Abstract
Atherogenic dyslipidemia, characterized by an increased level of lipoprotein (a) and a decreased level of adiponectin, is a major risk factor for cardiovascular diseases in diabetic patients. To reduce cardiovascular risk in diabetic patients, use of agents with antidiabetic and anti-atherogenic potential is required. Using an animal model of diabetes, we investigated the antiatherogenic potential of extracts of three medicinal plants: jujube, barberry, and saffron. For this, serum level of fasting blood glucose, lipid profile, malondialdehyde, total antioxidant capacity, adiponectin and lipoprotein (a) in diabetic control and extract treated groups were measured. Statistical analysis of measurements showed that serum levels of fasting blood glucose, triglyceride, and VLDL decreased significantly (P < 0.05) in all treated groups. Treatment with all extracts reduced lipid peroxidation and increased antioxidant capacity of the experimental diabetic groups. Serum adiponectin levels increased in all treated groups, whereas lipoprotein (a) levels decreased, most markedly when treated with jujube extract. Jujube, saffron, and barberry extracts are beneficial in ameliorating oxidative stress and atherogenic risk of diabetic rats. This highlights the benefits of further investigating the cardio-protective potential of medicinal plant extracts and evaluating their usefulness as cardio protective agents in clinical practice.
Keywords: Atherogenic dyslipidemia, adiponectin, antioxidant, diabetes, medicinal plants
Introduction
The increasing incidence of diabetes mellitus (DM) is a major global public health concern (Shane-McWhorter, 2005[32]). Diabetes requires long-term medical care for glycemic control; decreases the quality of life because of complications (such as retinopathy, neuropathy, and nephropathy); and leads to a large increase in medical expenditure (Chen et al., 2013[8]). In addition, diabetes increases the risk of life-threatening diseases, including cardiovascular diseases (CVDs) and cancer (Seshasai et al., 2011[31]). The prevention of diabetes is therefore an issue of high priority. There is considerable evidence that hyperglycemia-mediated oxidative stress is the major cause of complications of DM (Davi et al., 2005[11]; Pari and Latha, 2005[27]) and lipid peroxide-mediated tissue damage is observed in the development of type I and type II DM (Feillet-Coudray et al., 1999[13]). Dyslipidemia is a major CVD risk factor in DM. The characteristic features of diabetic dyslipidemia are high plasma triglyceride levels, low HDL cholesterol levels, and increased concentrations of small dense LDL-cholesterol particles (Cohn and Sernyak, 2006[10]). Several studies report high plasma levels of lipoprotein (a) [Lp(a)] in diabetic patients (Levitsky et al., 1991[21]). Lp(a) consists of a carbohydrate-rich protein named apo(a) linked by a single disulfide bond to apoB of an LDL-like lipoprotein. Like LDL, Lp(a) is thought to be atherogenic. Because of the structural homology of apo(a) to plasminogen, it is also thought to have thrombogenic properties. The possibility of proatherogenic and prothrombotic properties has led to a surge in research on the role of Lp(a) in atherosclerosis (Purnell et al., 1995[28]). The atherogenic index of plasma (AIP), defined as the logarithm (log) of the plasma concentration ratio of triglycerides to high-density lipoprotein (HDL) cholesterol, was recently proposed as a predictive marker for plasma atherogenicity, and is positively correlated with the risk of CVD (Geohas et al., 2007[15]). Adipose tissue is considered as an important endocrine organ that secretes many biologically active substances, collectively known as adipocytokines (Satoh et al., 2004[30]). The major adipocytokine, adiponectin, is thought to play an important role in the regulation of cardiovascular and metabolic homeostasis. Adiponectin increases tissue fat oxidation, leading to reduced levels of fatty acids and tissue triglyceride content, thus increasing insulin sensitivity (Satoh et al., 2004[30]). Paradoxically, diabetes patients have decreased plasma adiponectin concentrations (Ashiuchi et al., 2002[2]), suggesting