing the patient’s body temperature. Once this exceeds 34 °C, local treatment of the frostbite can begin (2).
In the case of NFCI, rapid warming of the involved parts is contraindicated because it usually leads to reperfusion damage. The affected blood vessels are not in the position to supply the injured cells with the required nutrients and remove noxious metabolites (5). In contrast to hypothermia, in which the priority is to increase the body temperature, patients with NFCI should be treated by slow warming of the affected area (5). An air current of 22 to 27 °C is recommended for this purpose (10). In the late phase of NFCI, administration of amitriptyline is indicated for analgesia, beginning with a dose of 10 to 25 mg at night and increasing to a maximum of 100 mg as required in the course of treatment (5).
In the event of acute frostbite, the affected extremity should be bathed for 15 to 60 min in water at a temperature of 37 to 39 °C containing an antibacterial agent. An animal experiment showed that rapid warming was clinically superior to slow warming in rats and dogs (evidence level 2b) (22). In the post-thaw phase, treatment proceeds as laid out in the Box.
Experimental approaches to the treatment of frostbite
Hyperbaric oxygen therapy
The literature on the treatment of frostbite contains no explicit recommendation of hyperbaric oxygen therapy. No superiority with regard to cell death could be demonstrated in controlled experimental animal studies. Individual case reports have described a favorable clinical outcome after hyperbaric oxygen therapy (23). There is insufficient evidence to justify a general treatment recommendation (24).
Sympathectomy
A controlled experimental animal study on rabbit ears showed that sympathectomy within 24 h of injury led to increased cell survival (25). A controlled prospective study in humans—without frostbite—revealed that a peripheral nerve blockade achieved significant increases in skin temperature and perfusion of the fingers. In a series of three cases of frostbite of the fingers, an increase of 0.8 to 1.3 °C in skin temperature was observed after selective sympathectomy (26). Sympathectomy within 24 h of injury thus appears to show promise for the treatment of frostbite. Performed later, however, sympathectomy exerts no positive influence on tissue survival (27). Sympathectomy can be achieved surgically or a catheter can be inserted for selective nerve blockade by means of drugs. In the acute treatment of frostbite injuries, local medicinal plexus blockade is the treatment of choice.
Anticoagulation
The administration of heparin in frostbite is indicated only when no risk factors for deep vein thrombosis are present (28).
Lysis (tPA)
A retrospective study showed that the use of tissue plasminogen activator within 24 h of injury was associated with a reduction in the rate of amputation of fingers or toes from 41% to 10% (29). Another retrospective study, in which 11 patients with scintigraphically confirmed perfusion disorders were treated with tissue plasminogen activator and heparin, reported amputation of 43 out of 73 underperfused fingers or toes threatened by amputation (30). The treatment selected was a bolus dose of 0.15 mg/kg tPA followed by 0.15 mg/kg/h tPA (maximum 100 mg) for 6 h (30, 31). Lysis is contraindicated by accompanying injuries such as fractures and craniocerebral trauma, thrombocytopenia, coagulation disorders, and pregnancy.
Vasodilators
In animal experiments a significant reduction of the tissue damage in frostbite was achieved by combining slow warming with infusion of prostaglandin E1 (PGE1). In contrast, reserpine and the bolus administration of PGE1 showed no therapeutic benefit (32). In a clinical case series of five patients with second-degree and third-degree frostbite of the fingers or toes treated with iloprost (prostaglandin I2), amputation of the affected digits could be avoided. Perfusion was restored (33). In a comparative animal study, isosorbide dinitrate (ISDN) showed improvement in angiogenesis, an increase in vascular diameter, and reduction of edema formation (34).
