Abstract
Background
About 4.6 million persons in Germany are now taking statins, i.e., drugs that inhibit the enzyme 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase. Statins lower the concentration of low-density lipoproteins (LDL) and thereby lessen the rate of cardiovascular events; the size of this effect depends on the extent of lowering of the LDL cholesterol concentration. Muscle symptoms are a clinically relevant side effect of statin treatment.
Methods
This review is based on pertinent publications retrieved by a selective literature search, and on the current recommendations of the European Atherosclerosis Society.
Results
At least 5% of patients taking statins have statin-associated muscle symptoms (SAMS). The etiology of SAMS is heterogeneous. SAMS may seriously impair quality of life and cause complications of variable severity, up to and including rhabdomyolysis (in about 1 in 100 000 cases). SAMS often lead to a reduction in the prescribed dose of the statin, while also negatively affecting drug adherence. More than 90% of patients with SAMS can keep on taking statins over the long term and gain the full clinical benefit of statin treatment after a switch to another type of statin or a readjustment of the dose or frequency of administration. If the LDL cholesterol concentration is not adequately lowered while the patient is taking a statin in the highest tolerable dose, combination therapy is indicated.
Conclusion
SAMS are important adverse effects of statin treatment because they lessen drug adherence. Patients with SAMS should undergo a thorough diagnostic evaluation followed by appropriate counseling. In most cases, statins can be continued, with appropriate adjustments, even in the aftermath of SAMS.
Low-density lipoproteins (LDL) are causally involved in the pathogenesis of atherosclerosis (e1– e5). Statins inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase and are among the most studied drugs. Both the efficacy of statins in reducing cardiovascular risk as well as their safety are undisputed (1, 2). Statins are therefore recommended in guidelines for cardiovascular prevention (e6– e8). Nonetheless, about one-third of those undergoing long-term treatment report difficulties with routinely taking their medication (3, 4). A reduced adherence to statin therapy is linearly associated with increased mortality (5). Improving statin adherence is associated with a reduction in cardiovascular morbidity and mortality, making this an important aim in medical care (Table) (5, e9).
Table. Direct correlation between statin adherence and overall mortality.
| Percentage of days taking statin during study period (%) | Hazard ratio (95% CI)* primary prevention cohort | Hazard ratio (95% CI)* secondary prevention cohort |
|---|---|---|
| <10 | 1 (Reference) | 1 (Reference) |
| 10–19 | 1.35 (1.22–1.50) | 1.28 (1.18–1.39) |
| 20–29 | 1.07 (0.95–1.21) | 0.98 (0.90–1.08) |
| 30–39 | 0.88 (0.77–1.00) | 0.81 (0.74–0.89) |
| 40–49 | 0.86 (0.75–0.98) | 0.73 (0.66–0.80) |
| 50–59 | 0.77 (0.67–0.88) | 0.69 (0.63–0.76) |
| 60–69 | 0.63 (0.55–0.73) | 0.67 (0.62–0.74) |
| 70–79 | 0.59 (0.51–0.68) | 0.61 (0.56–0.67) |
| 80–89 | 0.61 (0.53–0.69) | 0.54 (0.50–0.59) |
| ≥ 90 | 0.55 (0.49–0.61) | 0.49 (0.46–0.53) |
Data are from a retrospective study with 229 918 adults, conducted by Maccabi Healthcare Services (MHS) in Israel between 1998 and 2006 (5).
CI, confidence interval
*Adjusted for age, sex, marital status, nationality, socioeconomic status, years residing in Israel, location, chronic diseases, primary care physician visits within one year before the index date, number of hospital admissions within one year before the index date, cancer, diabetes mellitus, taking antihypertensive drugs and ?diuretics within one year before the index date, and the average value of the low-density lipoprotein cholesterol within one year before the index date; the index date is defined as the date of the initial statin dose. Modified from Shalev et al. (5)
Incidence of muscle symptoms during statin therapy
In clinical practice and observational studies, 10–30% of patients report statin-associated muscle symptoms (SAMS) (4, e10– e13). SAMS are relevant causes of reduced adherence (6, e14). However, there is a striking discrepancy between incidences reported and those documented in randomized trials. The incidence of muscle symptoms in randomized trials was low, with little difference between the statin and placebo groups (7– 9, e15, e16), which could be attributed to numerous factors. For instance, in some studies, patients were excluded if they presented muscle symptoms or statin intolerance. Slight muscle symptoms were not always systematically recorded. For observational studies without a control group, the actual incidence of SAMS can be overestimated. Evaluation of muscle symptoms in 420 statin-naïve people in one prospective randomized trial revealed that the incidence of myalgia (muscle pain) was 9.4% for individuals taking 80 mg of atorvastatin daily over six months, compared to 4.6% for those taking a placebo (e17). According to the Arzneiverordnungs-Report (Drug Prescription Report), about 4.7 million people were treated with statins in Germany in 2013, with 1.707 billion prescribed statin daily doses. Thus, with an estimated SAMS prevalence of 5–10%, approximately 235 000 to 470 000 people experience SAMS annually in Germany.
Definition
SAMS are clinically heterogeneous, with patients reporting proximal, symmetric pain, tension, stiffness, or cramps, which may be accompanied by muscle weakness. From our clinical experience, it seems that people who are physically active are particularly affected (10). Creatine kinase (CK; EC 2.7. 3.2) is often not increased; however, in physically active patients a slight post-exercise increase in CK (of up to 400 U/L) can often not be differentiated from a physiological increase. SAMS has been graded differently in the literature. Our proposal for grading is given in eTable 1.
eTable 1. Point scheme for SAMS diagnosis.
| Clinical symptoms | Points |
|---|---|
| Location/distribution | |
| Symmetrical, hip flexor/thighes | 3 |
| Symmetrical, calves | 2 |
| Symmetrical, shoulder girdle | 2 |
| Nonspecific asymmetrical, intermittent | 1 |
| Temporal association with initiation of statin therapy | |
| Symptoms after <4 weeks | 3 |
| Symptoms after 4–12 weeks | 2 |
| Symptoms after >12 weeks | 1 |
| After discontinuation | |
| Improvement within <2 weeks | 2 |
| Improvement within 2–4 weeks | 1 |
| No improvement >4 weeks | 0 |
| Rechallenge | |
| Symptoms re-appeared within <4 weeks | 3 |
| Symptoms re-appeared within 4–12 weeks | 1 |
| Diagnosis of statin-associated muscle problems | |
| Probable | 9–11 |
| Possible | 7–8 |
| Unlikely | <7 |
modified according to Rosenson et al. (26).