that hypoadiponectinemia is involved in the pathophysiology of DM. Another key element in development and progression of DM is oxidative stress, and use of antioxidants such as vitamins E and C improves the action of insulin in diabetic patients (Ceriello, 2000[7]; Maritim et al., 2003[23]). Accordingly, dietary supplementation with nutrients rich in antioxidants could ameliorate hyperglycemia-mediated stress in DM. Traditional antidiabetic plants might provide new oral hypoglycemic compounds which may counter the high cost and poor availability of the current medicines. B. vulgaris, C. sativus, and Z. jujuba have antidiabetic and antioxidant effects on diabetic patient (Farkhondeh and Samarghandian, 2014[12]; Kaleem et al., 2014[20]; Meliani et al. 2011[24]). There is a direct correlation between antioxidant activity and phenolic content of Z. jujuba (Li et al, 2005[22]). B. vulgaris has a high antioxidant capacity due to its high levels of total phenolic, flavonoid (such as anthocyanin), and other polyphenolic compounds (Özgen et al, 2012[26]). The methanolic extract of Crocus sativus and its components such as safranal, crocin, etc. are reported to have radical scavenging activity (Bhargava, 2011[6]). To better understand the action of B. vulgaris, C. sativus, and Z. jujuba in diabetes, and also to compare between effectiveness of the plants in treatment of diabetes, we studied the effects of hydroalcoholic and aqueous extracts of these plants on atherogenicity and risk of CVD in experimental diabetes.
Materials and Methods
Animal experiments
Adult male Wistar-derived rats with a mass of 200 to 220 g, bred and raised at the Birjand University of Medical Sciences animal quarters, housed at five rats per cage, were fed a rat chow diet (Pars Dam Co, Tehran, Iran) and given water ad libitum. Diabetes was induced by intraperitoneal injection of streptozotocin (60 mg/kg body mass) two weeks before starting treatment. Two weeks after injection, animals with plasma glucose levels exceeding 16.6 mM were considered to be diabetic. We randomly grouped 65 diabetic rats as follows: Groups 1 to 6 comprised of diabetic rats receiving hydroalcoholic extracts of barberry, saffron, and jujube at doses of 25 and 100 mg/kg, respectively. Groups 7 to 12 received an equal dose of aqueous extracts of these plants. Extracts were administered orally for 21 days. Diabetic (group 13) and healthy (group 14) control rats received water instead of extract. At the end of the 3-week treatment, blood samples were collected and biochemical parameters were measured. Different concentrations of aqueous and alcoholic extracts of the plants were prepared on the basis of previous studies (Taati et al., 2011[34]; Abdel-Zaher et al. 2005[1]). Results of the similar studies led us to use the selected doses of the medicinal plants (Meliani et al., 2011[24]; Gulfraz et al., 2008[17]).
Measurement of FBG and lipid profile, and assessment of the atherogenic index
Serum concentrations of FBG, triglyceride, total cholesterol, and HDL-C were measured by photometric methods using diagnostic kits (Pars Azmun, Iran). The atherogenic index of plasma (AIP), calculated as log [TG]/[HDL-C] is used as a significant predictor of atherosclerosis (Nwagha et al, 2010[25]).
Measurement of Adiponectin and Lp(a)
Serum adiponectin and Lp(a) concentrations were estimated using specific enzyme-linked immunosorbent assays (Glory Sciences, Taiwan). For each group, assays were performed in duplicate. The experimental protocol was approved by the ethics committee of the Birjand University of Medical Sciences.
Ferric Reducing Antioxidant Power (FRAP) Assay
Total serum antioxidant power was measured with the FRAP assay of Benzie and Strain (1996[5]). In brief, antioxidants in serum reduce ferric iron to its ferrous form at low pH, leading to the formation of a colored ferrous-tripyridyltriazine complex. Two ml of FRAP reagent (300 mM sodium acetate buffer pH 3.6, 10 mM 2,4,6-tris (2-pyridyl)-S-triazine (TPTZ) in HCl, and 20 mM FeCl3.6H2O, mixed in a proportion of 10:1:1 (v/v) was added to 50 µl of sample. After 15 min, the absorbance at 593 nm was read (Benzie and Strain, 1996[5]).