Discussion
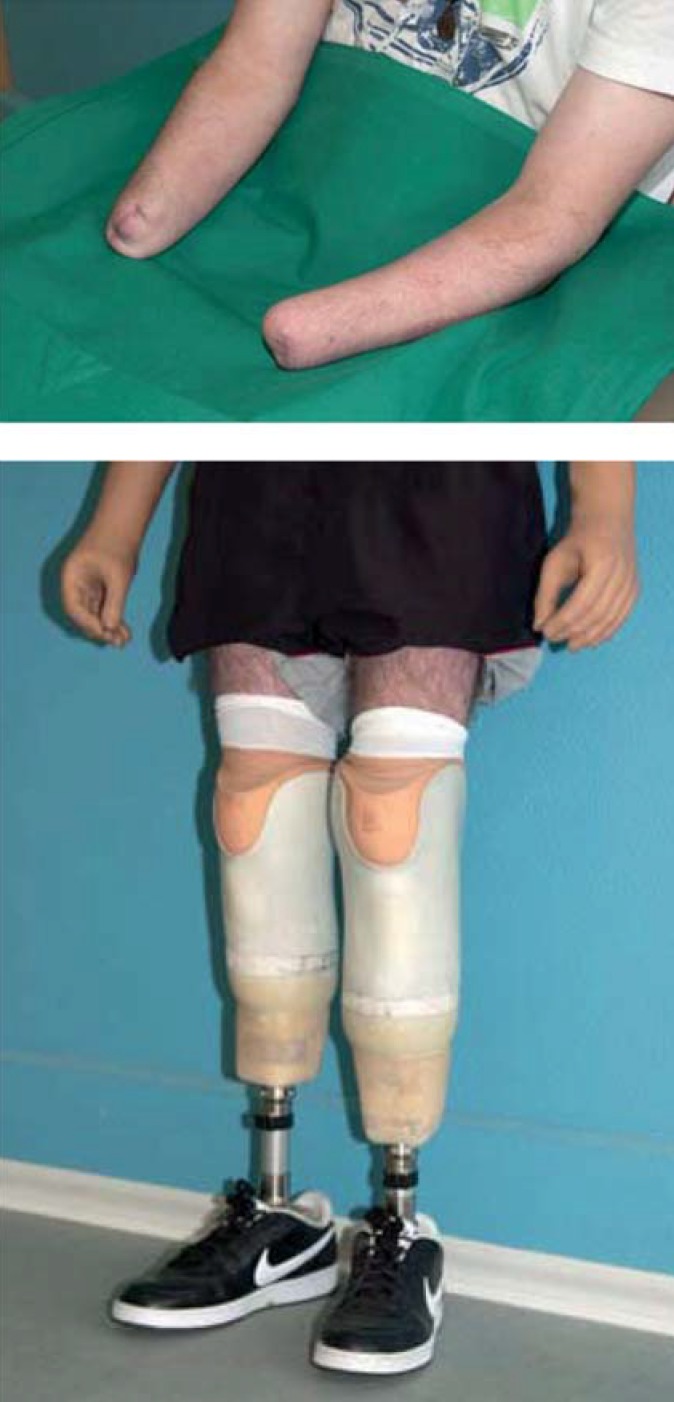
Cold-induced injuries are often severe with far-reaching consequences, so the correct treatment needs to be given as soon as possible. If reperfusion is initially not achieved, treatment as described above should be supplemented by additional measures, depending on the patient’s clinical situation; the indications must be carefully assessed. While the predominant symptoms in the posthyperemic phase of NFCI are chronic pain and paresthesia, the greatest dangers for patients who have suffered frostbite are loss of function and the possible necessity of amputation (Figure 4).
Figure 4.
Fourth-degree frostbite of the extremities requires amputation in most cases
The initial classification of frostbite injuries according to the above-mentioned degrees of severity permits only limited conclusions with regard to the extent of tissue loss that has to be expected and the treatment that is then indicated (10). In the absence of signs of infection, wound healing or the appearance of clear demarcation in the affected area should be monitored over a period of weeks.
New treatment approaches have shown promise in experimental studies and case reports.
The treatment strategies for frostbite and NFCI presented here correspond to recommendation strength C. No recommendation grade can yet be assigned for the alternative treatment approaches, because there is no general consensus as to their value and they have not been sufficiently validated by studies. If warming does not result in reperfusion of the affected tissues, these procedures should be employed with regular monitoring of perfusion by Doppler sonography, angiography, or checking for a pulse. Nothing is yet known about combinations of the various procedures. More studies are needed before further treatment recommendations can be formulated. Basic recommendations are provided by the Wilderness Medical Society Practice Guidelines (20, 40).
Summary
Although some interesting and plausible options for the treatment of frostbite have been described, the level of evidence is very low. Lysis seems to be distinctly superior to the other approaches.