SAMS, statin-associated muscle synodrome
CK levels that are elevated to more than ten times the upper limit of normal have an incidence of 1:1000 to 1:10 000 per year (11). Severe muscle damage with massive release of myoglobin (rhabdomyolysis) is even more rare (about 1 per 100 000 patients). Rhabdomyolysis is accompanied by very high CK activity and myoglobinuria, and it can lead to acute renal failure and death.
Macro CK—a CK variant with a high molecular mass—occurs in approximately 4% of asymptomatic patients with an incidental finding of increased CK levels. In this case, the values of troponin, myoglobin, lactate dehydrogenase (LDH), glutamic-oxaloacetic transaminase (GOT), and glutamate pyruvate transaminase (GPT) are within a normal range, and the CK level does not vary over time. Macro CK can be specifically diagnosed by isoenzyme electrophoresis. Macro CK type 1, in which CK-BB (a brain isoform) binds to specific antibodies, has no clinical significance. However, macro CK type 2, which is characterized by mitochondrial CK in an oligomeric form, occurs in malignomas, cirrhosis, and Lyell’s syndrome.
A typical sequence of events for SAMS is that CK activity decreases within a few weeks after statin therapy is discontinued, but the symptoms recur and/or CK increases within one to a maximum of four weeks after the statin therapy is reinstated. For physically active patients, a control examination should be carried out following a training break of one week. The clinical procedure depends on the symptoms and the extent of the CK increase. A distinction is made between patients reporting muscle problems who have a CK increase of less than four times the upper limit of normal and those who have it above this limit. Patients with a CK increase of more than 4 times the upper limit of normal should first discontinue their statin (2). This recommendation is not based on clinical data but rather comes from clinical experience about the potential risks of rhabdomyolysis.
Etiology
The pathophysiology of SAMS is not yet fully understood. Several molecular mechanisms have been proposed (Box 1) (12– 18), which include changes affecting the cellular energy metabolism, signaling protein isoprenylation, and mitochondria (11– 20, e18– e25). Muscle biopsy analyses support the hypothesis of impaired mitochondrial function, although this has yet to be confirmed (e18– e24).
Box 1. Possible cellular causes of statin-associated muscle symptoms.
Reduced concentrations of the final breakdown products of the mevalonate pathway (cholesterol biosynthesis)
Reduced cholesterol content in the sarcolemma and/or sarcoplasmic reticulum
Increased myocellular fat and/or sterols
Inhibition of production of prenylated proteins or guanosine triphosphatase
Alterations in muscle protein catabolism
Decreased myocellular creatine
Alterations in calcium homeostasis
Immune-mediated effects of statins and effects on mitochondrial function
The risk of SAMS increases with a number of clinical and constitutional factors (Box 2), and its frequency also depends on the statin dose and plasma concentration. Using standard dosages, such as 20–40 mg of simvastatin daily, leads to pronounced symptoms in one of 10 000 patients annually. However, the risk increases exponentially with higher dosages. Of particular clinical relevance, the effective concentration of statins can be increased by the concomitant use of medications that interfere with statin degradation, such as macrolide antibiotics or azole antifungals, or grapefruit juice (eBox 1) (11, 19).
Box 2. Risk factors for statin-associated muscle symptoms.
-
Patient characteristics
Age >80 years (caution advised from >75 years)
Female
Lower body mass index, frailty, cachexia
Asian descent
-
Concurrent conditions
Acute infection
Hypothyroidism (treated or untreated)
Impaired renal function (CKD stages 3, 4, and 5)
Liver impairment
Closure of bile ducts
Organ transplant recipient
Severe trauma
Human immunodeficiency virus (HIV)
Diabetes mellitus
Vitamin D deficiency
-
Operations
Major surgery or preoperative phase
-
Pre-existing conditions
Increased creatine kinase levels in medical history
Previous onset of muscle, joint, or tendon pain
Inflammatory or hereditary metabolic neuromuscular/muscular defects, such as McArdle disease, carnitine palmitoyltransferase (CPT-II) deficiency, myoadenylate deaminase deficiency, and malignant hyperthermia
Myopathy during another lipid-lowering therapy
Known cases of myopathy in the family
-
Genetic factors
Polymorphisms in genes for drug transporters
-
Other factors
High levels of physical activity
Excessive alcohol consumption
Diet (excessive grapefruit or cranberry juice)
Medication interactions (eBox 1)
Drug abuse (cocaine, amphetamines, heroin)
modified according to Mancini et al. (38); CKD, chronic kidney disease
eBox 1. Selected metabolic interactions between statins and other drugs.
-
CYP3A4*
Antifungals: itraconazole, posaconazole, miconazole
Macrolide antibiotics: erythromycin, telithromycin, clarithromycin
Protease inhibitors: amprenavir, atazanavir, fosamprenavir, indinavir, lopinavir, nelfinavir, ritonavir, tipranavir
Gemfibrozil
Verapamil, diltiazem
Warfarin
Amiodarone
-
Inhibitory to SLCO1B1 (OATP1B1)
Gemfibrozil
Cyclosporin
modified according to Mancini et al. (39)
*he combination of simvastatin and strong CYP3A4 inhibitors is contraindicated.
The differential diagnosis of statin-induced and exercise-induced muscle symptoms after endurance sports (such as bicycling or running) or strength training is difficult, but symptoms induced by statins persist longer and often prevent a further exercise session. For correct diagnosis, it is important to note that muscular stress can increase CK levels to 500–600 U/L. Including an exercise break and then monitoring the CK values can be useful for this. Further, alcohol abuse should be excluded, as this is a common cause of CK level increases and rhabdomyolysis.
In the search for causal genetic factors, a strong association between polymorphisms in the SLCO1B1 (solute carrier organic anion transporter family member 1B1) gene and SAMS has been found with simvastatin (20, e25). SLCO1B1 is a hepatic transporter responsible for the uptake of statins into the liver, among other things. A SLCO1B1 polymorphism (c.521T>C, p.V174A) increases the blood concentrations of statins (e26). When combined with simvastatin treatment, this genetic defect confers an increased risk for myopathy of 4.5-fold for heterozygous carriers and of 17-fold for homozygote carriers. This SLCO1B1 variant affects the plasma concentrations of the following statins (listed from most to least affected) (21, 22):
Simvastatin
Atorvastatin
Pravastatin
Rosuvastatin
Fluvastatin.