Measurement of Thiobarbituric Acid Reactive Substances (TBARS)
Malondialdehyde (MDA) levels were measured using the Thiobarbituric acid reactive substances (TBARS) method (Basu et al, 2009[4]) in blood, collected from the heart at the end of treatment period. Plasma samples (300 µl) were added to 3 ml TBARS reagent (7.5 g trichloroacetic acid, 187 mg TBA, and 6.25 ml chloridric acid), the mixture was heated in boiling water bath for 20 min, and the absorbance at 532 nm was determined.
Statistical analysis
Data was analyzed using One-Way ANOVA with SPSS version 16 software (SPSS Inc., Chicago, IL, USA). The statistical significance of differences in mean levels of FBS, Lp(a), etc. between the control and treated groups was evaluated using Student’s t-test. P-values of 0.05 or less were considered significant. Graphs were drawn with GraphPad Prism version 5 (GraphPad Software Inc., La Jolla, CA, USA).
Results
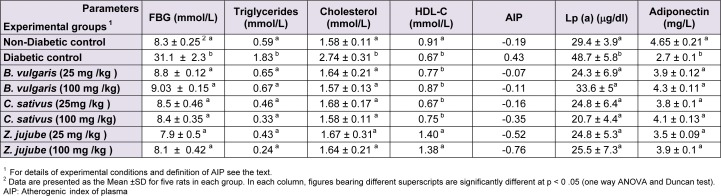
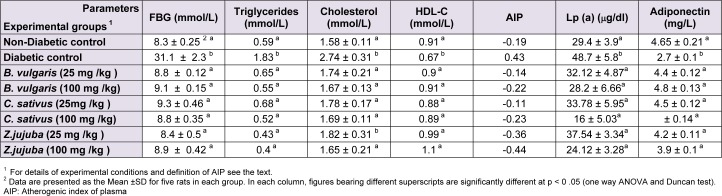
Compared with the diabetic control group, rats treated for 21 days with aqueous or hydroalcoholic extracts of B. vulgaris, C. sativus, and Z. jujuba had significantly reduced serum levels of FBG and triglyceride (p < 0.05) (Tables 1(Tab. 1) and 2(Tab. 2)). Oral administration of these extracts also significantly improved lipid profile of diabetic rats. Consumption of B. vulgaris, C. sativus, and Z. jujuba reduced total cholesterol levels to those of the normoglycemic control group (Tables 1(Tab. 1) and 2(Tab. 2)), and in contrast only Z. jujuba extracts at the two specified doses could increase HDL-C levels. Results represented in Tables 1 and 2, indicate hypolipidemic effects of the plants used in the present study. B. vulgaris, C. sativus, and Z. jujuba could improve increased level of triglycerides in diabetic rats. Compared to the nondiabetic control group, diabetic control rats had significantly increased serum Lp(a) levels (p < 0.05) (Table 1(Tab. 1)). Treatment with the plant extracts reduced their Lp(a) levels to those of the nondiabetic control group. The result presented in Table 1(Tab. 1) and 2(Tab. 2) show that diabetic control group had lower levels of adiponectin than normoglycemic group. Treatment with saffron, jujube, and barberry extracts increased their adiponectin levels. The AIP of diabetic rats decreased significantly following administration of saffron, jujube and barberry extracts (Tables 1(Tab. 1) and 2(Tab. 2)). In Tables 1(Tab. 1) and 2(Tab. 2), figures bearing different superscripts (a and b) are significantly different at p < 0 .05 (one way ANOVA and Duncan test).
Table 1. The effects of hydroalcoholic extracts of B. Vulgaris, C. Sativus and Z. Jujuba on lipid profile and AIP in diabetic groups.
Table 2. The effects of aqueous extracts of B. Vulgaris, C. Sativus and Z. Jujuba on lipid profile and AIP in diabetic groups.
