Abstract
Merkel cell carcinoma (MCC) is a rare and highly aggressive neuroendocrine tumor of the skin which almost exclusively presents as a solitary tumor. It is most often seen on sun-exposed regions, historically almost exclusively on the head and neck, with only rare case reports on the extremities. Although recent studies have shown increased incidence with up to 20% on the extremities, here we present one of these rare emerging presentations, with the addition of a unique treatment option. Our patient is an 80-year-old male with a 3-month history of multiple raised, rapidly enlarging tumors on the right ankle. Two separate biopsies were performed and demonstrated sheets and clusters of small blue cells filling the dermis with scant cytoplasm, dusty chromatin, and nuclear molding. Subsequent immunohistochemical stains confirmed the diagnosis of multiple primary MCC. Despite the characteristic immunohistochemical profile of primary MCC, the possibility of a metastatic neuroendocrine carcinoma from an alternate primary site was entertained, given his unusual clinical presentation. A complete clinical workup including CT scans of the chest, abdomen, and pelvis showed no evidence of disease elsewhere. Instead of amputation, the patient opted for nonsurgical treatment with radiation therapy alone, resulting in a rapid and complete response. This case represents an unusual presentation of primary MCC and demonstrates further evidence that radiation as monotherapy is an effective local treatment option for inoperable MCC.
Key Words: Merkel cell carcinoma, Neuroendocrine carcinoma of the skin, Radiation therapy, Immunohistochemistry
Case Report
An 80-year-old Caucasian male presented for evaluation and management of multiple pruritic, painful nodules on his right lower extremity. These lesions had been enlarging over a 3-month duration. Biopsies from the right lateral and medial lower leg revealed neuroendocrine tumors consistent with poorly differentiated Merkel cell carcinoma (MCC).
Physical examination revealed 3 lesions on the right ankle. These shiny erythematous nodules displayed some focal hyperpigmentation and ranged in size from 1 to 3 cm in diameter. On palpation, all lesions were warm and slightly tender. No inguinal adenopathy was identified, and 1+ pitting edema of the involved right lower extremity was present. His past medical history was noncontributory.
Histologic review of the biopsy revealed a tumor extensively involving the entire thickness of the dermis that was distributed in sheets, clusters, and single cells. Tumor cells showed features including scant cytoplasm, dusty chromatin, and nuclear molding, and demonstrated dot-like cytokeratin (CK) 20 positivity (fig. 1). Additionally, the tumor cells were positive for synaptophysin, chromogranin, neuron-specific enolase (NSE), and epithelial membrane antigen (EMA), and negative for CD45, PSA, CK7, and thyroid-transcription factor 1 (TTF-1). Taking histologic and immunohistochemical findings into account, we confirmed a diagnosis of multiple primary cutaneous MCC. Given the rarity of this entity and anatomic location of lesions on the lower extremities, a differential diagnosis of metastatic neuroendocrine carcinoma from an alternate primary site was still considered (table 1) [1]. A complete clinical workup including CT imaging of the chest, abdomen, and pelvis revealed no evidence of disease elsewhere, further supporting our diagnosis of an unusual case of multiple cutaneous MCC involving the lower extremity.
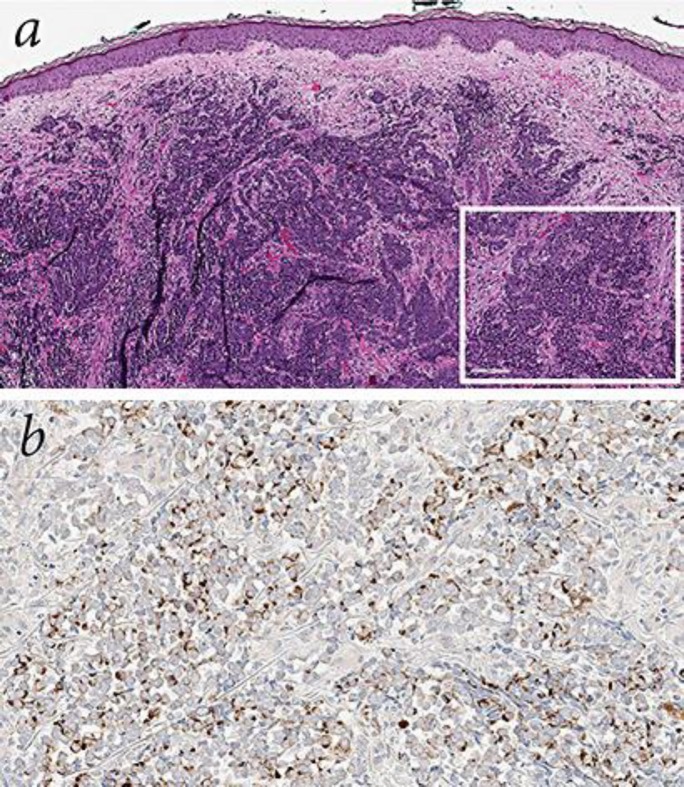
Fig. 1.