Further genetic variants that could potentially affect the effective concentration for statins have also been identified (15, 23, e27– e34).
Statins increase the detection rate of hemoglobin A1C (HbA1C) from >6.5% to about 9% (e35). In particular, patients with diabetes mellitus benefit from statin therapy (e36). There is no correlation between diabetes mellitus and an increased risk of SAMS under statin therapy.
Diagnosis
CK activity is neither sensitive to nor specific for SAMS. More appropriate laboratory parameters are not known. In our opinion, it is advisable to measure CK activity once prior to initiating therapy, to be able to better determine abnormal elevations of CK activity during therapy. Additionally, urine measurements for the thyroid stimulating hormone (TSH), transaminases, a calculated glomerular filtration rate, and protein should be made (24).
We do not believe that a general screening for the gene variant SLCO1B1 makes sense, although it can be considered in isolated cases if there is a high risk for SAMS. Such cases include:
Long-term treatment with drugs that increase the risk of myopathy (Box 2, eBox 1) (25)
An increased risk of SAMS during statin therapy (e.g., due to hypothyroidism, myopathies, SAMS in family members, high muscle stress) (Box 2)
Documentation of statin intolerance during assessment for indication of apheresis.
Key Messages.
Statin-associated muscle symptoms (SAMS) are the most clinically relevant adverse effects of statin therapy as they reduce quality of life, can trigger complications as severe as rhabdomyolysis, and lessen adherence to statin therapy.
The exact molecular mechanisms of SAMS are unknown. Therefore, there are no specific methods for their detection and treatment.
A clinical diagnosis is based on a detailed medical history, the typical form of presentation, the temporal association of the symptoms with statin intake, and the recurrence of symptoms when therapy is reinstated after a break. In the vast majority of SAMS cases, creatine kinase levels are not elevated.
Offering patients thorough explanations and guidance allows more than 90% of those affected by SAMS to comply with long-term statin therapy.
If the individual target values for LDL cholesterol are not attained with the maximally tolerated statin dose, therapy should be combined with other lipid-lowering agents.
Patients with a SLCO1B1 gene defect should avoid taking simvastatin (especially at doses >20 mg) and instead use another statin (23, 26).
Clinical
There are currently no imaging methods that can detect SAMS, although clinical diagnostic algorithms have been proposed in the literature (4, 27, e17). In accordance with the European Atherosclerosis Society (EAS), we recommend the criteria catalog from Rosenson et al. (eTable 1) (27). Diagnosis is based on a detailed medical history that includes the typical form of presentation as well as a clear temporal association of symptoms with statin intake, discontinuation, and rechallenge. Symptoms occur typically four to six weeks after therapy initiation, mostly affecting large muscle groups, such as thighs, shoulder girdle, and upper arm muscles. However, SAMS may even occur after many years of treatment. SAMS often occurs following a dose increase or if an interacting drug has been prescribed (eBox 1).
Neurological differential diagnoses
A pre-existing asymptomatic myopathy may be unmasked by statins (e37). The typical characteristics of this situation are a pre-existing CK increase or a lack of a decrease in elevated CK activities after statin discontinuation. Additionally, the delayed disappearance of muscle symptoms after discontinuation may indicate a subclinical myopathy of a different origin. As electromyograms (EMG) do not even give specific findings for SAMS with elevated CK levels, they are indicated to distinguish myopathies of other origins from SAMS. Additional differential diagnostic information on muscle symptoms and CK increases is given in Box 3a and Box 3b (28).
Box 3a. Differential diagnosis for muscle symptoms and elevated creatine kinase levels.
-
Muscle symptoms
Physical effort (particularly in untrained individuals)
Viral diseases
Vitamin D deficiency
Hypo- or hyperthyroidism
Cushing’s syndrome or adrenal insufficiency
Hypoparathyroidism
Fibromyalgia
Polymyalgia rheumatica
Polymyositis
Systemic lupus erythematosus
Tendon or joint disease
Trauma
Epileptic seizure or chills
Peripheral artery disease*1
Drugs (corticosteroids, anti-psychotics, anti-retroviral drugs, illicit drugs such as cocaine or amphetamines)
-
Elevated creatine kinase levels
Physical exertion
Hypothyroidism
Metabolic or inflammatory myopathies (McArdle disease, carnitine palmitoyltransferase II deficiency, myoadenylate deaminase deficiency, malignant hyperthermia)
Alcoholism
Neuropathy or radiculopathy
Ethnic background (African American)
Idiopathic increase in creatine kinase levels *2
Seizure or chills
Trauma
Drugs (antipsychotics, illicit drugs such as cocaine or amphetamines)
modified according to Joy and Hegele (40)
*1in patients with cramps in calves or thighs
*2increased creatine kinase concentrations that have no other apparent cause
Statin rechallenge
In the majority of cases, no identifiable specific muscle or neurological pathology, or relevant drug interaction is found (29, 30, e11). Large case series show however that patients with muscle complaints during statin therapy can still be treated with a statin. Thus, of 11 124 patients who discontinued a statin therapy due to muscle symptoms, 92% were able to resume statin therapy subsequently (e11). In the Cleveland Clinic (Ohio, USA) from 1995 to 2010, 1605 patients with a suspected diagnosis of statin intolerance were examined; 1165 patients were subsequently able to undergo continuous statin therapy (30). In so-called n-of-1 trials, patients took a medication break and then were individually assigned to either first a statin and then a placebo, or the reverse, in a double-blind and random manner (29). The patient-reported symptoms were not significantly different between the statin and placebo phases, and most patients were able to return to their original medication after rechallenge.
Taken together, these data show that a very large proportion of the muscle symptoms are not specifically associated with statin therapy. In agreement with this, results from the Heart Protection Study (HPS) showed that 32.9% of participants who received simvastatin (40 mg), and 33.2% of those who received a placebo, complained of myalgia or muscle weakness at least once (8). These results are consistent with experiences from other areas of pain research. The challenge in everyday clinical practice is thus to distinguish perception and psychological transfer phenomena, as well as the Hawthorne Effect, from classic, organic myopathies. Only an extremely small proportion of patients will need to permanently discontinue statin medications; for instance, for the HPS participants, only 0.5% had to discontinue of the more than 30% who complained of muscle problems (2). However, those affected frequently do not tolerate high statin dosages.