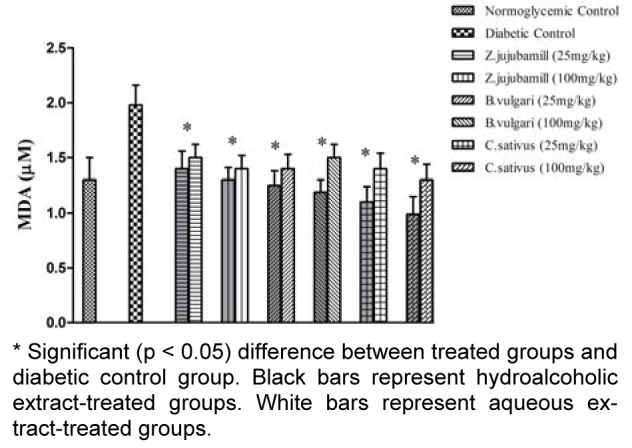
The plant extracts ameliorated hyperglycemia-mediated oxidative stress in diabetes and improved the antioxidant capacity of the experimental groups compared to diabetic control group (Figures 1(Fig. 1) and 2(Fig. 2)). As shown in Figure 1(Fig. 1), the hydroalcoholic extracts increased antioxidant capacity more effectively than the aqueous extracts. This effect was more notable when using the jujube extract than with the other extracts. As shown in Figure 2(Fig. 2), the medicinal plant extracts reduced lipid peroxidation. The saffron extracts were more effective than the other plant extracts, and the hydroalcoholic extract was more effective than the aqueous extract.
Figure 1. The effect of hydroalcoholic and aqueous extract of barberry, saffron and jujube on antioxidant capacity (Mean ± SD).
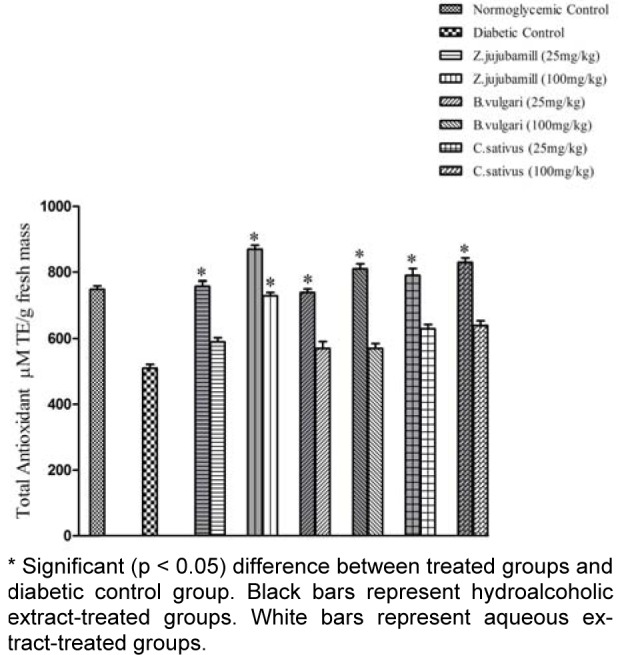
Figure 2. The effect of hydroalcoholic and aqueous extract of barberry, saffron and jujube on malondialdehyde (Mean ± SD).