Apart from further investigation into various promising experimental approaches, such as the use of tissue plasminogen activator, prostaglandin E1 (PGE1), iloprost (prostaglandin I2), or isosorbide dinitrate (ISDN), prospective randomized clinical trials are required to develop guidelines and treatment standards.
Key Messages.
Protracted exposure to low temperatures with slow cooling can lead to non-freezing cold injuries.
Frostbite arises from formation of ice crystals in tissue.
Particularly in the acute stage, frostbite should be treated at burn centers if at all possible
The affected parts of the body should not be warmed until renewed exposure to cold can be ruled out.
The treatment of frostbite and non-freezing cold injuries is barely standardized; there are no studies with large case numbers and no authoritative treatment guidelines.
Acknowledgments
Translated from the original German by David Roseveare.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Murphy JV, Banwell PE, Roberts AH, McGrouther DA. Frostbite: pathogenesis and treatment. J Trauma. 2000;48:171–178. doi: 10.1097/00005373-200001000-00036. [DOI] [PubMed] [Google Scholar]
- 2.Imray C, Grieve A, Dhillon S Caudwell Xtreme Everest Research Group. Cold damage to the extremities: frostbite and non-freezing cold injuries. Postgrad Med J. 2009;85:481–488. doi: 10.1136/pgmj.2008.068635. [DOI] [PubMed] [Google Scholar]
- 3.Goertz O, Hirsch T, Buschhaus B, et al. Intravital pathophysiologic comparison of frostbite and burn injury in a murine model. J Surg Res. 2011;167:e395–Ôe401. doi: 10.1016/j.jss.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 4.Petrone P, Asensio JA, Marini CP. Management of accidental hypothermia and cold injury. Curr Probl Surg. 2014;51:417–431. doi: 10.1067/j.cpsurg.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Imray CH, Richards P, Greaves J, Castellani JW. Nonfreezing cold-induced injuries. J R Army Med Corps. 2011;157:79–84. doi: 10.1136/jramc-157-01-14. [DOI] [PubMed] [Google Scholar]
- 6.Francis TJ, Golden FS. Non-freezing cold injury: the pathogenesis. J R Nav Med Serv. 1985;71:3–8. [PubMed] [Google Scholar]
- 7.Daanen HA, Ducharme MB. Finger cold-induced vasodilation during mild hypothermia, hyperthermia and at thermoneutrality. Aviation, space, and environmental medicine. 1999;70:1206–1210. [PubMed] [Google Scholar]
- 8.Daanen HA, Van de Linde FJ, Romet TT, Ducharme MB. The effect of body temperature on the hunting response of the middle finger skin temperature. Eur J Appl Physiol. 1997;76:538–543. doi: 10.1007/s004210050287. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien C, Young AJ, Lee DT, Shitzer A, Sawka MN, Pandolf KB. Role of core temperature as a stimulus for cold acclimation during repeated immersion in 20 degrees C water. J Appl Physiol. 2000;89:242–250. doi: 10.1152/jappl.2000.89.1.242. [DOI] [PubMed] [Google Scholar]
- 10.Hallam MJ, Cubison T, Dheansa B, Imray C. Managing frostbite. BMJ. 2010;341 doi: 10.1136/bmj.c5864. [DOI] [PubMed] [Google Scholar]
- 11.Su CW, Lohman R, Gottlieb LJ. Frostbite of the upper extremity. Hand Clin. 2000;16:235–247. [PubMed] [Google Scholar]
- 12.Marzella L, Jesudass RR, Manson PN, Myers RA, Bulkley GB. Morphologic characterization of acute injury to vascular endothelium of skin after frostbite. Plast Reconstr Surg. 1989;83:67–76. doi: 10.1097/00006534-198901000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Jurkovich GJ. Environmental cold-induced injury. Surg Clin North Am. 2007;87:247–267. doi: 10.1016/j.suc.2006.10.003. viii. [DOI] [PubMed] [Google Scholar]
- 14.Homann HH, Hauser J, Joneidi-Jafari H, Lehnhardt M, Goertz O. [Can the results of plastic surgery be improved by the modulation of reperfusion damage?] Dtsch Med Wochenschr. 2009;134:405–408. doi: 10.1055/s-0029-1243036. [DOI] [PubMed] [Google Scholar]