Therapy monitoring and prophylaxis
Prior to initiating a statin therapy, a detailed medical history and the basis investigations outlined should be carried out. The following advice can help prevent SAMS:
Observe the contraindications for statins and avoid interfering drugs (eBox 2)
Interrupt the statin therapy if a patient plans to undertake severe physical exertion, such as a marathon
CK does not need to be controlled on a regular basis if no muscle symptoms are present. However, controls are indicated for patients with an increased risk of myopathy, for example due to concomitant medication (Box 2, eBox 1) or due to an existing, low elevation of CK
Follow the dose recommendations in eTable 2 for patients with renal failure (from stage 3a) (31).
eBox 2. Recommendations of the US Food and Drug Administration for the treatment with simvastatin.
-
Simvastatin is contraindicated with
itraconazole, ketoconazole, posaconazole, erythromycin, clarithromycin, telithromycin, HIV protease inhibitors, nefazodone, gemfibrozil, cyclosporine, or danazol
-
Do not exceed 10 mg simvastatin daily when taking
verapamil or dilitiazem
-
Do not exceed 20 mg simvastatin daily when taking
amlodipine, amiodarone, or ranolazine
Avoid drinking large quantities of grapefruit juice (25).
HIV, human immunodeficiency virus
FDA Drug Safety Communication: New restrictions, contraindications, and dose limitations for Zocor (simvastatin) to reduce the risk of injuries muscles: 08/06/2011: www.fda.gov/Drugs/DrugSafety/ucm256581.htm
eTable 2. Recommended statin doses for adults with chronic kidney disease from stage 3a (glomerular filtration rate below 60 mL/min/1.73 m2, dialysis, kidney transplantation) (30).
| Statin | Dosage (mg per day) |
|---|---|
| Lovastatin | 20*1 |
| Fluvastatin | 80*2 |
| Atorvastatin | 20*3 |
| Rosuvastatin | 10*4 |
| Simvastatin/ezetimibe | 20/10*5 |
| Pravastatin | 40 |
| Simvastatin | 40 |
modified according to Wanner et al. (31).
―Prescribing information lovastatin-ratiopharm (de.oddb.org/de/drugs/fachinfo/uid/26190)
*2ALERT (Assessment of Lescol in Renal Transplantation) study
*3Deutsche Diabetes Dialyse (4D) study
*4AURORA: A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of survival and Cardiovascular Events
*5Study of Heart and Renal Protection (SHARP)
Course of action
Take enough time
Taking sufficient time for a patient is an important factor for the diagnosis and, in many cases, the key to success.
Many symptoms can be traced back to viral infections, unaccustomed physical activity, and drug interactions. Non–statin-associated muscle diseases also need to be considered (Box 3).
The doctor–patient interview should take into account fears and expectations, as well as the psychosocial situation, of the person concerned. A large proportion of allegedly statin-associated muscle symptoms can be otherwise re-assigned.
Rechallenge
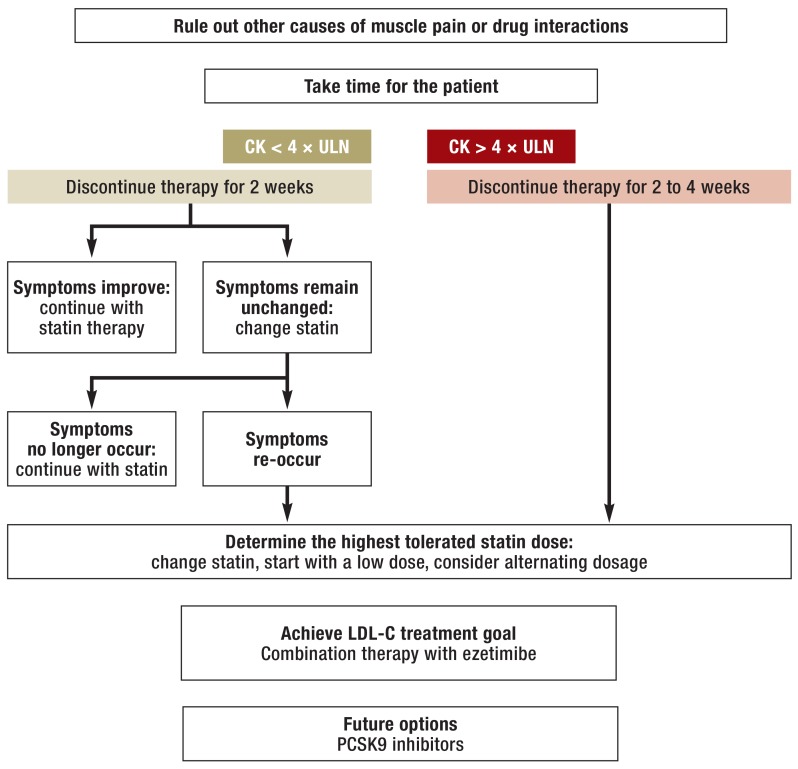
According to expert recommendations (2), further actions should differentiate between affected patients with a CK elevation of over four times the upper limit of normal and those with no or a low elevation of CK but who are still symptomatic (Figure). In any case, the statin medication should be interrupted if the patient is symptomatic. If the CK levels exceed four times the reference range limit, a rechallenge should be done cautiously under close supervision, using a lower dose or a less potent statin. If the CK levels are not elevated or only elevated to a low level, a statin rechallenge can be carried out more quickly and with the original preparation. This interruption and rechallenge can help the patient and doctor to determine whether the described symptoms are indeed related to the statin medication (eTable 1). However, this is usually not the case.
Figure.
Flow-chart for statin-associated muscle symptoms (modified according to Stroes et al. [2]). CK, creatine kinase; ULN, upper limit of normal (of the reference range); LDL, low-density protein; PCSK9, proprotein convertase subtilisin/kexin type 9; CETP, cholesteryl ester transfer protein
Clinical experience shows that it may be psychologically favorable to carry out the rechallenge with other statins. Due to simvastatin’s high interaction potential, more patients appear to report muscle symptoms when using it (eBox 2). Switching to the more potent atorvastatin is often successful, even though it shares the same degradation pathway via cytochrome P450 3A4 as simvastatin. However, there are no set rules for this. In fact, some patients presenting muscle symptoms when using atorvastatin can tolerate simvastatin better. It can be advantageous to use statins that are independent of the cytochrome P450 3A4 pathway, such as fluvastatin, pravastatin, and to a large extent rosuvastatin.