Discussion
It is well known that the incidence of CVD in patients with diabetes is high. Although CVD pathogenesis in diabetes is multifactorial, dyslipidemia is a powerful risk factor (Suryawanshi et al., 2006[33]). In addition, oxidative stress has a key role in this process (Hambali et al., 2011[19]). In the present study antiatherogenic and antioxidant effects of three medicinal plants B. vulgaris, C. sativus, and Z. jujuba were compared. Our results show that saffron, jujube, and barberry extracts may have antiatherogenic effects in diabetic rat models, which is likely to be related to the antioxidant capacities of the extracts. We found that treating diabetic rats with aqueous and hydroalcoholic extracts of B. vulgaris, C. sativus, and Z. jujuba effectively decreased their elevated FBS levels. The hypoglycemic effects of the medicinal plants were previously reported (Meliani et al., 2011[24]). Oral administration of hydroalcoholic extracts of C. sativus, B. vulgaris, and Z. jujuba significantly (P < 0.05) increased the serum adiponectin levels of diabetic rats. Considering the results of similar studies, we may conclude that adiponectin has a reverse relationship with glucose, triglyceride, VLDL, and cholesterol, and a direct relationship with HDL-C (Qiao et al., 2008[29]). Given the role of adiponectin in glucose and lipid metabolism, it seems that the hypoglycemic and hypolipidemic effects of the plants could be affected through changes in adiponectin levels. The AIPs of the different groups indicate that saffron, jujube and barberry extracts have anti-atherogenic effects (Table 1(Tab. 1), 2(Tab. 2)). Concentrations of Lp(a) were higher in the diabetic control group than in the nondiabetic control group (Table 1(Tab. 1)). Elevated levels of Lp(a) in type I DM were found in other studies (Haffner, 1993[18]; Giacco and Brownlee, 2010[16]). Serum Lp(a) levels significantly decreased in all treated groups (Tables 1(Tab. 1) and 2(Tab. 2)). These medicinal plant extracts may therefore reduce the high risk of cardiovascular complications in experimental diabetes. Use of antioxidant-rich nutrients to reduce atherogenic modifications has been studied previously (Aviram et al., 2000[3]). Our measurement of antioxidant capacity in diabetic rats revealed high glucose- mediated oxidative stress, which was relieved by oral consumption of jujube, saffron, and barberry extracts. Among the different plant extracts used in our study, jujube had a higher antioxidant capacity. It appears that use of natural sources of antioxidants such as barberry, saffron, and especially jujube, is useful in treatment of diabetes and associated CVD. It was suggested that the possible antioxidant activities of extracts are due to the presence of tannins, carotenes, and flavonoids in some Ziziphus species (Gao et al., 2012[14]; Taati et al., 2011[34]) and a direct correlation was found between the antioxidant activity and phenolic contents (Li et al., 2005[22]). Adiponectin as an antioxidant is inversely correlated with oxidative stress, inflammation, and chronic diseases such as diabetes (Chen et al., 2012[8]). Dyslipidemia is a common feature of DM. Our study supports the finding that the diabetic dyslipidemia comprising of elevated total cholesterol, TG, VLDL, Lp(a), and low HDL-C and adiponectin levels persists in diabetes. Lp(a) may be considered as an important independent risk marker to identify diabetes patients who may develop CVD. In this study, we found that jujube, saffron, and barberry extracts especially hydroalcoholic extract could reduce risk of CVD by improving the lipid profile, AIP, and Lp(a) levels. TG and HDL are the parameters that affect AIP. In the present study, herbal medicine consumption improved lipid profile, also AIP decreased to normoglycemic group. According to the role of Lp(a) in atherogenicity, and considering that consumption of the extracts especially jujube reduced Lp(a), we can expect reduced level of AIP in treatment groups. A more accurate explanation will obtain from consumption of the extracts by normoglycemic rats and investigation of changes in lipid profile and AIP of the experimental groups. According to the results of the FRAP assay, oral administration of the extracts increased total antioxidant capacity and AIP in treatment groups could be in associate with total antioxidant capacity. These beneficial effects especially for saffron and jujube could be taken into consideration when designing new drugs. Based on our data, adding jujube and saffron extracts to diets for diabetic patients may help to improve control of diabetic complications. Further experimental and clinical investigation is needed to determine the effective dosage of the plant extracts in clinical practice.
Acknowledgements
This investigation was supported by Grant No. 711 from the office of Vice Chancellor for Research, Birjand University of Medical Sciences.
Conflict of interest
The authors declare that they have no conflict interests.