- 15.Ozyazgan I, Tercan M, Melli M, Bekerecioglu M, Ustun H, Gunay GK. Eicosanoids and inflammatory cells in frostbitten tissue: prostacyclin, thromboxane, polymorphonuclear leukocytes, and mast cells. Plast Reconstr Surg. 1998;101:1881–1886. doi: 10.1097/00006534-199806000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Goertz O, Baerreiter S, Ring A, et al. Determination of microcirculatory changes and angiogenesis in a model of frostbite injury in vivo. J Surg Res. 2011;168:155–161. doi: 10.1016/j.jss.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Kennett RP, Gilliatt RW. Nerve conduction studies in experimental non-freezing cold injury: I. Local nerve cooling. Muscle Nerve. 1991;14:553–562. doi: 10.1002/mus.880140610. [DOI] [PubMed] [Google Scholar]
- 18.Large A, Heinbecker P. Nerve degeneration following prolonged cooling of an extremity. Ann Surg. 1944;120:742–749. doi: 10.1097/00000658-194411000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Endrich B, Laprell-Moschner C, Brendel W, Messmer K. Effects of prolonged cold injury on the subcutaneous microcirculation of the hamster. I. Technique, morphology and tissue oxygenation. Research in experimental medicine. 1982;181:49–61. doi: 10.1007/BF01850989. [DOI] [PubMed] [Google Scholar]
- 20.MAcIntosh SE, Hamonko M, Freer L, et al. Wilderness Medical Society practice guidelines for the prevention and treatment of frostbite. Wilderness Environmental Med. 2011;22:156–166. doi: 10.1016/j.wem.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Syme D, Commission IM. Position paper: on-site treatment of frostbite for mountaineers. High Alt Med Biol. 2002;3:297–298. doi: 10.1089/152702902320604296. [DOI] [PubMed] [Google Scholar]
- 22.Entin MA, Baxter H. Influence of rapid warming on frostbite in experimental animals. Plast Reconstr Surg. 1952;9:511–524. doi: 10.1097/00006534-195201000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Kemper TC, de Jong VM, Anema HA, van den Brink A, van Hulst RA. Frostbite of both first digits of the foot treated with delayed hyperbaric oxygen:a case report and review of literature. Undersea Hyperb Med[journal] 2014;41:65–70. [PubMed] [Google Scholar]
- 24.Handford C, Buxton P, Russell K, et al. Frostbite: a practical approach to hospital management. Extrem Physiol Med. 2014;3 doi: 10.1186/2046-7648-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hardenbergh E, Miles JA. The effect of sympathectomy on tissue loss after experimental frostbite of the rabbit ear. J Surg Res. 1972;13:126–134. doi: 10.1016/0022-4804(72)90055-8. [DOI] [PubMed] [Google Scholar]
- 26.Flatt AE. Digital artery sympathectomy. J Hand Surg Am. 1980;5:550–556. doi: 10.1016/s0363-5023(80)80104-3. [DOI] [PubMed] [Google Scholar]
- 27.Bouwman DL, Morrison S, Lucas CE, Ledgerwood AM. Early sympathetic blockade for frostbite-is it of value? J Trauma. 1980;20:744–749. [PubMed] [Google Scholar]
- 28.Grieve AW, Davis P, Dhillon S, Richards P, Hillebrandt D, Imray CH. A clinical review of the management of frostbite. J R Army Med Corps. 2011;157:73–78. doi: 10.1136/jramc-157-01-13. [DOI] [PubMed] [Google Scholar]
- 29.Bruen KJ, Ballard JR, Morris SE, Cochran A, Edelman LS, Saffle JR. Reduction of the incidence of amputation in frostbite injury with thrombolytic therapy. Arch Surg. 2007;142:546–551. doi: 10.1001/archsurg.142.6.546. discussion 51-3. [DOI] [PubMed] [Google Scholar]
- 30.Johnson AR, Jensen HL, Peltier G, DelaCruz E. Efficacy of intravenous tissue plasminogen activator in frostbite patients and presentation of a treatment protocol for frostbite patients. Foot Ankle Spec. 2011;4:344–348. doi: 10.1177/1938640011422596. [DOI] [PubMed] [Google Scholar]