To be able to assess the tolerability of individual statins, it is important to use equipotential doses. Fluvastatin and pravastatin have a low potency, with 40 mg fluvastatin corresponding to 5–10 mg atorvastatin, and 40 mg pravastatin, to 10–20 mg atorvastatin. Contrary to pharmacological considerations, no differences have been observed in clinical practice between the incidence of SAMS under therapy with hydrophilic (e.g., pravastatin and rosuvastatin) or lipophilic statins (e16).
Determine the maximum tolerated dose
If the complaints stop after a discontinuation break but re-occur after rechallenge, the next step is to determine the highest tolerated statin doses. It is advisable to start with a very low statin dose, for example 5 mg atorvastatin (note that the tablets must be divided in this case). Alternatively, statin can be taken only every other day or even less frequently (30). Because of the long half-life of atorvastatin and rosuvastatin, there will be no significant loss of activity. However, this can adversely affect therapy adherence (30). Finding the correct dose is time consuming, as the dosage should not be increased any more frequently than every two weeks.
Combination therapy for insufficient LDL reduction
Often, even the highest tolerated dosages are not sufficient to bring the LDL-C levels to its target. In these cases, combined therapy is indicated. Given the results of the IMPROVE-IT trial (32), we recommend ezetimibe as the first line treatment for patients with statin-associated muscle symptoms and high LDL-C values (2). The study showed that the combination of simvastatin with ezetimibe (10 mg) leads to a further reduction of myocardial infarction. No reduction was observed in overall mortality or mortality due to cardiovascular and cardiac events. If the concomitant use of a statin and ezetimibe does not bring the LDL-C to its target value, medication adherence may be the problem. Additionally, in selected cases, if the statin-ezetimibe combination does not achieve the target, bile acid-binding ion exchangers can be used. In complicated situations referral to a lipid clinic should be considered.
If the patient has familial hypercholesterolemia or a particularly pronounced statin intolerance and high cardiovascular risk, lipoprotein apheresis should be considered.
As with all pain conditions, a placebo treatment improves symptoms in many patients. In the literature, coenzyme Q10 and vitamin D have been proposed for treating SAMS. Nonetheless, as their effect is not supported by robust randomized trials, we can not recommend these (e38– e46).
Two new LDL-C–lowering drug classes are in advanced stages of clinical testing: cholesteryl ester transfer protein (CETP) (33– 35) and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors. The latter were specifically tested in patients with statin intolerance and are currently approved for the treatment. They lower LDL-C in this cohort significantly (36, 37, e47, e48). It remains to be determined whether they are superior in terms of their tolerability.
Box 3b. Examples of the etiology of neurological differential diagnoses of muscle pain.
-
Diseases of the central nervous system
Spastic increase of muscle tone
Basal ganglia disease
Projected myalgia
-
Peripheral nerve damage
Acute polyradiculitis (Guillain-Barré syndrome)
Autonomic disorders
-
Hereditary muscle diseases with myalgia
Myotonic dystrophy type 2
-
Hereditary metabolic myopathies
Glycogen storage disease (e.g., McArdle’s disease)
Disorders of fatty acid oxidation (e.g., carnitine palmitoyltransferase enzyme deficiency)
Disorders of purine metabolism (e.g., autosomal recessive myoadenylate deaminase deficiency)
Mitochondrial myopathies
-
Acquired muscle diseases with myalgia
Toxic myopathies (e.g., alcohol-induced, the most common toxic myopathy)
Associated with lipid-lowering drugs (e.g., statin-induced myopathy)
-
Pathogen-associated, infectious myositis
Staphylococcal infections
Viral infections (e.g., Coxsackie B5, influenza, parainfluenza)
Trichinosis
Borrelia burgdorferi infection
-
Immunogenic inflammatory myopathies
Dermatomyositis
-
Endocrine myopathies
Hypothyroidism
Hashimoto’s thyroiditis
Hypoparathyroidism
modified from Joy and Hegele (40)
Acknowledgments
Translated from the original German by Veronica A. Raker, PhD.
Footnotes
Conflict of interest statement
Prof. Laufs has received consultant and/or speaking fees, study support (third-party funds), travel expenses, or registration fee reimbursement from ABDA, AkdA, Amgen, AstraZeneca, Bayer, Berlin-Chemie, BNK, Boehringer-Ingelheim, DACH, Daiichi-Sankyo, i-cor, Lilly, Medtronik, MSD, Pfizer, Roche, Sanofi, Servier, Synlab, UdS and UKS.
Prof. Hall has received consulting and/or speaking honoraria and travel expenses and/or conference fee reimbursement from Sanofi-Aventis, MSD, BMS, Roche, AstraZeneca, Amgen, and Berlin-Chemie.
Prof. Windler has received speaking and consulting honoraria from Amgen, AstraZeneca, Bayer, Danone, MSD Sharp & Dohme, Novartis, Pfizer, Roche Pharma, Unilever, and Sanofi.
Prof. Endres has received consulting or speaking honoraria, study support (third-party funds), travel expenses, or registration fee reimbursement from Amgen, Bayer, Boston Scientific, BMS, Boehringer-Ingelheim, Ever, GSK, MSD, Novartis, Pfizer, and Sanofi.
Prof. März is employed by Synlab Services GmbH and is a shareholder of Synlab Holding GmbH, Augsburg. He has received speaking and consulting honoraria from the companies Aegerion Pharmaceuticals, Amgen, AstraZeneca, Danone Research, Sanofi, MSD, Synageva, and Unilever.
Assoc. Prof. Scharnagl declares that no conflict of interests exists.