References
- 1.Abdel-Zaher AO, Salim SY, Assaf MH, Abdel-Hady RH. Antidiabetic activity and toxicity of Zizyphus spina-christi leaves. J Ethnopharm. 2005;101:129–138. doi: 10.1016/j.jep.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Ashiuchi M, Kuwana E, Yamamoto T, Komatsu K, Soda K, Misono H. Glutamate racemase is an endogenous DNA gyrase inhibitor. J Biol Chem. 2002;277:39070–39073. doi: 10.1074/jbc.C200253200. [DOI] [PubMed] [Google Scholar]
- 3.Aviram M, Dornfeld L, Rosenblat M, Volkova N, Kaplan M, Coleman R, et al. Pomegranate juice consumption reduces oxidative stress, atherogenic modifications to LDL, and platelet aggregation: studies in humans and in atherosclerotic apolipoprotein E-deficient mice. Am J Clin Nutr. 2000;71:1062–1076. doi: 10.1093/ajcn/71.5.1062. [DOI] [PubMed] [Google Scholar]
- 4.Basu D, Khare G, Singh S, Tyagi A, Khosla S, Mande SC. A novel nucleoid-associated protein of Mycobacterium tuberculosis is a sequence homolog of GroEL. Nucleic Acids Res. 2009;37:4944–4954. doi: 10.1093/nar/gkp502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava V. Medicinal uses and pharmacological properties of Crocus sativus Linn (Saffron) Int J Pharm Pharmaceut Sci. 2011;3:22–26. [Google Scholar]
- 7.Ceriello A. Oxidative stress and glycemic regulation. Metabolism. 2000;49(2 Suppl 1):27–29. doi: 10.1016/s0026-0495(00)80082-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Zhang H, Broitman SL, Reiche M, Farrell I, Cooperman BS, et al. Dynamics of translation by single ribosomes through mRNA secondary structures. Nat Struct Mol Biol. 2013;20:582–588. doi: 10.1038/nsmb.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen SJ, Yen CH, Huang YC, Lee BJ, Hsia S, Lin PT. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One. 2012;7:e45693. doi: 10.1371/journal.pone.0045693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn TA, Sernyak MJ. Metabolic monitoring for patients treated with antipsychotic medications. Can J Psychiatry. 2006;51:492–501. doi: 10.1177/070674370605100804. [DOI] [PubMed] [Google Scholar]
- 11.Davì G, Falco A, Patrono C. Lipid peroxidation in diabetes mellitus. Antioxid Redox Signal. 2005;7:256–268. doi: 10.1089/ars.2005.7.256. [DOI] [PubMed] [Google Scholar]
- 12.Farkhondeh T, Samarghandian S. The effect of saffron (Crocus sativus L.) and its ingredients on the management of diabetes mellitus and dislipidemia. Afr J Pharm Pharmacol. 2014;8:541–549. [Google Scholar]
- 13.Feillet-Coudray C, Rock E, Coudray C, Grzelkowska K, Azais-Braesco V, Dardevet D, et al. Lipid peroxidation and antioxidant status in experimental diabetes. Clin Chim Acta. 1999;284:31–43. doi: 10.1016/s0009-8981(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 14.Gao QH, Wu CS, Yu JG, Wang M, Ma YJ, Li CL. Textural characteristic, antioxidant activity, sugar, organic acid, and phenolic profiles of 10 promising jujube (Ziziphus jujuba Mill.) selections. J Food Sci. 2012;77:C1218–C1225. doi: 10.1111/j.1750-3841.2012.02946.x. [DOI] [PubMed] [Google Scholar]
- 15.Geohas J, Daly A, Juturu V, Finch M, Komorowski JR. Chromium picolinate and biotin combination reduces atherogenic index of plasma in patients with type 2 diabetes mellitus: a placebo-controlled, double-blinded, randomized clinical trial. Am J Med Sci. 2007;333:145–153. doi: 10.1097/MAJ.0b013e318031b3c9. [DOI] [PubMed] [Google Scholar]
- 16.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulfraz M, Mehmood S, Ahmad A, Fatima N, Praveen Z, Williamson EM. Comparison of the antidiabetic activity of Berberis Lyceum root extract and berberine in alloxan-induced diabetic rats. Phytother Res. 2008;22:1208–1212. doi: 10.1002/ptr.2438. [DOI] [PubMed] [Google Scholar]