Histologic features of the lesion. a Hematoxylin-eosin: sheets and clusters of uniform, small, round blue cells filling the dermis, with scant cytoplasm, dusty chromatin, and nuclear molding (×9). Inset shows a higher-power view (×20). b Characteristic paranuclear, dot-like pattern of CK20 staining (×30). Synaptophysin, chromogranin, and NSE were expressed as well.
Table 1.
Immunohistochemical staining profile of MCC and its differentiation from neuroendocrine tumors and other entities in the differential diagnosis [2]
| CK20 | TTF-1 | CK7 | NSE | Chromo-granin | Synapto-physin | S100 | LCA/CD45 | AE1/AE3 | |
|---|---|---|---|---|---|---|---|---|---|
| MCC | + | − | − (+*) | + | + | + | − | − | + |
| SCLC | − | + | + | + | + | + | − | − | + |
| Lymphoma | − | − | − | − | − | − | − | + | − |
| Melanoma | − | − | − | + | − | − | + | − | − |
| Ewing sarcoma/primitive neuroectodermal tumor | − | − | − | + | − | +/− | +/− | − | +/− |
LCA = Leukocyte common antigen.
Rare cases of CK7-positive MCC have been reported [1].
Due to the rapid progression and location of these lesions, surgical management with excision and reconstruction would be an extensive endeavor. CT angiography studies in our patient demonstrated poor runoff below the knee, which indicated insufficient vascular support necessary for free flap reconstruction. When presented with amputation as the only surgical option, our patient instead elected radiation therapy for local control. Prior to the initiation of radiotherapy, he developed several small satellite lesions proximally on the same leg (fig. 2). A radiation plan was created to treat the entire lower leg with 46 Gy in 23 fractions using intensity modulated photons, sparing a small strip along the lateral edge to decrease the risk of lymphedema. The gross residual disease was boosted with electron fields for a total of 54 Gy in 27 fractions. The patient had a rewarding response to treatment with clearing of the small satellite lesions and marked decrease of the large lesions during treatment and final resolution at the end of treatment (fig. 3). The patient will continue to be closely monitored to confirm complete remission.
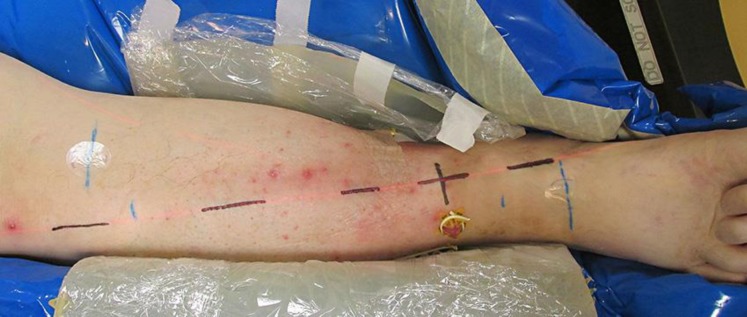
Fig. 2.
Clinical features of the lesions of the right anterior lower leg prior to treatment.
Fig. 3.
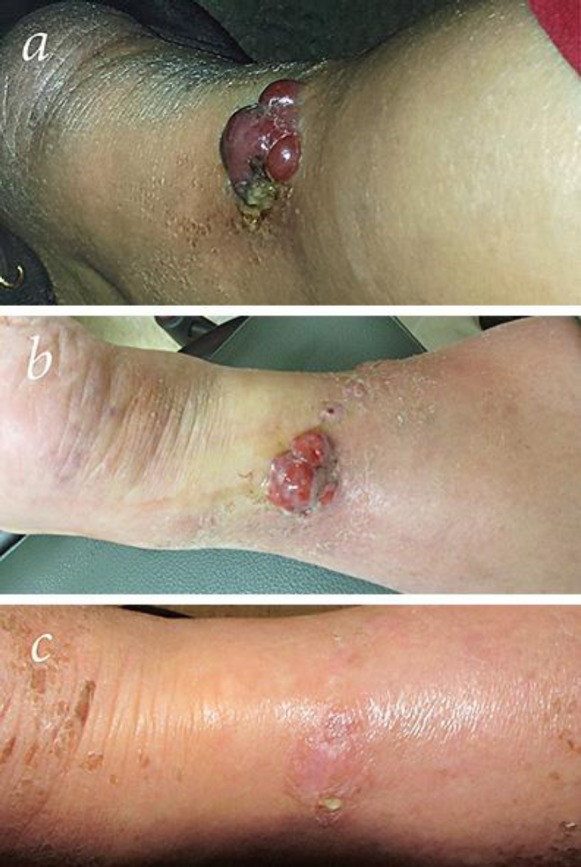
Clinical features of the right posterior lower leg prior to treatment (a), 2 weeks into radiation therapy (b), and 5 weeks after treatment (c).