References
- 1.Fulcher J, O’Connell R, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of LDL-lowering therapy among men and women: meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet. 2015;385:1397–1404. doi: 10.1016/S0140-6736(14)61368-4. [DOI] [PubMed] [Google Scholar]
- 2.Stroes ES, Thompson PD, Corsini A, et al. Statin-associated muscle symptoms: impact on statin therapy-European atherosclerosis society consensus panel statement on assessment, aetiology and management. Eur Heart J. 2015;36:1012–1022. doi: 10.1093/eurheartj/ehv043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Laufs U, Rettig-Ewen V, Böhm M. Strategies to improve drug adherence. Eur Heart J. 2011;32:264–268. doi: 10.1093/eurheartj/ehq297. [DOI] [PubMed] [Google Scholar]
- 4.Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients-the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–414. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 5.Shalev V, Chodick G, Silber H, Kokia E, Jan J, Heymann AD. Continuation of statin treatment and all-cause mortality: a population-based cohort study. Arch Intern Med. 2009;169:260–268. doi: 10.1001/archinternmed.2008.552. [DOI] [PubMed] [Google Scholar]
- 6.Chodick G, Shalev V, Gerber Y, et al. Long-term persistence with statin treatment in a not-for-profit health maintenance organization: a population-based retrospective cohort study in Israel. Clin Ther. 2008;30:2167–2179. doi: 10.1016/j.clinthera.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Ganga HV, Slim HB, Thompson PD. A systematic review of statin-induced muscle problems in clinical trials. Am Heart J. 2014;168:6–15. doi: 10.1016/j.ahj.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 8.Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 10.Goto Y. [AFCAPS/TexCAPS [The Air Force/Texas Coronary Atherosclerosis Prevention Study]] Nihon Rinsho. 2001;59(Suppl 3):398–403. [PubMed] [Google Scholar]
- 11.Law M, Rudnicka AR. Statin safety: a systematic review. Am J Cardiol. 2006;97:52C–60C. doi: 10.1016/j.amjcard.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Madsen CS, Janovitz E, Zhang R, et al. The Guinea pig as a preclinical model for demonstrating the efficacy and safety of statins. J Pharmacol Exp Ther. 2008;324:576–586. doi: 10.1124/jpet.107.131615. [DOI] [PubMed] [Google Scholar]
- 13.Mikus CR, Boyle LJ, Borengasser SJ, et al. Simvastatin impairs exercise training adaptations. J Am Coll Cardiol. 2013;62:709–714. doi: 10.1016/j.jacc.2013.02.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naba H, Kakinuma C, Ohnishi S, Ogihara T. Improving effect of ethyl eicosapentanoate on statin-induced rhabdomyolysis in Eisai hyperbilirubinemic rats. Biochem Biophys Res Comm. 2006;340:215–220. doi: 10.1016/j.bbrc.2005.11.179. [DOI] [PubMed] [Google Scholar]
- 15.Needham M, Mastaglia FL. Statin myotoxicity: a review of genetic susceptibility factors. Neuromuscul Disord. 2014;24:4–15. doi: 10.1016/j.nmd.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Obayashi H, Nezu Y, Yokota H, et al. Cerivastatin induces type-I fiber-, not type-II fiber-, predominant muscular toxicity in the young male F344 rats. J Toxicol Sci. 2011;36:445–452. doi: 10.2131/jts.36.445. [DOI] [PubMed] [Google Scholar]
- 17.Sidaway J, Wang Y, Marsden AM, et al. Statin-induced myopathy in the rat: relationship between systemic exposure, muscle exposure and myopathy. Xenobiotica. 2009;39:90–98. doi: 10.1080/00498250802585539. [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Verity MA, Reue K. Lipin-1 regulates autophagy clearance and intersects with statin drug effects in skeletal muscle. Cell Metab. 2014;20:267–279. doi: 10.1016/j.cmet.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armitage J. The safety of statins in clinical practice. Lancet. 2007;370:1781–1790. doi: 10.1016/S0140-6736(07)60716-8. [DOI] [PubMed] [Google Scholar]
- 20.Link E, Parish S, et al. Search Collaborative Group. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 21.Niemi M. Transporter pharmacogenetics and statin toxicity. Clin Pharmacol Ther. 2010;87:130–133. doi: 10.1038/clpt.2009.197. [DOI] [PubMed] [Google Scholar]
- 22.Niemi M, Pasanen MK, Neuvonen PJ. Organic anion transporting polypeptide 1B1: a genetically polymorphic transporter of major importance for hepatic drug uptake. Pharmacol Rev. 2011;63:157–181. doi: 10.1124/pr.110.002857. [DOI] [PubMed] [Google Scholar]
- 23.DeGorter MK, Tirona RG, Schwarz UI, et al. Clinical and pharmacogenetic predictors of circulating atorvastatin and rosuvastatin concentrations in routine clinical care. Circ Cardiovasc Gen. 2013;6:400–408. doi: 10.1161/CIRCGENETICS.113.000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tonelli M, Wanner C Kidney Disease. Improving Global Outcomes Lipid Guideline Development Work Group Members: Lipid management in chronic kidney disease: synopsis of the kidney disease: Improving Global Outcomes 2013 clinical practice guideline. Ann Intern Med. 2014;160 doi: 10.7326/M13-2453. [DOI] [PubMed] [Google Scholar]
- 25.Dreier JP, Endres M. Statin-associated rhabdomyolysis triggered by grapefruit consumption. Neurology. 2004;62 doi: 10.1212/wnl.62.4.670. [DOI] [PubMed] [Google Scholar]
- 26.Wilke RA, Ramsey LB, Johnson SG, et al. The clinical pharmacogenomics implementation consortium: CPIC guideline for SLCO1B1 and simvastatin-induced myopathy. Clin Pharmacol Ther. 2012;92:112–117. doi: 10.1038/clpt.2012.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenson RS, Baker SK, Jacobson TA, Kopecky SL, Parker BA. The National Lipid Association’s Muscle Safety Expert Panel: An assessment by the Statin Muscle Safety Task Force: 2014 update. J Clin Lipidol. 2014;8:58–71. doi: 10.1016/j.jacl.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Gertz K, Dreier JP, Endres M. [The causes of statin induced myopathies] Nervenarzt. 2005;76:1006–1007. doi: 10.1007/s00115-005-1934-5. [DOI] [PubMed] [Google Scholar]
- 29.Joy TR, Monjed A, Zou GY, Hegele RA, McDonald CG, Mahon JL. N-of-1 (single-patient) trials for statin-related myalgia. Ann Intern Med. 2014;160:301–310. doi: 10.7326/M13-1921. [DOI] [PubMed] [Google Scholar]