- 18.Haffner SM. Lipoprotein (a) and diabetes. An update. Diabetes Care. 1993;16:835–840. doi: 10.2337/diacare.16.5.835. [DOI] [PubMed] [Google Scholar]
- 19.Hambali Z, Ahmad Z, Arab S, Khazaai H. Oxidative stress and its association with cardiovascular disease in chronic renal failure patients. Indian J Nephrol. 2011;21:21–25. doi: 10.4103/0971-4065.75218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaleem WA, Muhammad N, Khan H, Rauf A. Pharmacological and phytochemical studies of Genus Zizyphus. Middle-East J Sci Res. 2014;21:1243–1263. [Google Scholar]
- 21.Levitsky LL, Scanu AM, Gould SH. Lipoprotein (a) levels in black and white children and adolescents with IDDM. Diabetes Care. 1991;14:283–287. doi: 10.2337/diacare.14.4.283. [DOI] [PubMed] [Google Scholar]
- 22.Li JW, Ding SD, Ding XL. Comparison of antioxidant capacities of extracts from five cultivars of Chinese jujube. Process Biochem. 2005;40:3607–3613. [Google Scholar]
- 23.Maritim AC, Sanders RA, Watkins JB., 3rd Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 24.Meliani N, Dib MEA, Allali H, Tabti B. Hypoglycaemic effect of Berberis vulgaris L. in normal and streptozotocin-induced diabetic rats. Asian Pac J Trop Biomed. 2011;1:468–471. doi: 10.1016/S2221-1691(11)60102-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nwagha UI, Ikekpeazu EJ, Ejezie FE, Neboh EE, Maduka IC. Atherogenic index of plasma as useful predictor of cardiovascular risk among postmenopausal women in Enugu, Nigeria. Afr Health Sci. 2010;10:248–252. [PMC free article] [PubMed] [Google Scholar]
- 26.Özgen M, Saraçoğlu O, Geçer EN. Antioxidant capacity and chemical properties of selected barberry (Berberis vulgaris L.) fruits. Horticult Environ Biotech. 2012;53:447–451. [Google Scholar]
- 27.Pari L, Latha M. Effect on lipid peroxidation in streptozotocin diabetes. Gen Physiol Biophys. 2005;24:13–26. [PubMed] [Google Scholar]
- 28.Purnell JQ, Marcovina SM, Hokanson JE, Kennedy H, Cleary PA, Steffes MW, et al. Levels of lipoprotein (a), apolipoprotein B, and lipoprotein cholesterol distribution in IDDM: results from follow-up in the Diabetes Control and Complications Trial. Diabetes. 1995;44:1218–1226. doi: 10.2337/diab.44.10.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiao L, Zou C, van der Westhuyzen DR, Shao J. Adiponectin reduces plasma triglyceride by increasing VLDL triglyceride catabolism. Diabetes. 2008;57:1824–1833. doi: 10.2337/db07-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satoh N, Naruse M, Usui T, Tagami T, Suganami T, Yamada K, et al. Leptin-to-adiponectin ratio as a potential atherogenic index in obese type 2 diabetic patients. Diabetes Care. 2004;27:2488–2490. doi: 10.2337/diacare.27.10.2488. [DOI] [PubMed] [Google Scholar]
- 31.Seshasai SR, Kaptoge S, Thompson A, Di Angelantonio E, Gao P, Sarwar N, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shane-McWhorter L. Botanical dietary supplements and the treatment of diabetes: what is the evidence? Current Diabetes Rep. 2005;5:391–398. doi: 10.1007/s11892-005-0099-8. [DOI] [PubMed] [Google Scholar]
- 33.Suryawanshi NP, Bhutey AK, Nagdeote AN, Jadhav AA, Manoorkar GS. Study of lipid peroxide and lipid profile in diabetes mellitus. Indian J Clin Biochem. 2006;21:126–130. doi: 10.1007/BF02913080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taati M, Alirezaei M, Meshkatalsadat M, Rasoulian B, Kheradmand A, Neamati S. Antioxidant effects of aqueous fruit extract of Ziziphus jujuba on ethanol-induced oxidative stress in the rat testes. Iranian J Vet Res. 2011;12:39–45. [Google Scholar]