Discussion
MCC is a rare, aggressive neuroendocrine tumor of the skin that most commonly presents in white males in their 7th or 8th decade of life [2]. It is a highly metastatic disease, with a 5-year disease-specific survival rate of 64% [3]. As survival has been shown to decrease rapidly with advancing disease stage, early recognition by clinicians may improve survival [3,4]. The most common site of presentation is on sun-exposed skin, usually the head and neck region, with 29-53% located here according to several studies [2,3,4]. Approximately 35-38% of cases involve the extremities [2,3], further broken down to 21% on the upper extremities and only 14-24% on the lower extremities [4,5]. Classically known to be a tumor of the head and neck region, primary cutaneous MCC has rarely been reported on the lower leg, with a review of the literature revealing only 5 case reports [5,6,7,8,9].
MCC often has a nonspecific clinical appearance and can present as persistent, asymptomatic pink to red solitary or grouped dome-shaped nodule(s), smaller than 2 cm, that grow rapidly over several months [2,4]. Interestingly, Heath et al. [4] found that the majority of initial clinical impressions at the time of biopsy were benign, with cyst or acneiform lesion being the most common presumptive diagnosis of later confirmed MCC. To assist clinicians in the recognition of lesions concerning for MCC, the authors described a mnemonic entitled ‘AEIOU’ (A for asymptomatic/lack of tenderness, E for expanding rapidly, I for immune suppression, O for older than 50 years, and U for ultraviolet-exposed site on a person with fair skin) [4].
Because of its often nonspecific clinical presentation, histologic confirmation is necessary to help diagnose and differentiate MCC from a metastasis emanating from another neuroendocrine carcinoma, such as small-cell lung cancer (SCLC), lymphoma, or melanoma. Histologically, MCC presents as uniform, small blue cells filling the dermis, with scant cytoplasm, dusty chromatin, and nuclear molding. The cells arrange in sheets and nests, spreading to the reticular dermis and subcutis, with occasional epidermal involvement [2]. MCC possesses both neuroendocrine and epithelial features, expressing neuroendocrine markers such as chromogranin, synaptophysin, NSE, and neurofilament, as well as epithelial markers like AE1/AE3, EMA, CAM 5.2, and CK20 [2,5]. The morphologic similarity between these cancers has made immunohistochemical staining invaluable, with dot-like, paranuclear expression of CK20 being a key differentiating marker for MCC [2]. In contrast, MCC is negative for CK7 and TTF-1, leukocyte common antigen/CD45, and S100, differentiating it from SCLC, lymphoma, and melanoma, respectively [2].
More recently, Merkel cell polyomavirus (MCPyV) has been found to be present in approximately 80% of all MCC [10,11]. This viral infection appears to play a role in the pathogenesis of MCC, as it is seen more frequently than expected among immunosuppressed transplant and AIDS patients, and was found to be integrated into the genome before clonal expansion of the tumor cells [10]. Two MCPyV-specific stains, Ab3 and CM2B4, have been developed for use in diagnosis [11,12].
Despite the aid that such immunohistochemical stains provide in the diagnosis of MCC, the possibility of a metastatic neuroendocrine carcinoma from an alternate primary site should be entertained. A complete clinical workup including CT scans of the chest, abdomen, and pelvis or PET scan can be performed to rule out this possibility.
Due to the low incidence of MCC, there are no clear treatment guidelines validated by prospective randomized controlled clinical trials. The historical standard of care has thus far been surgical excision with 2-3 cm margins and sentinel lymph node biopsy [3,13]. There is strong evidence for the addition of adjuvant radiation, but not for adjuvant chemotherapy [14]. Systemic therapies such as etoposide, vincristine, anthracyclines, antimetabolites, and platinum derivatives have all been used to treat advanced disease with high remission rates; however, multiple studies have shown no significant increase in survival time and even decreased survival, with a high degree of toxicity, given the elderly treatment population [3,13,14]. For this reason, adjuvant chemotherapy is generally not recommended. While adjuvant radiation has been shown to have clear added benefit when combined with surgical excision, more recent studies have found that radiation therapy as an exclusive treatment for unresectable disease has a control rate similar to that of conventional treatment (surgical excision plus adjuvant radiation) [13,14,15]. Doses of 50 Gy or more for subclinical disease and 55 Gy or more for gross disease are recommended to obtain field disease control [13,15].