- 30.Mampuya WM, Frid D, Rocco M, et al. Treatment strategies in patients with statin intolerance: the Cleveland Clinic experience. Am Heart J. 2013;166:597–603. doi: 10.1016/j.ahj.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanner C, Tonelli M, Kidney Disease Improving global outcomes lipid guideline development work group members: KDIGO Clinical practice guideline for lipid management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Intern. 2014;85:1303–1309. doi: 10.1038/ki.2014.31. [DOI] [PubMed] [Google Scholar]
- 32.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015 doi: 10.1056/NEJMoa1410489. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Cannon CP, Shah S, Dansky HM, et al. Safety of anacetrapib in patients with or at high risk for coronary heart disease. N Engl J Med. 2010;363:2406–2415. doi: 10.1056/NEJMoa1009744. [DOI] [PubMed] [Google Scholar]
- 34.Davidson M, Liu SX, Barter P, et al. Measurement of LDL-C after treatment with the CETP inhibitor anacetrapib. J Lipid Res. 2013;54:467–472. doi: 10.1194/jlr.M032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholls SJ, Brewer HB, Kastelein JJ, et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA. 2011;306:2099–2109. doi: 10.1001/jama.2011.1649. [DOI] [PubMed] [Google Scholar]
- 36.Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 37.Sabatine MS, Giugliano RP, Wiviott SD, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. doi: 10.1056/NEJMoa1500858. [DOI] [PubMed] [Google Scholar]
- 38.Mancini GB, Tashakkor AY, Baker S, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can J Med. 2013;29:1553–1568. doi: 10.1016/j.cjca.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Mancini GB, Baker S, Bergeron J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Can J Cardiol. 2011;27:635–662. doi: 10.1016/j.cjca.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Joy TR, Hegele RA. Narrative review: statin-related myopathy. Ann Intern Med. 2009;150:858–868. doi: 10.7326/0003-4819-150-12-200906160-00009. [DOI] [PubMed] [Google Scholar]
- e1.Glass CK, Witztum JL. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- e2.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e3.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- e4.Chew DP, Anderson FA, Avezum A, et al. Six-month survival benefits associated with clinical guideline recommendations in acute coronary syndromes. Heart. 2010;96:1201–1206. doi: 10.1136/hrt.2009.184853. [DOI] [PubMed] [Google Scholar]
- e5.Chowdhury R, Khan H, Heydon E, et al. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- e6.Arzneimittelkommission der deutschen Ärzteschaft Auf einen Blick. Fettstoffwechselstörungen. Handlungsleitlinie Fettstoffwechselstörungen aus Empfehlungen zur Therapie von Fettstoffwechselstörungen (3rd edition) www.akdae.de/Arzneimitteltherapie/TE/A-Z/PDF_Kurzversion/Fettstoffwechselstoerungen_k.pdf. (last accessed on 12 August 2015)
- e7.Bundesärztekammer, Kassenärztliche Vereinigung, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. Programm für Nationale Versorgungsleitlinien. Nationale Versorgungsleitlinie Chronische KHK. Langfassung. www.leitlinien.de/mdb/downloads/nvl/khk/khk-3aufl-vers1-lang.pdf. 3rd Edition. Version 1, 2014. (last accessed on 12 August 2015)
- e8.European Association for Cardiovascular Prevention and Rehabilitation, Reiner Z, Catapano AL et. al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS) Eur Heart J. 2011;32:1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- e9.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288:462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- e10.Buettner C, Rippberger MJ, Smith JK, Leveille SG, Davis RB, Mittleman MA. Statin use and musculoskeletal pain among adults with and without arthritis. Am J Med. 2012;125:176–182. doi: 10.1016/j.amjmed.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e11.Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–534. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e12.Bitzur R, Cohen H, Kamari Y, Harats D. Intolerance to statins: mechanisms and management. Diabetes Care. 2013;36(Suppl 2):325–330. doi: 10.2337/dcS13-2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e13.Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23:1182–1186. doi: 10.1007/s11606-008-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e14.Cohen JD, Brinton EA, Ito MK, Jacobson TA. Understanding statin use in america and gaps in patient education (USAGE): an internet-based survey of 10,138 current and former statin users. J Clin Lipidol. 2012;6:208–215. doi: 10.1016/j.jacl.2012.03.003. [DOI] [PubMed] [Google Scholar]
- e15.Kashani A, Phillips CO, Foody JM, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–2797. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- e16.Newman CB, Tobert JA. Statin intolerance: reconciling clinical trials and clinical experience. JAMA. 2015;313:1011–1012. doi: 10.1001/jama.2015.1335. [DOI] [PubMed] [Google Scholar]
- e17.Parker BA, Capizzi JA, Grimaldi AS, et al. Effect of statins on skeletal muscle function. Circulation. 2013;127:96–103. doi: 10.1161/CIRCULATIONAHA.112.136101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e18.Mohaupt MG, Karas RH, Babiychuk EB, et al. Association between statin-associated myopathy and skeletal muscle damage. Can Med Assoc J. 2009;181:E11–E18. doi: 10.1503/cmaj.081785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.Phillips PS, Haas RH, Bannykh S, et al. Statin-associated myopathy with normal creatine kinase levels. Ann Intern Med. 2002;137:581–585. doi: 10.7326/0003-4819-137-7-200210010-00009. [DOI] [PubMed] [Google Scholar]
- e20.Lamperti C, Naini AB, Lucchini V, et al. Muscle coenzyme Q10 level in statin-related myopathy. Arch Neurol. 2005;62:1709–1712. doi: 10.1001/archneur.62.11.1709. [DOI] [PubMed] [Google Scholar]
- e21.Knauer MJ, Urquhart BL, et al. Meyer zu Schwabedissen HE. Human skeletal muscle drug transporters determine local exposure and toxicity of statins. Circ Res. 2010;106:297–306. doi: 10.1161/CIRCRESAHA.109.203596. [DOI] [PubMed] [Google Scholar]