- 31.Twomey JA, Peltier GL, Zera RT. An open-label study to evaluate the safety and efficacy of tissue plasminogen activator in treatment of severe frostbite. J Trauma. 2005;59:1350–1354. doi: 10.1097/01.ta.0000195517.50778.2e. discussion 4-5. [DOI] [PubMed] [Google Scholar]
- 32.Yeager RA, Campion TW, Kerr JC, Hobson RW, 2nd, Lynch TG. Treatment of frostbite with intra-arterial prostaglandin E1. Am Surg. 1983;49:665–667. [PubMed] [Google Scholar]
- 33.Groechenig E. Treatment of frostbite with iloprost. Lancet. 1994;344:1152–1153. doi: 10.1016/s0140-6736(94)90657-2. [DOI] [PubMed] [Google Scholar]
- 34.Goertz O, Haddad H, von der Lohe L, et al. Influence of ISDN, l-NAME and selenium on microcirculation, leukocyte endothelium interaction and angiogenesis after frostbite. Burns. 2014 doi: 10.1016/j.burns.2014.05.022. http://dx.doi.org/10.1016/j.burns.2014.05.022 (last accessed on 8 June 2015) [DOI] [PubMed] [Google Scholar]
- 35.Saemi AM, Johnson JM, Morris CS. Treatment of bilateral hand frostbite using transcatheter arterial thrombolysis after papaverine infusion. Cardiovasc Intervent Radiol. 2009;32:1280–1283. doi: 10.1007/s00270-009-9584-9. [DOI] [PubMed] [Google Scholar]
- 36.Sheridan RL, Goldstein MA, Stoddard FJ, Walker TG. Case records of the Massachusetts General Hospital Case 41-2009. A 16 year-old boy with hypothermia and frostbite. New Engl J Med. 2009;361:2654–2662. doi: 10.1056/NEJMcpc0910088. [DOI] [PubMed] [Google Scholar]
- 37.Wagner C, Pannucci CJ. Thrombolytic therapy in the acute management of frostbite injuries. Air Medical Journal. 2011;30:39–44. doi: 10.1016/j.amj.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cauchy E, Cheguillaume B, Chetaille E. A controlled trial of a prostacyclin and rt-PA in the treatment of severe frostbite. New Engl J Med. 2011;364:189–190. doi: 10.1056/NEJMc1000538. [DOI] [PubMed] [Google Scholar]
- 39.Woo EK, Lee JW, Hur GY, et al. Proposed treatment protocol for frostbite: a retrospective analysis of 17 cases based on a 3-year single-institution experience. Arch Plast Surg. 2013;40:510–516. doi: 10.5999/aps.2013.40.5.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McIntosh SE, Opacic M, Freer L, et al. Wilderness medical society practice guidelines for the prevention and treatment of frostbite: 2014 update. Wilderness Environmental Med. 2014;25:43–54. doi: 10.1016/j.wem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- e1.Basbaum CB. Induced hypothermia in peripheral nerve: electron microscopic and electrophysiological observations. J Neurocytol. 1973;2:171–187. doi: 10.1007/BF01474719. [DOI] [PubMed] [Google Scholar]
- e2.Denny-Brown D, Adams RD, Brenner C, Doherty MM. The pathology of injury to nerve induced by cold. J Neuropathol Exp Neurol. 1945;4:305–323. doi: 10.1097/00005072-194504040-00001. [DOI] [PubMed] [Google Scholar]
- e3.Nukada H, Pollock M, Allpress S. Experimental cold injury to peripheral nerve. Brain. 1981;104:779–811. doi: 10.1093/brain/104.4.779. [DOI] [PubMed] [Google Scholar]
- e4.Kennett RP, Gilliatt RW. Nerve conduction studies in experimental non-freezing cold injury: II. Generalized nerve cooling by limb immersion. Muscle Nerve. 1991;14:960–967. doi: 10.1002/mus.880141006. [DOI] [PubMed] [Google Scholar]
- e5.Irwin MS. Nature and mechanism of peripheral nerve damage in an experimental model of non-freezing cold injury. Ann R Coll Surg Engl. 1996;78:372–379. [PMC free article] [PubMed] [Google Scholar]
- e6.Montgomery H, Horwitz O, Peirce G, Sayen A. Experimental immersion foot I. The effects of prolonged exposure to water at 3 degrees C. on the oxygen tension and temperature of the rabbit leg. J Clin Invest. 1954;33:361–369. doi: 10.1172/JCI102908. [DOI] [PMC free article] [PubMed] [Google Scholar]