In our patient, advanced age and anatomic location precluded the possibility of wide-local excision, leaving amputation as the only surgical option. He was offered radiation therapy alone and received a total of 54 Gy in 27 fractions to the bulky disease area, with 46 Gy in 23 fractions to the remaining leg. Our patient obtained an excellent clinical response, lasting 2 months at present despite extensive disease. This case highlights definitive radiotherapy as an effective treatment option for inoperable MCC due to advanced age, comorbidities, and/or cosmetic or functional deficits.
Statement of Ethics
The patient was informed of and agreed to the use of his health information with regard to this case and its possible publication and presentation at national conferences.
Disclosure Statement
The authors indicate no potential conflicts of interest.
References
- 1.Pilloni L, Manieli C, Senes G, Ribuffo D, Faa G. Merkel cell carcinoma with an unusual immunohistochemical profile. Eur J Histochem. 2009;53:e33. doi: 10.4081/ejh.2009.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong HH, Wang J. Merkel cell carcinoma. Arch Pathol Lab Med. 2010;134:1711–1716. doi: 10.5858/2009-0165-RSR2.1. [DOI] [PubMed] [Google Scholar]
- 3.Allen PJ, Bowne WB, Jaques DP, Brennan MF, Busam K, Coit DG. Merkel cell carcinoma: prognosis and treatment of patients from a single institution. J Clin Oncol. 2005;23:2300–2309. doi: 10.1200/JCO.2005.02.329. [DOI] [PubMed] [Google Scholar]
- 4.Heath M, Jaimes N, Lemos B, Mostaghimi A, Wang LC, Penas PF, Nghiem P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: the AEIOU features. J Am Acad Dermatol. 2008;58:375–381. doi: 10.1016/j.jaad.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strobel ES, Feyer P, Steingraber M, Schmitt-Graff A, Kohl PK. An unusual case of Merkel cell carcinoma. J Cancer Res Clin Oncol. 2008;134:119–123. doi: 10.1007/s00432-007-0257-2. [DOI] [PubMed] [Google Scholar]
- 6.Chatzinasiou F, Papadavid E, Korkolopoulou P, Levidou G, Panayiotides I, Theodoropoulos K, Pogka V, Asimakopoulos C, Rigopoulos D. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282–286. doi: 10.1111/dth.12238. [DOI] [PubMed] [Google Scholar]
- 7.Bassi A, Arunachalam M, Galeone M, Scarfi F, Maio V, Moretti S, Difonzo EM. Multiple clustered nodules on the leg. Diagnosis: Merkel cell carcinoma. J Clin Oncol. 2014;32:e61–e62. doi: 10.1200/JCO.2012.48.4204. [DOI] [PubMed] [Google Scholar]
- 8.Mota-Burgos A, Castillo-Munoz R, Herrera-Ceballos E. Tumor-like lesions grouped on a patient's leg. Actas Dermosifiliogr. 2013;104:159–160. doi: 10.1016/j.ad.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 9.Roda DJ, Albano B, Rathore B, Zhou L. Merkel cell carcinoma of the lower extremity: a case report. J Am Podiatr Med Assoc. 2014;104:422–425. doi: 10.7547/0003-0538-104.4.422. [DOI] [PubMed] [Google Scholar]
- 10.Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodig SJ, Cheng J, Wardzala J, DoRosario A, Scanlon JJ, Laga AC, Martinez-Fernandez A, Barletta JA, Bellizzi AM, Sadasivam S, Holloway DT, Cooper DJ, Kupper TS, Wang LC, DeCaprio JA. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J Clin Invest. 2012;122:4645–4653. doi: 10.1172/JCI64116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Busam KJ, Jungbluth AA, Rekthman N, Coit D, Pulitzer M, Bini J, Arora R, Hanson NC, Tassello JA, Frosina D, Moore P, Chang Y. Merkel cell polyomavirus expression in merkel cell carcinomas and its absence in combined tumors and pulmonary neuroendocrine carcinomas. Am J Surg Pathol. 2009;33:1378–1385. doi: 10.1097/PAS.0b013e3181aa30a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Curr Opin Oncol. 2012;24:141–149. doi: 10.1097/CCO.0b013e32834fc9fe. [DOI] [PubMed] [Google Scholar]
- 14.Garneski KM, Nghiem P. Merkel cell carcinoma adjuvant therapy: current data support radiation but not chemotherapy. J Am Acad Dermatol. 2007;57:166–169. doi: 10.1016/j.jaad.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prewett SL, Ajithkumar T. Merkel cell carcinoma: current management and controversies. Clin Oncol (R Coll Radiol) 2015;27:436–444. doi: 10.1016/j.clon.2015.04.007. [DOI] [PubMed] [Google Scholar]