- e22.Rodrigues AC. Efflux and uptake transporters as determinants of statin response. Exp Opin Drug Metab Toxicol. 2010;6:621–632. doi: 10.1517/17425251003713519. [DOI] [PubMed] [Google Scholar]
- e23.Bersot T. Drug therapy for hypercholesterolemia and dyslipidemia. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman & Gilman’s pharmacological basis of therapeutics. 12th edition. New York: McGraw-Hill; 2011. pp. 877–905. [Google Scholar]
- e24.Sakamoto K, Kimura J. Mechanism of statin-induced rhabdomyolysis. J Pharmacol Sci. 2013;123:289–294. doi: 10.1254/jphs.13r06cp. [DOI] [PubMed] [Google Scholar]
- e25.Gong IY, Kim RB. Impact of genetic variation in OATP transporters to drug disposition and response. Drug Metab Pharmacokinet. 2013;28:4–18. doi: 10.2133/dmpk.dmpk-12-rv-099. [DOI] [PubMed] [Google Scholar]
- e26.Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genom. 2006;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- e27.Vladutiu GD, Simmons Z, Isackson PJ, et al. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve. 2006;34:153–162. doi: 10.1002/mus.20567. [DOI] [PubMed] [Google Scholar]
- e28.Vladutiu GD, Isackson PJ, Kaufman K, et al. Genetic risk for malignant hyperthermia in non-anesthesia-induced myopathies. Mol Genet Metab. 2011;104:167–173. doi: 10.1016/j.ymgme.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e29.Baker SK, Vladutiu GD, Peltier WL, Isackson PJ, Tarnopolsky MA. Metabolic myopathies discovered during investigations of statin myopathy. Can J Neurol Sci. 2008;35:94–97. doi: 10.1017/s0317167100007630. [DOI] [PubMed] [Google Scholar]
- e30.Ruano G, Windemuth A, Wu AH, et al. Mechanisms of statin-induced myalgia assessed by physiogenomic associations. Atheroscler. 2011;218:451–456. doi: 10.1016/j.atherosclerosis.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e31.Mangravite LM, Engelhardt BE, Medina MW, et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature. 2013;502:377–380. doi: 10.1038/nature12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e32.Carr DF, Alfirevic A, Johnson R, Chinoy H, van Staa T, Pirmohamed M. GATM gene variants and statin myopathy risk. Nature. 2014;513 doi: 10.1038/nature13628. [DOI] [PubMed] [Google Scholar]
- e33.Oh J, Ban MR, Miskie BA, Pollex RL, Hegele RA. Genetic determinants of statin intolerance. Lipids Health Dis. 2007;6 doi: 10.1186/1476-511X-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e34.Isackson PJ, Ochs-Balcom HM, Ma C, et al. Association of common variants in the human eyes shut ortholog (EYS) with statin-induced myopathy: evidence for additional functions of EYS. Muscle Nerve. 2011;44:531–538. doi: 10.1002/mus.22115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e35.Naci H, Brugts J, Ades T. Comparative tolerability and harms of individual statins: a study-level network meta-analysis of 246 955 participants from 135 randomized, controlled trials. Circ Cardiovasc Qual Outcomes. 2013;6:390–399. doi: 10.1161/CIRCOUTCOMES.111.000071. [DOI] [PubMed] [Google Scholar]
- e36.Kearney PM, Blackwell L, et al. Cholesterol Treatment Trialists Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- e37.Ghatak A, Faheem O, Thompson PD. The genetics of statin-induced myopathy. Atheroscler. 2010;210:337–343. doi: 10.1016/j.atherosclerosis.2009.11.033. [DOI] [PubMed] [Google Scholar]
- e38.Bookstaver DA, Burkhalter NA, Hatzigeorgiou C. Effect of coenzyme Q10 supplementation on statin-induced myalgias. Am J Cardiol. 2012;110:526–529. doi: 10.1016/j.amjcard.2012.04.026. [DOI] [PubMed] [Google Scholar]
- e39.Caso G, Kelly P, McNurlan MA, Lawson WE. Effect of coenzyme q10 on myopathic symptoms in patients treated with statins. Am J Cardiol. 2007;99:1409–1412. doi: 10.1016/j.amjcard.2006.12.063. [DOI] [PubMed] [Google Scholar]
- e40.Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Intern. 2002;13:187–194. doi: 10.1007/s001980200012. [DOI] [PubMed] [Google Scholar]
- e41.Michalska-Kasiczak M, Sahebkar A, Mikhailidis DP, et al. Analysis of vitamin D levels in patients with and without statin-associated myalgia - a systematic review and meta-analysis of 7 studies with 2420 patients. Intern J Cardiol. 2015;178:111–116. doi: 10.1016/j.ijcard.2014.10.118. [DOI] [PubMed] [Google Scholar]
- e42.Zlatohlavek L, Vrablik M, Grauova B, Motykova E, Ceska R. The effect of coenzyme Q10 in statin myopathy. Neuro Endocrinol Lett. 2012;33(Suppl 2):98–101. [PubMed] [Google Scholar]
- e43.Fedacko J, Pella D, Fedackova P, et al. Coenzyme Q(10) and selenium in statin-associated myopathy treatment. Can J Physiol Pharmacol. 2013;91:165–170. doi: 10.1139/cjpp-2012-0118. [DOI] [PubMed] [Google Scholar]
- e44.Glueck CJ, Budhani SB, Masineni SS, et al. Vitamin D deficiency, myositis-myalgia, and reversible statin intolerance. Curr Med Res Opin. 2011;27:1683–1690. doi: 10.1185/03007995.2011.598144. [DOI] [PubMed] [Google Scholar]
- e45.Kurnik D, Hochman I, Vesterman-Landes J, et al. Muscle pain and serum creatine kinase are not associated with low serum 25(OH) vitamin D levels in patients receiving statins. Clinical Endocrinol. 2012;77:36–41. doi: 10.1111/j.1365-2265.2011.04321.x. [DOI] [PubMed] [Google Scholar]
- e46.Riphagen IJ, van der Veer E, Muskiet FA, DeJongste MJ. Myopathy during statin therapy in the daily practice of an outpatient cardiology clinic: prevalence, predictors and relation with vitamin D. Curr Med Res Opin. 2012;28:1247–1252. doi: 10.1185/03007995.2012.702102. [DOI] [PubMed] [Google Scholar]
- e47.Moriarty PM, Jacobson TA, Bruckert E, et al. Efficacy and safety of alirocumab, a monoclonal antibody to PCSK9, in statin-intolerant patients: design and rationale of ODYSSEY ALTERNATIVE, a randomized phase 3 trial. J Clin Lipidol. 2014:554–561. doi: 10.1016/j.jacl.2014.09.007. [DOI] [PubMed] [Google Scholar]
- e48.Stroes E, Colquhoun D, Sullivan D, et al. Anti-PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS-2 randomized, placebo-controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. doi: 10.1016/j.jacc.2014.03.019. [DOI] [PubMed] [Google Scholar]